Abstract
Lanreotide and Octreotide are used to treat various endocrine and neoplastic diseases. This study aims to compare the adverse event profiles of Lanreotide and Octreotide in somatostatin-responsive diseases using FAERS data. FAERS data from Q1 2004 to Q2 2024 were reviewed for AE reports related to Lanreotide and Octreotide. Reports were categorized using MedDRA system organ classes (SOCs). Disproportionality analysis was conducted using Reporting Odds Ratios (ROR), Proportional Reporting Ratios (PRR), and Information Components (IC) to identify significant AEs. The top 20 AEs for each drug were analyzed, and chi-square tests assessed differences in AE frequencies between the drugs. Detailed comparisons were made across gastrointestinal, cardiovascular, and neoplastic AEs. Lanreotide was more associated with gastrointestinal AEs, such as diarrhea (1457 reports) and cholelithiasis (198 reports), with a notable signal for cholelithiasis (ROR 12.03, 95% CI 10.46–13.85). Octreotide had higher reports of cardiovascular AEs, including systolic (1483 reports) and diastolic (541 reports) blood pressure increases. Additionally, Octreotide was linked to neoplasm progression (1735 reports) and more frequent malignant neoplasms. Injection site reactions, including pain and nodules, were more common with Lanreotide (ROR 19.09, 95% CI 17.2–21.19). Lanreotide and Octreotide exhibit distinct adverse event profiles, with gastrointestinal signals more frequently observed for Lanreotide, and cardiovascular/neoplastic signals more apparent for Octreotide. These patterns should be interpreted with caution due to limitations of the FAERS data.
Similar content being viewed by others
Introduction
Neuroendocrine tumors (NETs) are a diverse group of neoplasms that originate from neuroendocrine cells, which are found throughout the body, particularly in organs such as the gastrointestinal tract, pancreas, and lungs. These tumors can range from slow-growing to highly aggressive, with the potential to metastasize, especially to the liver. The complexity of NETs, both in their presentation and progression, presents significant challenges for diagnosis and management1. Many NETs are asymptomatic in the early stages, and when symptoms do occur, they are often subtle and nonspecific, making early detection difficult. As a result, treatment strategies must address not only the tumor’s biological behavior but also the varied clinical manifestations that arise from hormone secretion, requiring a comprehensive, multidisciplinary approach to optimize outcomes2.
In the context of treatment, somatostatin analogs (SSAs) such as Lanreotide and Octreotide have emerged as cornerstone therapies for managing NETs, particularly in patients with unresectable or metastatic disease. These drugs work by mimicking the effects of somatostatin, a hormone that inhibits the release of other hormones and growth factors. By binding to somatostatin receptors on neuroendocrine cells, Lanreotide and Octreotide help control symptoms like diarrhea and flushing, commonly seen in patients with functioning NETs, while also slowing tumor progression3. Despite their shared mechanism of action, Lanreotide and Octreotide differ in their pharmacokinetics and administration methods, which may influence patient adherence and overall treatment outcomes. For example, Lanreotide is administered as a long-acting subcutaneous injection every four weeks, while Octreotide is available in both short-acting and long-acting formulations, requiring more frequent dosing4,5.
Given the chronic nature of NETs and the need for long-term therapy, it is essential to thoroughly understand the safety profiles of both Lanreotide and Octreotide in real-world clinical settings. Although clinical trials provide valuable information on efficacy, they may not capture the full range of adverse events (AEs) experienced by patients in everyday practice. Therefore, real-world data from the FDA Adverse Event Reporting System (FAERS) can provide a broader perspective on how these drugs perform in diverse populations. This study aims to analyze FAERS data to compare the adverse event profiles of Lanreotide and Octreotide, focusing on major system organ classes (SOCs) such as gastrointestinal, cardiovascular, and neoplastic reactions. By evaluating these real-world outcomes, we hope to offer insights that will help clinicians make more informed treatment decisions, ultimately improving patient care in the management of NETs.
Methods
Data source and extraction
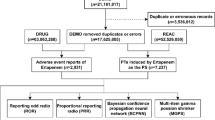
The data for this study were sourced from the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS). FAERS is a publicly available pharmacovigilance database that collects information on adverse events (AEs) related to drugs and therapeutic biological products. Reports involving Lanreotide and Octreotide from Q1 2004 to Q2 2024 were extracted. Only cases where these drugs were the primary suspect were included, and duplicate reports were removed based on case identification numbers.
Classification of adverse events
Adverse events were classified using the Medical Dictionary for Regulatory Activities (MedDRA) system organ classes (SOCs) and preferred terms (PTs). The top 20 most frequently reported AEs for both Lanreotide and Octreotide were identified. SOCs of interest, such as gastrointestinal, cardiovascular, neoplastic, and endocrine disorders, were further investigated. For the purpose of top 20 AE analysis, only clinically interpretable adverse events with plausible pharmacologic or pathophysiologic relevance were included. Non-specific or administrative terms such as “off-label use,” “metastases to liver,” or “product use issue” were excluded.
Disproportionality analysis
Disproportionality analysis was performed to detect potential safety signals using multiple statistical methods6,7:
-
1.
Reporting Odds Ratio (ROR): Calculated as the odds of reporting a specific AE with Lanreotide or Octreotide compared to all other drugs in the FAERS database. A signal is considered significant if the ROR is greater than 1, with a 95% confidence interval (CI) excluding 1.
-
2.
Proportional Reporting Ratio (PRR): This ratio compares the proportion of reports of a specific AE for Lanreotide or Octreotide to the proportion of the same AE for all other drugs in the FAERS database. A PRR > 2, with a chi-square (χ2) ≥ 4 and at least 3 reports, is considered a signal.
-
3.
Information Component (IC): The IC uses a Bayesian method to compare observed and expected numbers of AE reports. An IC value above 0 signals a higher-than-expected number of AEs for the drug.
-
4.
Chi-square (χ2): Chi-square tests were used to compare the frequency of AEs between Lanreotide and Octreotide. A chi-square statistic with a value ≥ 4 was used as part of the signal detection criteria for PRR, indicating a significant association between the drug and AE.
-
5.
Empirical Bayes Geometric Mean (EBGM): EBGM is an advanced Bayesian method that adjusts for the variability in reporting. It calculates the observed-to-expected AE reporting ratios, with EBGM05 being the lower 90% confidence bound. An EBGM05 greater than 1 indicates a statistically significant signal for a specific AE.
Statistical analysis
Chi-square tests were employed to assess statistical significance between AE frequencies for Lanreotide and Octreotide. A p-value < 0.05 was considered statistically significant. Descriptive statistics were also generated to summarize demographic data, including patient age, gender, and geographical distribution of the AE reports.
Signal detection
Signal detection was based on a combination of ROR, PRR, IC, chi-square, and EBGM. Only adverse events that met all of the following predefined thresholds were considered significant and included in Table 1: Reporting Odds Ratio (ROR) > 1 with a 95% confidence interval not including 1, Proportional Reporting Ratio (PRR) > 2, chi-square (χ2) ≥ 4, and EBGM05 > 1. A signal was considered more robust if these criteria were also supported by elevated IC values. EBGM was further used to strengthen confidence in signal detection, particularly in cases with smaller sample sizes.
Results
Descriptive analyses
Between Q1 2004 and Q2 2024, a total of 4040 reports related to Lanreotide and 9291 reports related to Octreotide were identified from the FAERS database (Table 1). In terms of gender distribution, 52.68% of Lanreotide-related reports involved female patients, while 43.27% involved males. For Octreotide, 49.25% of the reports involved female patients, with 41.25% involving males. A higher percentage of reports for both drugs were from patients aged 60 years or older, with Lanreotide reports showing 35.58% and Octreotide 34.38% in this age group. The data showed hospitalization rates of 30.11% for Lanreotide and 29.28% for Octreotide, indicating that many adverse events (AEs) were serious enough to require hospital admission. The fatal outcome rate was higher in the Octreotide group, with 22.56% of cases reporting death, compared to 17.10% in the Lanreotide group, underscoring the severity of AEs associated with Octreotide. Geographically, the majority of reports were from North America, followed by Europe, with a smaller number of reports from Asia and other regions.
Indications and concomitant medications
The primary indications for Lanreotide and Octreotide were both dominated by Neuroendocrine Tumors (NETs), but the frequency varied: 35.6% of Lanreotide reports and 27.34% of Octreotide reports were for NETs (Table 2). For Lanreotide, Acromegaly was the second most common indication, reported in 26.16% of cases, while for Octreotide, Carcinoid Tumor was the second most common, with 15.91% of reports. Additional indications for Lanreotide included Carcinoid Tumor (16.31%), Malignant Neoplasm (2.55%), and Pituitary Tumor (1.21%). Octreotide had similar secondary indications, including Acromegaly (13.32%), Malignant Neoplasm (1.72%), and Diarrhea (1.30%).
The top five concomitant medications revealed a substantial overlap between the two drugs. Metformin and Levothyroxine sodium were the most frequently used concomitant medications for both Lanreotide and Octreotide, with Metformin reported in 340 Lanreotide cases and 650 Octreotide cases. Levothyroxine sodium was used in 268Lanreotide cases and 634 Octreotide cases, indicating their frequent use in managing underlying metabolic and endocrine conditions alongside the primary treatment with somatostatin analogs (Table 2).
Top 20 adverse events
An analysis of the top 20 adverse events (AEs) revealed distinct profiles for the two drugs (Table 3). To ensure clinical relevance, non-specific terms (e.g., “off-label use”) were excluded from this list. Only pharmacologically interpretable AEs were retained. For Lanreotide, diarrhea was the most frequently reported AE, occurring in 1457 cases. Other significant AEs included injection site pain (595 cases), fatigue (493 cases), and cholelithiasis (198 cases). These results highlight Lanreotide’s tendency to cause gastrointestinal disturbances and localized injection site reactions.
For Octreotide, increased blood pressure was the most frequently reported AE, with 1834 cases, reflecting its strong association with cardiovascular complications. “Malignant neoplasm progression” was the second most reported AE (1735 cases); however, this likely reflects the natural course of the underlying disease rather than a direct adverse effect of Octreotide. This was followed by abdominal pain (1526 cases) and fatigue (778 cases). These data emphasize Octreotide’s higher risk of neoplastic and cardiovascular AEs compared to Lanreotide.
Disproportionality analyses
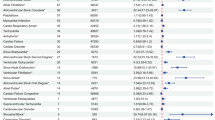
Disproportionality analysis provided further insight into the distinct safety profiles of the two drugs (Table 3). Lanreotideexhibited a strong association with gastrointestinal events, particularly cholelithiasis (gallstone formation), with a Reporting Odds Ratio (ROR) of 12.03 (95% CI: 10.46–13.85) and an EBGM05 of 11.88, indicating a significant and robust safety signal. Additionally, diarrhea was frequently reported, with significant reporting in over 1457 cases, and abdominal distension was also common.
In contrast, Octreotide showed stronger signals for cardiovascular adverse events, with increased systolic blood pressure being the most significant AE, with a ROR of 37.45 (95% CI: 35.50–39.51). Increased diastolic blood pressure was also reported frequently, with a ROR of 34.78 (95% CI: 31.85–37.98). Furthermore, malignant neoplasm progression was another major concern for Octreotide, reported in 1735 cases, with a significant ROR of 9.96 (95% CI: 8.66–11.46).
Time scans of safety signals
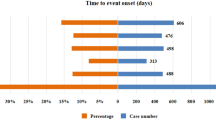
The time scan analysis demonstrated evolving trends in the reporting of adverse events for both drugs (Figs. 1 and 2). For Lanreotide, the safety signal for cholelithiasis began to rise steadily in 2015, with a clear upward trend observed in the Information Component (IC), which continued to rise over time with a narrowing confidence interval, confirming a stable and robust association with gallstone formation (Fig. 1). Additionally, injection site pain and mass also showed consistent increases over the years, reflecting the growing recognition of these localized reactions among patients treated with Lanreotide.
For Octreotide, the signal for increased systolic blood pressure peaked around 2018, with a continuous rise in the number of reported cases. The IC values also rose steadily, further confirming the cardiovascular risks associated with Octreotide use (Fig. 2). Reports of malignant neoplasm progression maintained consistently high levels throughout the reporting period, indicating a persistent concern for long-term users.
System organ class (SOC) comparison
The comparison of safety signals across four key system organ classes (SOCs)—gastrointestinal, cardiovascular, neoplastic, and injection site reactions—revealed clear differences between the two drugs (Table 3). Lanreotide showed a strong association with gastrointestinal disorders, particularly cholelithiasis, with 198 cases reported. Other gastrointestinal AEs, such as diarrhea and abdominal pain, were also frequently reported, underscoring the drug’s impact on the gastrointestinal system. Additionally, injection site reactions, including injection site pain and injection site mass, were prominent, with significant signals reported for both.
On the other hand, Octreotide was more frequently associated with cardiovascular events, including increased systolic blood pressure and increased diastolic blood pressure, both of which were reported in high numbers and displayed substantial ROR values. Neoplasm progression, particularly malignant neoplasm progression, was another notable safety concern, with 1735 cases reported. This suggests a higher risk of cancer-related AEs in patients treated with Octreotide compared to those receiving Lanreotide. Overall, these findings highlight the distinct safety profiles of the two drugs, with Lanreotide posing higher risks of gastrointestinal and injection site issues, while Octreotide is more strongly linked to cardiovascular and neoplastic concerns (Figs. 1, 2 and 3).
Discussion
This study aimed to compare the adverse event (AE) profiles of Lanreotide and Octreotide in real-world settings, focusing on gastrointestinal, cardiovascular, neoplastic, and injection site reactions. Our findings show that gastrointestinal adverse events, such as diarrhea and abdominal pain, were more frequently associated with Lanreotide, whereas cardiovascular events, including increased blood pressure and bradycardia, were more commonly reported with Octreotide. Both drugs showed some associations with neoplastic progression, and injection site reactions were more prevalent with Lanreotide. Overall, these distinct safety profiles provide valuable insights into how these medications can be tailored for specific patient populations, particularly in the management of neuroendocrine tumors (NETs), where individual risk factors may dictate the choice of therapy.
Lanreotide’s association with gastrointestinal side effects is consistent with previous clinical trials, where diarrhea and abdominal pain were identified as common but generally mild and manageable AEs. For example, in a study by Paulson et al., 19.2% of patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) experienced treatment-related AEs, although none were severe8. These real-world findings affirm Lanreotide’s mild gastrointestinal profile, as further supported by Pillarisetty et al., who found no significant gastrointestinal AEs in their trial on postoperative pancreatic fistula prevention9. Together, these results support the gastrointestinal safety profile observed in controlled settings, confirming Lanreotide’s suitability for NET patients, even in routine clinical practice10,11.
On the other hand, our study observed a higher frequency of cardiovascular AEs, such as bradycardia and increased blood pressure, with Octreotide. This finding contrasts with many clinical trials, where cardiovascular risks were not prominently highlighted. However, Tasnim et al. reported cases of bradycardia and asystole related to intravenous Octreotide administration, especially in elderly patients12. This suggests that Octreotide’s effects on cardiac conduction and vascular resistance may present high risks in real-world settings, especially in older or comorbid populations, where such events are likely underreported in trial settings13,14. In addition, the observed higher incidence of cardiovascular AEs in Octreotide users may, in part, be attributed to underlying disease heterogeneity. For example, conditions such as acromegaly and phaeochromocytoma—both indications for Octreotide—are themselves associated with cardiovascular complications including hypertension, arrhythmias, and cardiomyopathy. This confounding by indication could inflate the cardiovascular risk signal in pharmacovigilance analyses and should be carefully considered when interpreting our findings.
It is also important to clarify that comparisons between the RORs of Lanreotide and Octreotide should not be interpreted as formal statistical comparisons between the two drugs. In pharmacovigilance methodology, RORs are calculated independently for each drug-AE pair using the entire FAERS database as the background comparator. Therefore, they reflect disproportionality within each drug’s own context and are not suitable for direct cross-drug comparison without cohort-level adjustments or shared denominators.
Despite the known antiproliferative effects of Lanreotide and Octreotide, our analysis found evidence of neoplastic progression in both groups. This result is likely due to the complex biology of NETs, which can exhibit resistance to somatostatin analogs (SSAs)15. Hessert-Vaudoncourt et al. highlighted how resistance mechanisms, such as activation of alternative pathways like PI3K/mTOR, could limit the antiproliferative effects of SSAs. Thus, combination therapies, particularly those involving mTOR inhibitors, may enhance the treatment of advanced or high-grade NETs, where single-agent therapy may not be sufficient. These findings underscore the importance of personalized treatment strategies for patients at risk of tumor progression11,16. It is also worth noting that “malignant neoplasm progression,” while frequently reported for Octreotide, likely represents the natural progression of neuroendocrine tumors rather than a pharmacologic adverse effect. In the context of FAERS, such outcomes may be recorded due to indication overlap and should be interpreted with caution.
In terms of injection site reactions, Lanreotide was associated with higher rates, consistent with past research. For example, the SODA registry study on acromegaly patients showed more frequent injection site reactions with self-administration compared to healthcare-provider administration. However, these reactions were mild and did not lead to treatment discontinuation, supporting the notion that while injection site reactions are common, they do not significantly impact long-term adherence. This consistency across studies strengthens the argument that Lanreotide remains a viable option for patients despite these minor reactions10,16.
Finally, both Lanreotide and Octreotide demonstrated acceptable long-term tolerability, aligning with prior clinical trials. Ito et al. reported that Lanreotide was well-tolerated over a median exposure of nearly three years in Japanese NET patients, and long-term safety data for Octreotide confirmed a manageable profile, with diarrhea and cholelithiasis being the most common AEs17. Serious adverse events, particularly those related to the gallbladder, were more frequent with Octreotide, as noted by Pivonello et al.18. Overall, both drugs offer viable long-term treatment options, but patient preferences and individual responses should guide the selection between the two, ensuring that treatment is aligned with the patient’s specific risk profile11,14.
The distinct AE profiles of Lanreotide and Octreotide uncovered in this study have significant clinical implications. Patients prone to gastrointestinal issues may benefit more from Octreotide, while those at greater risk for cardiovascular complications should be carefully monitored when prescribed Octreotide. Additionally, Lanreotide’s higher rate of injection site reactions, while typically mild, may affect its suitability for patients who value self-administration convenience or prefer less frequent dosing schedules. This study’s strength lies in its use of FAERS real-world data, which provides a broader and more diverse patient population compared to traditional clinical trials. However, the voluntary nature of FAERS reporting, along with the lack of detailed data on dosage and treatment duration, introduces limitations, particularly in terms of causality. Future research should focus on controlled studies that further investigate the cardiovascular risks associated with Octreotide, as well as exploring combination therapies to enhance the antiproliferative effects of SSAs. Long-term observational studies will also be essential for clarifying the full risk-benefit profiles of these therapies across various patient populations.
It is important to acknowledge the limitations inherent in using the FAERS database. Since FAERS is a spontaneous reporting system, it lacks denominator data (i.e., the total number of exposed patients), which precludes the calculation of incidence rates. Moreover, reporting bias, underreporting, and heterogeneity in patient populations receiving Lanreotide or Octreotide limit the ability to make direct comparisons between the two drugs. These factors can significantly influence the detection and strength of safety signals, and thus, findings from such data should be interpreted with caution.
In conclusion, our analysis suggests distinct adverse event patterns for Lanreotide and Octreotide, with gastrointestinal signals being more prominent for Lanreotide and cardiovascular/neoplastic signals more apparent for Octreotide. These findings are indicative rather than definitive, and should be interpreted cautiously due to inherent limitations in the FAERS data, including underreporting, indication bias, and lack of exposed population data.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Giulia, A. et al. Diagnostic and therapeutic management of primary orbital neuroendocrine tumors (NETs): Systematic literature review and clinical case presentation. Biomedicines https://doi.org/10.3390/biomedicines12020379 (2024).
Alex, H. et al. Management of neuroendocrine tumor liver metastases. Am. J. Surg. https://doi.org/10.1016/j.amjsurg.2023.08.011 (2023).
Anna La, S. et al. Targeting neuroendocrine tumors with octreotide and Lanreotide: Key points for clinical practice from NET specialists. Cancer Treat. Rev. 117, 102560–102560. https://doi.org/10.1016/j.ctrv.2023.102560 (2023).
Edward, M. W. et al. Lanreotide depot: an antineoplastic treatment of carcinoid or neuroendocrine tumors. J. Gastrointest. Cancer. 47 (4), 366–374. https://doi.org/10.1007/S12029-016-9866-9 (2016).
Amandine, M. et al. Pharmacokinetic differences between subcutaneous and intramuscular administration of Lanreotide: results from a phase I study. J. Clin. Oncol. https://doi.org/10.1200/JCO.2015.33.15_SUPPL.E15186 (2015).
Bate, A. & Evans, S. J. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf Jun. 18 (6), 427–436. https://doi.org/10.1002/pds.1742 (2009).
van Puijenbroek, E. P. et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf Jan-Feb. 11 (1), 3–10. https://doi.org/10.1002/pds.668 (2002).
Paulson, S. et al. Lanreotide depot to treat gastroenteropancreatic neuroendocrine tumors in a US community oncology setting: A prospective, observational study. Oncol. Therapy. 10 (2), 463–479. https://doi.org/10.1007/s40487-022-00208-1 (2022).
Venu, G. P., Arezou, A., James, O. P. & Jonathan, G. S. A phase II trial of Lanreotide for the prevention of postoperative pancreatic fistula. Hpb 24 (11), 2029–2034. https://doi.org/10.1016/j.hpb.2022.07.011 (2022).
Strosberg, J. R., Al-Toubah, T., El-Haddad, G., Reidy Lagunes, D. & Bodei, L. Sequencing of Somatostatin-Receptor-Based therapies in neuroendocrine tumor patients. J Nucl. Med Mar. 1 (3), 340–348. https://doi.org/10.2967/jnumed.123.265706 (2024).
Stueven, A. K. et al. Somatostatin analogues in the treatment of neuroendocrine tumors: past, present and future. Int J. Mol. Sci Jun. https://doi.org/10.3390/ijms20123049 (2019).
Saria, T., Tarek, Z. S., Hamsa, A-J. & Thien, V. Octreotide-induced Asystole in a 70-year-old woman. Chest 162 (4), A695–A695. https://doi.org/10.1016/j.chest.2022.08.545 (2022).
Al-Toubah, T. & Strosberg, J. Somatostatin analogs and interferon in the treatment of neuroendocrine tumors. In: (eds Yalcin, S. & Öberg, K.) Neuroendocrine Tumours: Diagnosis and Management. Springer International Publishing; :619–630. (2024).
Culler, M. D. et al. Somatostatin analogs for the treatment of neuroendocrine tumors. Cancer and Metastasis Reviews. /03/01 2011;30(1):9–17. (2011). https://doi.org/10.1007/s10555-011-9293-0
von Claus, H-V. et al. Concomitant Inhibition of PI3K/mTOR signaling pathways boosts antiproliferative effects of Lanreotide in bronchopulmonary neuroendocrine tumor cells. Front. Pharmacol. https://doi.org/10.3389/fphar.2024.1308686 (2024).
Sidéris, L., Dubé, P. & Rinke, A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist 17 (6), 747–755. https://doi.org/10.1634/theoncologist.2011-0458 (2012).
Tetsuhide, I. et al. Long-term safety and efficacy of Lanreotide autogel in Japanese patients with neuroendocrine tumors: final results of a phase II open-label extension study. Asia-Pac. J. Clin. Oncol. https://doi.org/10.1111/AJCO.13371 (2021).
Rosario, P. et al. Long-term safety of long-acting octreotide in patients with diabetic retinopathy: results of pooled data from 2 randomized, double-blind, placebo-controlled phase 3 studies. Endocrine 60 (1), 65–72. https://doi.org/10.1007/S12020-017-1448-5 (2018).
Funding
This research was funded by the 2024 Institutional Research Project of Wannan Medical College (WK2024ZQNZ08), and the School level scientific research project of Wannan Medical College in 2022 (jxyy202293).
Author information
Authors and Affiliations
Contributions
L.W. and S.C. (co-first authors) contributed equally to the study. L.W. and S.C. conceived and designed the study. L.W. performed data collection and analysis. M.W. contributed to statistical analysis and interpretation of results. L.Z. assisted in literature review and manuscript preparation. L.W. and S.C. wrote the main manuscript text, and M.W. and L.Z. revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Chen, S., Wu, M. et al. Comparative analysis of adverse event profiles of lanreotide and octreotide in somatostatin-responsive endocrine and neoplastic diseases. Sci Rep 15, 18641 (2025). https://doi.org/10.1038/s41598-025-03850-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03850-7