Abstract
Boron neutron capture therapy (BNCT) is a highly targeted radiation therapy that shows great promise for treating tumors that are challenging to address with conventional methods. The dose deposited in the tumor during a treatment can be monitored by detecting prompt gamma rays at 478 keV generated by boron neutron capture reactions within the tumor cells. However, this task is highly challenging due to the significant background of neutrons and gamma rays present during treatment that risk to mask the useful signal. An additional challenge is represented by borated polyethylene typically used for radioprotection purposes in the walls of the treatment rooms, which generates gamma rays of the same energy of the ones of clinical interest. To address these issues, we propose a scintillator-based detection system, integrating a pinhole collimator, an artificial neural network for gamma-ray position reconstruction and a multi-layer shielding strategy. This system successfully imaged borated samples with concentrations as low as 1843 ppm of 10B, achieving a spatial resolution of approximately 1 cm, during neutron irradiation with a fluence rate of 107 n/cm2/s at the accelerator-based neutron facility at Nagoya University, demonstrating its potential for dose monitoring in clinical-like BNCT environments.
Similar content being viewed by others
Introduction
Cancer is a complex disease involving multiple cell types and genetic mutations and it ranks among the top causes of mortality worldwide1. Over recent decades, innovative radiotherapy modalities have emerged, with “hadron therapy” standing out for its ability to target tumors with high precision2. It employs charged particles, with protons and carbon ions as the most used, which exhibit a unique energy deposition profile known as the Bragg peak3. This characteristic enables the delivery of high radiation dose precisely to the tumor region, minimizing the exposure of the surrounding healthy tissues. Despite its success with well-defined tumors, hadron therapy faces challenges in treating diffuse or infiltrative cancers, especially those near critical structures or with irregular geometries. In this context, boron neutron capture therapy (BNCT) has emerged as a promising, highly tailored therapeutic option4.
BNCT exploits the interaction between a non-radioactive boron-10 (10B) compound, which is administered to the patient and selectively binds to tumor cells, and low-energy thermal neutrons used for irradiation5. Due to the high thermal neutron cross section of 10B, boron neutron capture reactions 10B(n,\(\alpha\))7Li occur, generating two high linear energy transfer (LET) alpha and lithium particles. These particles deposit their energy over a short range, confining the radiation dose to the tumor cell where the neutron capture reaction occurs6. This selective mechanism forms the basis of BNCT’s personalized treatment approach.
Although the concept of BNCT dates back to the 1930s, proposed by Locher7 after Chadwick’s discovery of neutrons8, it was not until the late 20th century that clinical trials began9. Initially, BNCT had limited diffusion due to its reliance on nuclear reactors for generating high-flux neutron beams, which posed logistic and safety challenges for medical use. Recent advancements in high-power accelerator technology have made hospital-based neutron sources available, re-highlighting the interest in BNCT10.
According to the International Society for Neutron Capture Therapy (ISNCT), there are currently 29 BNCT facilities either operational or under construction worldwide11. As a result, there is the critical need to further develop BNCT-related technologies to support its clinical implementation.
One of the main challenge is the development of a real-time dose monitoring system to track the patient’s response during treatment. The therapeutic efficacy of BNCT is directly linked to the variability in the distribution of 10B neutron-capture reactions within and between cells, which depends on factors such as the tissue type, vascularization, tumor biology, and neutron fluence at the target12. Indirect estimation methods are currently used13,14, but they are ineffective in capturing dynamic changes in boron distribution and neutron fluence rate during treatment.
One promising approach proposed is the use of prompt gamma imaging to detect the 478 keV gamma rays promptly emitted in 94% of the 10B(n,\(\alpha\))7Li reactions15. Several studies have explored systems for this purpose, proposing SPECT systems based on both collimators16,17,18,19,20,21,22 and Compton cameras23,24,25.
A major issue for the detection system is that the neutron beam used for treatment is extremely intense (in the order of 109 n/cm2/s), creating a high background of gamma rays and neutrons. Given the low concentration of boron typically present in tumor cells (in the order of tens of ppm), the resulting signal is often weak compared to the substantial background. One specific challenge involves annihilation photons at 511 keV, originating from pair production of high-energy gamma rays (such as the 2.2 MeV gamma rays generated by hydrogen neutron capture reactions). The narrow energy difference of 33 keV between these gamma rays and the 478 keV signal defines an energy resolution target of at least 6.9%, calculated as the ratio of the energy difference to the signal energy at 478 keV. However, since the 478 keV gamma ray is emitted in motion by the 7Li nuclei, it causes Doppler broadening of the energy peak, making the achievement of better energy resolution desirable. Another crucial issue for prompt gamma-ray monitoring in BNCT is the presence of background signals at the exact energy of the relevant signal, generated by boron neutron capture reactions occurring outside of the target. Boron is commonly incorporated into the walls of treatment rooms for radioprotection purposes, as it effectively absorbs thermal neutrons and reduces radiation exposure to surrounding areas26. Additionally, boron may also be found in various materials present in the room, including instrumentation, equipment and medical devices, further contributing to the background signal.
We propose a scintillator-based gamma-ray detector, combined with a pinhole collimator and custom shielding, as a single module of a tomographic BNCT-SPECT system made of several units. This detection system has previously successfully tracked borated samples during irradiation in a nuclear reactor27,28. In this study, we took a significant step towards clinical validation by conducting experimental measurements at the BNCT accelerator-based neutron facility at Nagoya University (Japan). To our knowledge, these are the first boron monitoring measurements performed in a facility with borated walls, a scenario that significantly heightens detection challenges and validates the feasibility of our system towards clinical-like conditions.
Methods
The experimental measurements were performed with the Nagoya University Accelerator-driven Neutron Source (NUANS) in Nagoya, Japan29. The detection unit used for the acquisitions consisted of a lanthanum bromide scintillator crystal coupled with silicon photomultipliers and custom readout electronics, combined with a pinhole collimator and an artificial neural network for position reconstruction, specifically designed for boron distribution monitoring27,28. Different shielding configurations were explored to enhance the signal-to-background ratio, especially considering the boron content in the walls of the irradiation site and in the detector’s electronics.
Detection system
The gamma-ray detector, visible in Fig. 1(a), is based on a monolithic lanthanum bromide scintillator crystal co-doped with cerium and strontium (LaBr3(Ce+Sr)), provided by Saint Gobain. The crystal has dimensions of 5 × 5 × 2 cm3 and is coupled with an 8 × 8 matrix of Near Ultraviolet High-Density (NUV-HD) silicon photomultipliers (SiPMs) with 30 µm × 30 µm cell size, from Fondazione Bruno Kessler (FBK), Trento, Italy30. The SiPM signals are processed by custom readout electronics based on application-specific integrated circuits (ASICs) and field-programmable gate array (FPGA). The detector features high energy resolution of approximately 2.9% at 662 keV and 4% at 478 keV, which is enough to isolate the BNCT photopeak from the adjacent photopeak at 511 keV of annihilation photons27. The crystal’s 2 cm thickness provides good detection efficiency of about 60% at 478 keV.
The detector has a dead time of approximately 5.4 µs, which includes both the integration of the current signal of the incoming event and its subsequent digitization. Specifically, the charge-to-voltage conversion of the outputs from the 64 SiPMs is performed by four 16-channels ASICs31, which allow for selectable integration time and sequentially output the signals for external digitization. This digitization is performed by four 13-bit analog-to-digital converters (ADCs), one per ASIC, working at 5 Msps. Given the intense neutron fluence rate required for BNCT treatments, and the consequent high background of gamma rays and neutrons present during the measurements, the detector features an energy discrimination mechanism to handle the high count rate. This mechanism uses an electronic circuit which senses the total current of the SiPMs and compares it with a selectable threshold, whose function is to activate the integration and the subsequent processing of one event only if it falls within a desired energy range. The ASICs output a current signal proportional to the energy of the incoming event, which is then processed by the circuit to determine whether it meets the preset energy thresholds defined by voltage comparators. These thresholds can be set by the user via a graphical user interface, allowing the system to effectively filter out most background events that could otherwise saturate the detector’s response. For the measurements, the energy thresholds were set to restrict the acquisition to the photopeaks at 478 keV and 511 keV. Specifically, they were configured to ADC bin values of 1400 and 2400 (out of a full-scale range of 8191 bins), corresponding to an energy range that spanned approximately from 350 keV up to 570 keV.
To create 2-dimensional (2D) images of the gamma-rays emitting source, the detector is coupled with a custom-designed channel edge pinhole collimator28, visible in Fig. 1(a). The collimator is made of lead and has dimensions of 9.4 × 9.4 × 10 cm3, with a hole diameter of 0.5 cm and an aperture angle of 9.52°. This configuration provides a field of view (FOV) of 5 cm, matching the detector crystal’s surface area, when both the detector and the source are positioned 25 cm from the collimator’s surface, on opposite sides. The collimator features a spatial resolution of 8 mm and a geometric efficiency of 3.84 × 10-6 at 478 keV in simulations. The reconstruction of the gamma-ray interaction positions within the 2 cm-thick monolithic crystal is performed by a specifically designed artificial neural network (ANN)32. The ANN analyzes the SiPMs light patterns for each impinging event, and maps them to specific 2D interaction coordinates on the crystal surface. The network runs on a personal computer, with a processing time of approximately 1.8 µs per event32. The network architecture, visible in Fig. 1(b), consists of an input layer with 64 neurons, each dedicated to one of the 64 SiPM signals, followed by two hidden layers with 25 neurons each, with hyperbolic tangent activation functions. Finally, the output layer comprises two neurons with linear activation functions to predict the x and y interaction coordinates. The ANN was trained using data obtained by scanning the crystal’s surface with a 1 mm-collimated cesium source (emitting gammas at 662 keV) at 481 positions. The model achieved a spatial resolution of 2.5 mm at 662 keV in the center of the crystal, with a slight decrease towards the edges, resulting in an overall mean spatial resolution of approximately 2.9 mm32.
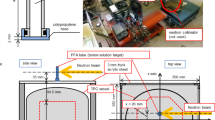
Single unit prototype for a BNCT-SPECT system designed for boron dose monitoring. The system integrates a scintillator-based gamma-ray detector with silicon photomultipliers (SiPMs) and custom electronics, a channel edge pinhole collimator and an artificial neural network (ANN) algorithm for gamma-ray position reconstruction. The 3D images are created with Autodesk Fusion 360 (v.2.0.20981 - https://www.autodesk.com/products/fusion-360/overview).
Irradiation site
The NUANS is an accelerator-based neutron source that uses a 7Li(d,n)8Be reaction to produce neutrons. The accelerator operates with a maximum acceleration voltage of 2.8 MeV and a beam current up to 15 mA for protons29. Although the NUANS is not used for patient treatment, its beam shaping assembly (BSA) has been specifically designed to meet the requirements for human treatment in BNCT. For the measurements reported here, a nozzle was installed to reduce the beam diameter to 120 mm and the accelerator was set at about 200 µA, resulting in a neutron fluence rate at the sample position of approximately 1 × 107 n/cm2/s.
The irradiation room features standard Portland concrete walls that are internally covered with 10 cm of borated polyethylene for shielding thermal neutrons. During the measurements, the floor was also covered with borated polyethylene blocks.
Experimental configuration
For irradiation, we used a 20 × 20 × 20 cm3 acrylic box filled with distilled water as a phantom. Borated vials could be inserted in a sample holder block, positioned 3 cm from the surface closest to the beam entrance to maximize the thermal neutron fluence rate. The vials were cylindrical, with dimensions of 26.5 mm in height and an internal diameter of 6 mm, and were filled with varying boron concentrations. The highest boron concentration was achieved using natural boron powder, which consists of approximately 20% of the isotope 10B. The other samples contained 10B dissolved in distilled water: a saturated solution with approximately 7371 ppm, and progressively diluted solutions with approximately 3686 ppm and 1843 ppm. To acquire background measurements, acquisitions were also performed using a vial filled with distilled water only.
The phantom was placed in front of the beam nozzle, with the vial aligned with the center of the aperture. The detector was positioned perpendicularly to the direction of the beam, at a distance of 50 cm from the center of the vial. The collimator was placed between the detector and the phantom, resulting in a FOV, for this configuration, of 4.16 cm. The detector-vial distance and the FOV (50 cm and 4.16 cm, respectively) were slightly smaller than the standard values used for the detection module (60 cm and 5 cm) due to space constraints in the irradiation room. The overall setup is illustrated in Fig. 2. The shielding configuration surrounding the detector, mainly consisting of lead and polyethylene blocks and cadmium foils, is detailed in the following section.
Initial measurements were acquired using the vials containing the highest boron concentrations, i.e., boron powder and 7371 ppm. During these measurements, the phantom was positioned so that the vial’s center was perfectly aligned with the detector’s crystal center. Subsequently, the phantom was manually shifted approximately 1 cm closer to the beam aperture, along the x-axis illustrated in Fig. 2(a), introducing a slight displacement relative to the detector’s center. This displacement is still within the FOV of the collimator, and should be detectable given its spatial resolution fo about 0.8 cm.
Additional measurements were then performed with the vial and the phantom returned to the central position, this time using lower boron concentrations of 3686 ppm and 1843 ppm.
Final measurements were conducted by inserting in the phantom’s sample holder two vials simultaneously: the one filled with boron powder and the one with 3686 ppm. The vials were arranged so that the interface between them aligned with the center of the detector’s crystal, with the powder positioned on the bottom and the solution-filled vial on the top. This configuration ensured that the FOV captured the lower half of the solution vial and the upper half of the boron powder vial.
All the spectra were measured for 2 min or 5 min period and to enhance statistical accuracy multiple acquisitions were performed for each boron concentration and vial position. An energy calibration source, specifically a gold (198Au) source emitting at 412 keV, was positioned close to the detector (see Fig. 2b) to ensure accurate energy calibration during the measurements.
Experimental setup for the measurements. The sectional diagram (a) illustrates the distances between the detector, collimator and vial, as well as the overall geometry of the configuration. The picture (b) shows the real setup with half of the shielding removed to allow a direct view of the components.
Shielding options
The main goal of the shielding was to reduce background radiation, particularly near the 478 keV energy region, maximizing the signal-to-background ratio. The background radiation in this energy range primarily originates from two sources: neutron interaction with the detector and with the surrounding room. Detector activation occurred due to both thermal and epithermal neutrons, which interacted with the detector material, producing gamma rays. Neutrons interacted with 10B present in the glass fiber substrates of printed circuit boards33, generating 478 keV gamma rays, with the 139La in the scintillator, producing 487 keV gamma rays27 and with 79Br in the scintillator, resulting in the emission of gamma rays at 467 keV. On the other hand, room activation contributed to additional gamma radiation at 478 keV due to the boron present in the surrounding walls. Monte Carlo simulations were used to assess the background radiation and explore different shielding configurations. To streamline the study, several simplifications were applied, including a reduced geometric model of the experimental room, excluding additional structures such as the BSA. The neutron spectrum used corresponds to the epithermal neutron spectrum of the facility29, with a fluence rate of 107 n/cm2/s. The phantom was included in the model to properly account for neutron interactions within the room. Under these conditions, the estimated count rate in the detector region, without any shielding and when irradiating a vial filled with only water, was \(3938\pm 2\) cps, primarily due to gamma rays from the borated walls. When the vial contained 7371 ppm of 10B, the count rate increased only slightly to \(3955\pm 2\) cps. This minimal difference indicates that even with a significant amount of 10B, the signal was barely distinguishable from the background, highlighting the necessity of effective shielding to ensure proper detection of the signal. To address this, a multi-layer shielding design was developed to enclose both the detector and the collimator as a single unit. The shielding was structured in successive layers, each optimized for neutron and gamma attenuation, and different configurations were tested. Starting from the outermost layer, 5 cm-thick polyethylene blocks were used to moderate epithermal neutrons down to thermal energies, due to their high hydrogen content. According to29, 3 cm in water is sufficient to thermalize the NUANS epithermal field, therefore, the available 5 cm-blocks were employed to ensure effective moderation. Beneath this layer, a 1 mm-thick cadmium foil was placed to efficiently absorb thermal neutrons. Because of its high radiative capture reaction cross section (\(\sigma\)th = 19852 b for 113Cd), this thickness was more than enough to stop all residual thermal neutrons. Finally, a 5 cm-thick lead layer was added to attenuate gamma radiation from both room activation and neutron capture reactions in the shielding materials. Theoretical calculations using the NIST XCOM database predicted that this thickness would provide at least a 104 reduction in background gamma rays at 478 keV from the borated walls. Additionally, the lead shield played a crucial role in attenuating secondary gamma rays generated in the outer layers, particularly those at 558 keV and 651 keV, emitted due to cadmium neutron capture reactions.
Three different configurations were tested. The first configuration, referred as Shield1, comprised the lead and cadmium layers, with just a few blocks of polyethylene placed in front of the collimator. These blocks are needed for efficiently thermalizing the epithermal scattered neutrons from the phantom. The second configuration, Shield2, was made by surrounding the entire unit with borated polyethylene blocks. Borated polyethylene was chosen to reduce as much as possible neutrons interacting with the detector, as this material is effective in moderating epithermal neutrons but also absorbing thermal neutrons (\(\sigma\)th = 3840 b for 10B). Nevertheless this configuration added a background contribution at the energy of interest rendering the lead shield less effective. For the third configuration, Shield3, the layer of borated polyethylene was replaced with boron-free polyethylene. The configurations are illustrated in Fig. 3.
(a) The basic shielding consists of lead blocks surrounding both the detector and collimator, with an external cadmium foil. (b) Shield1 includes also polyethylene blocks positioned in front of the collimator, Shield2 incorporates additional borated polyethylene around the entire unit, while Shield3 consists of polyethylene blocks enclosing the full unit.
Data processing
Energy spectra
The acquired spectra where calibrated in energy by fitting known photopeaks, specifically the 412 keV photopeak from the gold source and the 511 keV photopeak from annihilation photons, with Gaussian functions in Matlab. This fitting procedure enabled accurate conversion of ADC values into corresponding energy levels. Additionally, the detected counts were normalized to the measurement real time, ensuring consistent and comparable data across different acquisitions. To assess the contribution of each photopeak and separate it from the background continuum of scattered photons and neutrons, a fitting procedure for background subtraction was employed. Given the vicinity and the intersection of the two photopeaks at 478 keV and 511 keV, in order to account for them separately, an analytical model fitting simultaneously the two photopeaks with Gaussian functions plus a linear term to account for the background continuum was used. The energy range used for the fit was from 440 keV to 540 keV in order to include both peaks and to keep valid the assumption of linear background. Given the high count rate registered during the measurements, a correction factor that accounted for the detector’s dead time (DT) losses was used to obtain accurate counting measurements. This factor was calculated as the ratio between the input count rate (ICR) and the output count rate (OCR) at the detector, with \(OCR = \frac{ICR}{1+(ICR\times DT)}\)34.
Image reconstruction
For each acquired spectrum, the events falling under the photopeak at 478 keV, identified as ADC bin values ranging from 1950 to 2100, were selected. Each event, represented as a 1 × 64 array corresponding to the peak signal values of the 64 SiPMs, was fed to the ANN. The estimated interaction coordinates were imaged as a 32 × 32 matrix, with a pixel size of 1.56 mm. To obtain a clear visualization of the borated sample, a pixel-by-pixel subtraction of the background image (i.e., measurement with water-filled vial) from the image with the boron source was applied. In order for the background image to be properly subtracted, it was rescaled to match the counting rate of the background to the rate of the single measurements. The rescaling factor was determined by aligning the signal and background spectra at the 511 keV photopeak and calculating the ratio of the areas under the respective peaks. Following image subtraction, a Gaussian filter was applied in Matlab to reduce noise and enhance image quality. Additionally, all the events outside the 4.2 cm circular FOV were set to zero, allowing the signal of interest to stand out more clearly against the background. To quantify spatial resolution, 1D histograms were generated by summing the pixel values of the images along the y-axis. These histograms were fitted using Matlab with a Gaussian function to determine the spatial resolution expressed as FWHM of the distribution of the reconstructed points.
Spectra reconstruction
The energy spectra acquired and plotted during the measurements included all detected events, embracing both signal and background contributions. These spectra were used to calculate the counts per second and reconstruct the images. To enhance the signal-to-noise ratio, the position information from the images was utilized to generate a refined and less noisy energy spectrum. By selecting and plotting only the events originating within the FOV of the collimator, it was possible to minimize background events and improve the clarity of the signal.
The process involved feeding all the events from the acquired spectrum as input to the ANN. The ANN then predicted the x and y coordinates for each event. Events with coordinates falling within the interval -12.5 mm and 12.5 mm along both axes were identified as originating within the FOV of the collimator. An energy spectrum was then reconstructed exclusively from the subset of events.
Results
Initial spectra acquired by irradiating the water-filled vial show a high gamma-ray background. The most pronounced peaks were attributed to background boron neutron capture reactions and from pair annihilation at 511 keV. The detector was able to clearly distinguish the photopeak at 478 keV, with an energy resolution of approximately 4%. The energy discrimination mechanism was used to handle the high count rate. Figure 4 compares the full spectrum (blue curve) with the spectrum acquired using the energy discrimination mechanism (red curve), both acquired over a two-minute measurement time. The detected count rate decreased from 40245 Hz for the first measurement, to 15504 Hz for the second measurement, enhancing the detector’s performance. Notably, in the region of interest, the spectrum obtained with the energy discrimination mechanism active shows a higher count rate, demonstrating its efficacy in enhancing the detection of the desired signal. However, this effect impacts both the photopeaks and the surrounding background continuum. To address this, improvements to the energy discrimination circuit are currently underway to more precisely define the energy boundaries. This refinement will enable better isolation of the photopeaks from the background, reducing spectral tails. The increase in the counting rate at 478 keV, calculated by integrating the number of events within an energy range defined by the centroid of the 478 keV Gaussian \(\pm 1\sigma\) (to avoid including the nearby 511 keV photopeak), is approximately 14%.
The energy discrimination mechanism allowed the detector to efficiently handle the high count rate. By filtering out irrelevant events, the detector allocated more processing capability to events in the desired energy range, resulting in a greater number of detected events, whose number is normalized on the acquisition time. The gold source was not present during these acquisitions.
Shielding effectiveness
The spectra acquired with the three different shielding configurations -Shield1, Shield2 and Shield3- and irradiating the water-filled vial are visible in Fig. 5. The dotted lines represent the fitted spectra, obtained with a two-minute measurement time each, while the dash-dotted lines at the bottom highlight the fitting of the 478 keV photopeaks alone. The spectra clearly display three photopeaks: the 412 keV photopeak from the 198Au source, at 478 keV photopeak from boron interactions, and the 511 keV photopeak from annihilation photons. The photopeaks at 412 keV and 511 keV were used for energy calibration of the x-axes. The height reduction of the 412 keV peak can be attributed to the natural decay of the gold source (\(T_{\frac{1}{2}}= 2.69\) days), as the three measurements were taken over three consecutive days. However, source displacement likely contributed as well, since the source was not fixed to the table. The more pronounced drop between the first and second day (Shield1 and Shield2) is likely due to the fact that multiple shielding configurations were tested in between, making it probable that the source shifted, particularly moving farther from the detector, resulting in a lower detected gamma-ray count. Conversely, the decrease in the 478 keV photopeak demonstrates the effectiveness of the different shielding configurations in lowering thermal and epithermal neutron interaction with the detector, as well as minimizing the gamma-ray background from the irradiation room. Quantitatively, the number of counts at 478 keV decreases progressively across the configurations: from \(144.5\pm 5.4\) cps for Shield1, to \(83.8\pm 1.6\) cps for Shield2 and further to \(59.8\pm 2.2\) cps for Shield3.
Background measurements acquired with water-filled vial and different shielding configurations, with a measurement time of 2 min. Corresponding Matlab fitting curves are shown as dotted lines. Shield2 and Shield3 configurations reduced the overall background compared to Shield1, with Shield3 being particularly effective in lowering the background at 478 keV. The bin width of the histogram is 0.36 keV.
Imaging of borated samples
The reconstructed images of the vials filled with boron powder and 7371 ppm of 10B, along with the corresponding 1D histograms showing the distribution of the reconstructed points, are reported in Fig. 6. In each image the counts are normalized by the measurement times, which are reported in the figure captions. The varying durations are due to accelerator instabilities, with the selected time slots chosen to minimize these fluctuations and ensure stable irradiation conditions. All measurement times remain within the typical BNCT treatment duration, which generally ranges from 30 to 60 min35. Figure 6(a) and (c) show the results for the measurements acquired with the vials in the central position, while Fig. 6(e) and (g) present the measurements acquired when the phantom was shifted 1 cm closer to the nozzle, along x-axis of Fig. 2(a). Due to the pinhole geometry of the collimator, the shift of the sample along x direction is reconstructed on the image on the opposite side, thus corresponding to a negative x-coordinate.
Figure 7 shows the results obtained for the measurements with lower boron concentrations of 3686 ppm of 10B and 1843 ppm of 10B, acquired with the vial in the central position. In all measurements, the thermal neutron flux is slightly attenuated in the sample due to the high boron concentration, but the sample is irradiated isotropically in the water phantom and the prompt gamma-ray distribution correctly reflects the sample center.
The measurement conducted with two adjacent vials, one filled with 3686 ppm of 10B and the other one with boron powder is illustrated in Fig. 8. Again, due to the collimator’s flipping effect, the powder-filled vial positioned at the bottom of the sample holder, as depicted in Fig. 8(a), is reconstructed on the top of the image and vice versa for the solution-filled one (Fig. 8b). The different boron concentrations in the vials can be retrieved as differences in pixel intensities within the image.
The values of the FWHMs and the centroids of the histograms corresponding to each image are reported in Table 1.
Reconstructed images of borated vials containing boron powder and 7371 ppm of 10B in the central position (a, c) and shifted position (e, g), along with their respective histograms. The histograms have a bin width of 1.56 mm. Measurement times are 24 min for (a), 18 min for (c), 16 min for (e), and 45 min for (g).
Boron peak extraction
The energy spectra used to reconstruct the image of the vial filled with 3686 ppm of 10B displayed in Fig. 7(a), are shown in Fig. 9(a). They are presented as representative spectra for all the other measurements with different concentrations. The blue curve represents the spectrum acquired with the boron-filled vial, while the red curve corresponds to the background spectrum from the water-filled vial. To enable image reconstruction, and in particular background subtraction, the background spectrum was rescaled using the area under the 511 keV photopeak in order to match the count rate of the boron measurement. Despite the resulting image successfully revealed the vial (Fig. 7a), no visible difference in the boron photopeak at 478 keV is visible in the spectra of Fig. 9(a). This can be attributed to the fact that the useful signal is projected onto only a small area of the crystal surface due to the limited FOV of the setup, while background radiation is distributed across the entire detection surface of the crystal. As a result, the background dominates the recorded signal, leading to a poor signal-to-noise ratio that masks the boron signal. To address this, we reconstructed the energy spectrum using only the events identified by the ANN as originating within the collimator’s FOV. This selective filtering enhances the signal-to-noise ratio, allowing the boron signal at 478 keV to emerge from the background. The spectra generated with this subset of events are displayed in Fig. 9(b), where a clear difference is now visible in the 478 keV photopeak, with the boron measurement exhibiting a higher signal compared to the background measurement.
Energy spectra analysis for boron peak extraction. By reconstructing the spectra using only the events that the neural network predicted to originate within the field of view, a difference in the 478 keV photopeak becomes visible between the boron and water measurements. The bin width of the histograms is 0.36 keV.
Discussion
The detector was able to successfully map the signal from borated vials during irradiation at the Nagoya University BNCT facility, characterized by borated walls for radioprotecion purposes. Despite the high background present in the measurements, the system detected boron concentrations as low as 1843 ppm of 10B under a neutron fluence rate of approximately 1 × 107 n/cm2/s generated by the accelerator-based neutron source.
The gamma-ray background observed during the measurements was very intense, both due to neutron and gamma-ray interactions within the small irradiation site and contamination from the beam itself. Moreover, the background continuously increased over time, due to activation of all the different materials present in the room, including those of the beam structure. To manage this high count rate at the detector, the energy discrimination mechanism was essential. Energy thresholds were set on a broader range and not only on the 478 keV, to ensure the detection of all the relevant events and also to include the photopeak at 412 keV from 198Au source and at 511 keV from annihilation photons for energy calibration. This strategy reduced the detector count rate by about 60%. In the future, a narrower window around the 478 keV peak can be implemented.
As expected, a very high background signal was observed at 478 keV. This signal came from neutron capture reactions involving boron in the surrounding walls, as well as in the electronic boards, and also from neutron interaction with the scintillator crystal itself. All these contributions masked the signal from the vial. The goal of the shielding configuration was to reduce the contribution of gamma rays coming from the walls and to prevent both thermal and epithermal neutrons from interacting with the detector and its electronic boards. However, the initial configuration Shield1 still exhibited a significant background at 478 keV as visible in Fig. 5, suggesting that the dominant source of this background was neutron interaction with the detector. Although with 1 mm-thick cadmium foil the thermal neutron transmission should theoretically be zero, we tried to improve neutron shielding with Shield2 configuration. This version expanded the shielding by surrounding the cadmium layer with borated polyethylene blocks. Boron efficiently captures thermal neutrons because of its high cross section, while polyethylene thermalizes epithermal neutrons, which can be subsequently captured by the cadmium layer. This configuration significantly reduced the counts under the photopeak at 478 keV and the overall background radiation compared to Shield1. However, borated polyethylene increased the generation of background gamma rays at 478 keV due to its boron content. Although the lead layer should theoretically attenuate these gamma rays, the high amount of gamma rays from the room and the detector shielding meant that even a small percentage passing through could be significant. In the final configuration Shield3, the borated polyethylene was replaced with boron-free polyethylene. Considering the 1 mm-thick cadmium layer sufficient to stop thermal neutrons, polyethylene had the only goal to moderate epithermal neutrons. This configuration proved the most effective in minimizing gamma-ray background at 478 keV. In summary, the different shielding strategies aimed to optimize neutron moderation and gamma attenuation, with the second and third setups offering more comprehensive neutron suppression strategies. The final choice involved balancing effective neutron absorption with minimizing additional background radiation. It should be noted that, the exploited shield reduced the background without completely removing a 478 keV component. In spite of this, the overall system is capable of acquiring images of the different vials. This achievement encourages further exploration of innovative shielding solutions, potentially aimed at reducing weight and size, and/or modifications to the collimator design. While reducing the shielding might lead to an increase in background levels, this could be acceptable from the perspective of image acquisition, as the system has demonstrated its capability to produce reliable results even under non-zero background conditions. Such improvements would enable the development of a more compact and lightweight system capable of acquiring multiple projections efficiently for building a SPECT system.
The detector, coupled with the ANN, successfully tracked vial displacements (Fig. 6). The centroids of the histograms reported in Table 1 show a displacements of about 6.6 mm for the vial with borated power and 6.1 mm for the 7371 ppm vial. Although we stated a 1 cm displacement, the shifts were performed manually, thus introducing slight variability that can be considered consistent with the results obtained. The spatial resolutions of the reconstructed images are around 1 cm, aligning with the expected geometric resolution of the collimator of 8 mm and meeting the 1 cm target of the design27. A lower value is observed for the 7371 ppm shifted vial and this can be attributed to inaccuracies in the background subtraction process. Background subtraction required an alignment process to match the count rate of the boron measurement with the one of the water-only measurement (used as background measurement). This step is crucial because the image obtained during the water-only measurement registered a higher count rate, as more neutrons interact with the environment, particularly with the water in the phantom, rather than with the boron atoms. Consequently, in order to subtract the two images accurately, the count rates of both must be aligned. However, this alignment process can result in mismatches between the two images, potentially causing a loss of useful signal after subtraction, as it happens for the 7371 ppm shifted vial (Fig. 6g). On the other hand, some background signal may remain, as evidenced by the bright signal on the left side of the reconstructed images displayed in Fig. 7.
Although the ANN successfully imaged the borated vials, no significant changes were observed in the number of counts under the photopeak at 478 keV for the different boron concentrations irradiated or when only water was irradiated (Fig. 9a). This is because the small changes in the number of detected events are drowned in the statistical fluctuations of the background. However, by reducing the number of background events and considering only those predicted within the FOV by the ANN, the photopeak corresponding to the signal becomes clearly visible (Fig. 9b). Currently, the ANN runs on a personal computer, processing each detected event in approximately 1.8 µs. This allows the image on the graphical user interface to be continuously updated during the measurement, achieving near real-time reconstruction. Efforts are underway to implement the ANN directly on the detector’s FPGA, enabling fully integrated real-time processing, which would be particularly beneficial for the final tomographic SPECT configuration with multiple detectors simultaneously detecting events. Looking ahead, an on-chip implementation could further optimize processing time, power consumption, and memory requirements36. While the reconstruction process is already near real-time, background subtraction is still performed offline in post-processing. As a result, the final image is currently obtained only after both background and actual measurements are completed. To achieve true real-time imaging in clinical applications, future developments should integrate background subtraction as an initial calibration phase, removing the need for offline processing and further enhancing system efficiency.
The system was able to detect down to 1843 ppm of 10B (Fig. 7). Although this concentration is significantly higher than what is typically of clinical interest, where concentrations are on the order of tens of ppm, the thermal neutron fluence rate of 1 × 107 n/cm2/s used is nearly two orders of magnitude lower than the typical clinical fluence rate, which is approximately 109 n/cm2/s, thus scaling the measured concentration of 1843 ppm to a few ppm which is clinically realistic.
Figure 8 demonstrates the system’s ability to distinguish between different boron concentrations represented as differences in the pixel intensities. This is a key feature for clinical application, where the boron distribution can vary significantly within and between cells depending on the type of tissues and the biological characteristics of tumor cells.
The proposed detector has already demonstrated its capability to detect boron signals during neutron irradiation, as shown in previous studies27,28. Similarly, other systems designed for BNCT dose monitoring have also reported successful experimental results18,37. Among the various detector technologies explored for this application, CdTe and CZT detectors stand out due to their very good energy resolution (\(\sim 1\%\) at 662 keV17), which is crucial given the proximity of the 511 keV gamma-ray peak17,20,21. However, so far, it has been difficult to develop detection modules with this type of detectors that can simultaneously provide all the characteristics required by a BNCT-SPECT system, namely high efficiency (thickness \(>=2\) cm), large detection area (\(\sim 5\times 5\) cm2) and good energy resolution necessary to separate the 478 keV peak from the 511 keV peak. This is due to the fact that in thick detectors the peak tailing effect, resulting from charge trapping within the detector material, usually affects the quality of the spectrum. Additionally, cadmium-based detectors could introduce background radiation due to neutron capture reactions, further complicating the identification of useful signals. For these reasons, scintillator-based detectors, particularly LaBr3, can be considered a good solution for this application18,19,22, since they are a well-established technology that combines good detection efficiency and large detection area with an energy resolution adequate to reliably distinguish the boron signal from adjacent photopeaks. Moreover, this research takes a critical step forward by presenting the first measurements conducted in a clinical-like BNCT scenario. This includes irradiating small boron samples in a water phantom to simulate a tumor in a patient, employing a fast neutron field generated by an accellerator-based neutron source then shaped and epithermalized with a BSA (which fullfills the International Atomic Energy Agency requirements for BNCT), and, importantly, accounting for the presence of other borated materials in the irradiation room. It should be stressed that the presence of the BSA introduces other sources of background due mainly to some unwanted neutron leakages and to the activation processes during irradiation. The high background of neutrons and gamma rays during BNCT measurements is a well-known challenge for boron dose monitoring. However, less attention has been given to the significant boron content that may exist in clinical environments. Boron is often present in the walls of treatment rooms for radioprotection purposes, as well as in instruments, equipment, and other items commonly found in clinical rooms, such as glasses, cameras, or monitors. These sources contribute additional complexity to the already challenging background conditions.
This study demonstrates the potential of our system to map boron distribution in a BNCT-like clinical environment, addressing the challenges posed by realistic background conditions. However, further measurements at a clinical neutron flux of approximately 109 n/cm2/s and with clinically relevant boron concentrations are still required. The results highlight the demanding count rate expected in clinical settings, pointing out the need to enhance the detector’s count rate capability to effectively handle such conditions. To achieve this, ongoing efforts focus on optimizing the system’s performance by shortening integration time, implementing faster ADCs for digitization, and improving communication protocols between the detector and the external PC. Additionally, refinements to the energy discrimination circuit aim to better isolate the 478 keV peak, reducing the overall count rate at the detector. While setting a precise clinical count rate target remains challenging due to variables such as treatment room configuration, shielding, and energy discrimination precision, our objective is to increase the data throughput from the current 40 kHz to the MHz range, ensuring the system can efficiently detect the boron signal under clinical conditions.
Conclusions
The proposed detection system, comprising a LaBr3-based gamma-ray detector, a pinhole collimator and an artificial neural network algorithm for position reconstruction, has demonstrated its capability to successfully track borated vials under the challenging conditions of a BNCT facility. A multi-layer shielding approach was employed to effectively detect the vial’s signal despite the severe background, especially from boron contaminants in the electronic boards and treatment room walls. The detector was able to detect boron concentrations as low as 1843 ppm with a neutron fluence rate of approximately 1 × 107 n/cm2/s. It also demonstrated the ability to map 1 cm displacements and distinguish different boron concentrations within a single measurement. Future improvements will focus on optimizing the shielding, which should ideally be able to completely eliminate the background at 478 keV, keeping compact dimensions. Additionally, further measurements will be conducted with higher neutron fluence rate and lower boron concentrations. Although several factors must still be addressed for clinical implementation, this study marks a significant step towards the development of effective boron dose SPECT monitoring technologies.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Durante, M. & Loeffler, J. S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 7, 37–43. https://doi.org/10.1038/nrclinonc.2009.183 (2010).
Durante, M., Debus, J. & Loeffler, J. S. Physics and biomedical challenges of cancer therapy with accelerated heavy ions. Nat. Rev. Phys. 3, 777–790. https://doi.org/10.1038/s42254-021-00368-5 (2021).
Skwierawska, D., López-Valverde, J. A., Balcerzyk, M. & Leal, A. Clinical viability of boron neutron capture therapy for personalized radiation treatment. Cancers 14, 2865. https://doi.org/10.3390/cancers14122865 (2022).
Sauerwein, W. A., Wittig, A., Moss, R. & Nakagawa, Y. Neutron Capture Therapy: Principles and Applications (Springer, 2012).
Barth, R. F. et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 7, 1–21. https://doi.org/10.1186/1748-717X-7-146 (2012).
Locher, G. L. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol. 36, 1–13 (1936).
Chadwick, J. The existence of a neutron. Proc. R. Soc. Lond. Ser. A Containing Papers of a Mathematical and Physical Character 136, 692–708. https://doi.org/10.1098/rspa.1932.0112 (1932).
Slatkin, D. N. A history of boron neutron capture therapy of brain tumours: Postulation of a brain radiation dose tolerance limit. Brain 114, 1609–1629. https://doi.org/10.1093/brain/114.4.1609 (1991).
Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 25, 43–50. https://doi.org/10.1007/s10147-019-01480-4 (2020).
ISNCT. Accelerator-based BNCT Projects, 2024. https://isnct.net/bnct-boron-neutron-capture-therapy/accelerator-based-bnct-projects-2021/ (2024).
Sato, T., Masunaga, S.-I., Kumada, H. & Hamada, N. Microdosimetric modeling of biological effectiveness for boron neutron capture therapy considering intra-and intercellular heterogeneity in 10B distribution. Sci. Rep. 8, 988. https://doi.org/10.1038/s41598-017-18871-0 (2018).
Laakso, J. et al. Atomic emission method for total boron in blood during neutron-capture therapy. Clin. Chem. 47, 1796–1803. https://doi.org/10.1093/clinchem/47.10.1796 (2001).
Ishiwata, K. 4-borono-2-18F-fluoro-L-phenylalanine PET for boron neutron capture therapy-oriented diagnosis: Overview of a quarter century of research. Ann. Nucl. Med. 33, 223–236. https://doi.org/10.1007/s12149-019-01347-8 (2019).
Kobayashi, T., Sakurai, Y. & Ishikawa, M. A noninvasive dose estimation system for clinical BNCT based on PG-SPECT-conceptual study and fundamental experiments using HPGe and CdTe semiconductor detectors. Med. Phys. 27, 2124–2132. https://doi.org/10.1118/1.1288243 (2000).
Murata, I. et al. Design of SPECT for BNCT to measure local boron dose with GAGG scintillator. Appl. Radiat. Isot. 181, 110056. https://doi.org/10.1016/j.apradiso.2021.110056 (2022).
Abbene, L. et al. Potentialities of high-resolution 3-D CZT drift strip detectors for prompt gamma-ray measurements in BNCT. Sensors 22, 1502. https://doi.org/10.3390/s22041502 (2022).
Minsky, D. M. et al. First tomographic image of neutron capture rate in a BNCT facility. Appl. Radiat. Isot. 69, 1858–1861. https://doi.org/10.1016/j.apradiso.2011.01.030 (2011).
Okazaki, K. et al. Development of a prompt gamma-ray detector with an 8×8 array LaBr 3(Ce) scintillator and a multi-pixel photon counter for boron neutron capture therapy. Nucl. Instrum. Methods Phys. Res. Sect. A 1055, 168546. https://doi.org/10.1016/j.nima.2023.168546 (2023).
Hales, B. et al. Predicted performance of a PG-SPECT system using CZT primary detectors and secondary Compton-suppression anti-coincidence detectors under near-clinical settings for boron neutron capture therapy. Nucl. Instrum. Methods Phys. Res. Sect. A 875, 51–56. https://doi.org/10.1016/j.nima.2017.09.009 (2017).
Manabe, M., Nakamura, S. & Murata, I. Study on measuring device arrangement of array-type CdTe detector for BNCT-SPECT. Rep. Pract. Oncol. Radiother. 21, 102–107. https://doi.org/10.1016/j.rpor.2015.04.002 (2016).
Silarski, M. et al. A new detector concept based on the prompt gamma radiation analysis for in vivo boron monitoring in bnct. Radiat. Prot. Dosimetry. 199, 1932–1936. https://doi.org/10.1093/rpd/ncac245 (2023).
Moktan, H., Lee, C. L. & Cho, S. H. Prompt gamma ray detection and imaging for boron neutron capture therapy using CdTe detector and novel detector shield-Monte Carlo study. Med. Phys. 50, 1736–1745. https://doi.org/10.1002/mp.16207 (2023).
Nutter, K., Price, T., Kokalova, T., Green, S. & Phoenix, B. A feasibility study using an array of LaBr 3(Ce) scintillation detectors as a Compton camera for prompt gamma imaging during BNCT. Front. Phys. 12, 1347929. https://doi.org/10.3389/fphy.2024.1347929 (2024).
Hou, Z. et al. Boron concentration prediction from Compton camera image for boron neutron capture therapy based on generative adversarial network. Appl. Radiat. Isot. 186, 110302. https://doi.org/10.1016/j.apradiso.2022.110302 (2022).
Magni, C. et al. Radiation protection aspects in the design of a boron neutron capture therapy irradiation room. Radiat. Phys. Chem. 218, 111621. https://doi.org/10.1016/j.radphyschem.2024.111621 (2024).
Caracciolo, A. et al. A compact scintillator-based detector with collimator and shielding for dose monitoring in boron neutron capture therapy. Phys. Imaging Radiat. Oncol. 29, 100556. https://doi.org/10.1016/j.phro.2024.100556 (2024).
Ferri, T. et al. Imaging study and first measurements of a LaBr 3 gamma detector for BNCT applications. IEEE Trans. Nucl. Sci. https://doi.org/10.1109/TNS.2024.3385067 (2024).
Nishitani, T. et al. Neutronics analyses of the radiation field at the accelerator-based neutron source of Nagoya University for the BNCT study. J. Nucl. Eng. 3, 222–232. https://doi.org/10.3390/jne3030012 (2022).
Gola, A. et al. Nuv-sensitive silicon photomultiplier technologies developed at fondazione bruno kessler. Sensors 19, 308. https://doi.org/10.3390/s19020308 (2019).
Buonanno, L., Di Vita, D., Carminati, M. & Fiorini, C. Gamma: A 16-channel spectroscopic asic for sipms readout with 84-db dynamic range. IEEE Trans. Nucl. Sci. 68, 2559–2572. https://doi.org/10.1109/TNS.2021.3107333 (2021).
Ferri, T. et al. Gamma-ray position-of-interaction estimation in a thick monolithic LaBr 3 detector using artificial neural networks. IEEE Trans. Radiat. Plasma Med. Sci. https://doi.org/10.1109/TRPMS.2024.3492674 (2024).
Colombo, G. et al. Study of the thermal neutron activation of a gamma-ray detector for BNCT dose monitoring. J. Instrum. 19, P05047. https://doi.org/10.1088/1748-0221/19/05/P05047 (2024).
Knoll, G. F. Radiation Detection and Measurement (Wiley, 2010).
Hu, N. et al. Improvement in the neutron beam collimation for application in boron neutron capture therapy of the head and neck region. Sci. Rep. 12, 13778. https://doi.org/10.1038/s41598-022-17974-7 (2022).
Di Giacomo, S. et al. Implementing an integrated neural network for real-time position reconstruction in emission tomography with monolithic scintillators. IEEE Trans. Radiat. Plasma Med. Sci. https://doi.org/10.1109/TRPMS.2024.3378421 (2024).
Sakai, M. et al. Experimental study on Compton camera for boron neutron capture therapy applications. Sci. Rep. 13, 22883. https://doi.org/10.1038/s41598-023-49955-9 (2023).
Acknowledgements
Part of this work has been financed by INFN (CSN5), Italy (SPOC Project), and by PRIN PNRR 2022 funding program of Ministero Dell’Università e della Ricerca (MUR), Italy (BNCT-SPECT project).
Author information
Authors and Affiliations
Contributions
A.C., D.M., L.G., K.T., Y.I. and C.F. conducted the experiment. The detector was developed by A.C., T.F., F.G., M.P., G.B., M.C. and C.F., while D.M, L.G. and S.A. designed the shielding. A.C., D.M., T.F. and L.G. analyzed the results. G.B., M.C., S.A., K.T., T.N., Y.I., A.U., S.A., N.P. and C.F. supervised the findings of this work. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Caracciolo, A., Mazzucconi, D., Ferri, T. et al. Prompt gamma-ray imaging in realistic background conditions of a boron neutron capture therapy facility. Sci Rep 15, 18626 (2025). https://doi.org/10.1038/s41598-025-03764-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03764-4