Abstract
Smoking negatively impacts aerobic capacity, primarily by reducing V̇O2max, the gold standard measure of cardiorespiratory fitness. While smoking cessation is known to improve vascular function, exercise performance, and oxygen uptake, its specific impact on V̇O2max remains underexplored. Specifically, no research has yet evaluated V̇O2max changes following a switch to electronic cigarettes (ECs) or heated tobacco products (HTPs). This is a secondary analysis of the CEASEFIRE trial, a 12-weeks randomized controlled switching trial comparing the impact of ECs or HTPs on changes in smoking behaviour. The trial offers a unique opportunity to prospectively examine the relationship between smoking behavior and aerobic capacity, and to examine—for the first time—the specific impact of exclusive EC or HTP use on V̇O2max. Changes in VO₂max were analized across three smoking phenotypes: continuous smokers, those who reduced smoking, and those who abstained from smoking Additionally, VO2max was also evaluated specifically in participants who completely abstained from smoking tobacco cigarettes, evaluating outcomes in exclusive EC and HTP users. Quitters showed the greatest improvement in VO2max at both week 4 (2.4 ± 1.7 mL kg−1 min−1) and week 12 (2.7 ± 1.9 mL kg−1 min−1). Reducers also exhibited significant VO2max increases (1.3 ± 1.9 mL kg−1 min−1 at week 4: 1.9 ± 1.8 mL kg−1 min−1 at week 12), while Failures (i.e. those who continued smoking) showed no change. Exclusive use of EC and HTP resulted in statistically significant and clinically relevant improvements in V̇O2max. Compared to baseline, V̇O2max significantly increased at week 4 (EC: 38.4 ± 5.9 to 41.0 ± 6.1 mL kg−1 min−1; HTP: 39.2 ± 6.7 to 41.4 ± 6.4 mL kg−1 min−1, both p < 0.0001) and week 12 (EC: 38.4 ± 5.9 to 41.4 ± 6.3; HTP: 39.2 ± 6.7 to 41.6 ± 6.5 mL kg−1 min−1, both p < 0.0001). No significant differences between EC and HTP were observed at either time point. Rapid improvements in V̇O2max can happen when healthy smokers switch to exclusive use of ECs or HTPs. These findings reinforce the potential cardiorespiratory benefits of smoking cessation and harm reduction strategies.
Similar content being viewed by others
Introduction
Chronic exposure to the harmful chemicals in tobacco cigarette smoke significantly impairs physical fitness, primarily by reducing oxygen availability at the tissue level and diminishing both aerobic and anaerobic exercise capacity1,2.
Smoking is well-documented to decrease endurance performance in healthy individuals and negatively impacts physical fitness test outcomes, with reduced oxygen uptake capacity playing a central role3,4. Furthermore, an inverse relationship has been observed between smoking history and maximal aerobic capacity (V̇O2max)2,5, the gold standard for assessing cardiorespiratory fitness6.
V̇O2max represents the maximum rate of oxygen consumption measured during incremental exercise and reflects the efficiency of the respiratory, cardiovascular, musculoskeletal, and metabolic systems in oxygen transport and utilization. A higher V̇O2max indicates improved aerobic capacity, which is associated with better cardiorespiratory fitness and reduced cardiovascular and all-cause mortality risk7,8.
Importantly, the negative effects of smoking on cardiorespiratory performance can be reversed upon cessation. Studies have shown that smoking cessation leads to significant improvements in vascular endothelial function, exercise performance and cardiovascular responses to physical activity9,10,11,12. These benefits can be observed as early as 12 weeks post-cessation and persist for up to three years9,10,11,12. Notably, the rapid improvements in V̇O2max observed post cessation suggest that this parameter may serve as a sensitive biomarker of physiological recovery, reflecting early changes in cardiorespiratory health. This makes V̇O2max particularly valuable in the context of smoking cessation and switching trials involving alternative nicotine and tobacco products. However, the physiological effects and health benefits of smoking cessation—especially those measured by V̇O2max—remain underexplored, with only one study to date having rigorously assessed this outcome11. Moreover, no research has yet evaluated V̇O2max changes following a switch to electronic cigarettes (ECs) or heated tobacco products (HTPs), representing a critical gap in the literature.
ECs and HTPs, battery-powered devices that deliver nicotine without combustion, have gained popularity as alternatives to conventional cigarettes13,14. These products are widely used by smokers aiming to reduce their exposure to harmful chemical emissions from combustion15,16,17. The key to their reduced harm potential lies in the elimination of combustion18, which is the primary source of toxicants in cigarette smoke. Although HTPs contain tobacco and may not offer the same level of harm reduction as ECs, both products have been explored for their potential to lower smoking-related harm19,20 and their effectiveness as smoking cessation aids remains subject to ongoing research21,22.
We hypothesize that smokers who transition from combustible tobacco cigarettes to non-combustible nicotine/tobacco products (N–C NTPs) such as ECs and HTPs may experience measurable improvements in cardiorespiratory performance as a result of eliminating tobacco smoke exposure23. While previous research has documented the physiological effects and health improvements associated with switching to these products24,25,26 the specific impact on V̇O2max remains insufficiently explored. Given that V̇O2max is a well-established indicator of aerobic capacity, any observed improvements following smoking cessation could provide valuable insights into the potential respiratory and cardiovascular benefits of smoking substitution.
This study examines changes in V̇O2max, measured using the sub-maximal Chester Step Test, in relation to smoking reduction and smoking abstinence among participants in the CEASEFIRE trial27. CEASEFIRE is a large prospective randomized controlled trial designed to assess smoking reduction and cessation rates among adult smokers transitioning from conventional cigarettes to N–C NTPs (ECs and HTPs). V̇O2max was assessed at baseline and monitored at multiple follow-up visits.
The CEASEFIRE trial offers a unique opportunity to prospectively investigate the impact of smoking reduction or cessation on aerobic capacity. This study presents a secondary analysis of the trial data, specifically examining changes in V̇O2max across three groups: continuous smokers, those who reduced smoking, and those who abstained from smoking. Additionally, no research has ever documented V̇O2max changes after switching to ECs or HTPs. Therefore, we conducted a separate analysis to assess the differential impact of exclusive EC use versus exclusive HTP use on V̇O2max, offering unique insights into the effects of these non-combustible nicotine and tobacco products.
Methods
The current study serves as a secondary analysis of a large prospective randomized control non-inferiority trial that focused on examining the quit and reduction rates among adult smokers who transitioned from conventional tobacco cigarettes to non-combustible nicotine/tobacco products (N–C NTP) namely HTPs and ECs. The specifics regarding the population sample, study design, Ethics Review Board approval, study registration, and CONSORT reporting standards were previously detailed28.
Research was performed in accordance with the relevant guidelines/regulations and according to the Declaration of Helsinki. Informed consent was obtained from all participants and/or their legal guardians.
The de-identified datasets from the trial were sourced from the open science repository maintained by the Center of Excellence for the Acceleration of Harm Reduction (CoEHAR) at the University of Catania, and subsequently utilized for this analysis: https://zenodo.org/records/7941030
Since the study exclusively used publicly available, de-identified data, it was exempt from ERB review. Only complete and reliable data were extracted to ensure analytical integrity.
Study participants
Adult smokers of ≥ 10 cigarettes per day (regularly smoking for at least the past year) and with exhaled carbon monoxide (eCO) levels of ≥ 7 ppm, not planning to quit soon (within the next 30 days from screening), but open to switching to HTPs or ECs were recruited from hospital and university staff, via social media, and through word of mouth. They confirmed no quit intention by answering “No” to these two questions: “Do you plan to quit smoking within the next 30 days?” and “Do you wish to participate in a smoking cessation program?”. They also met specific exclusion criteria including: (1) history of mental disease, (2) history of alcoholism or drug abuse, (3) presence of clinical diseases that, in the opinion of the investigator, would jeopardize the safety of the participant or impact the validity of the study results, (4) use of any N–C NTPs within the last 3 months, and (5) use of nicotine replacement therapy or other smoking cessation therapies within the last 3 months. Subjects were informed that the purpose of the study was to quantify the impact of reductions in cigarette consumption on cardio-respiratory performance. The study was approved by the local Ethical Review Board (Comitato Etico, Azienda Ospedaliero Universitaria “Policlinico-V. Emanuele,” Università di Catania, Italy; approval no. 215/2017/PO). All participants provided written informed consent prior to participation in the study. The study was registered at ClinicalTrial.gov (trial registration ID: NCT03569748).
Trial design and study visits
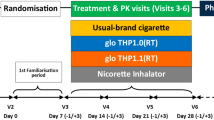
Eligible subjects were enrolled into a 12-week, randomized, two parallel arm, controlled trial consisting of seven study visits (one screening visit and six study visits) at smoking cessation clinics of the University of Catania (Centro per la Prevenzione e Cura del Tabagismo – CPCT) designed to compare cessation and reduction rates between HTPs and ECs study arms. The trial also measured maximal aerobic capacity by Chester step test at baseline, week-4, and week-12.
At the baseline visit, subjects were randomized to either the HTP (IQOS 2.4) group or the EC (JustFog Q16 Starter Kit) group and were instructed to use the assigned product to assist in abstaining from cigarette smoking. Participants were asked to return to the CPCT for follow-up visits to obtain regular supplies of tobacco sticks (for IQOS 2.4 users) and e-liquid refills (for JustFog Q16 users). During these visits, subjects were required to report their tobacco cigarette and EC/HTP consumption, undergo measurements of eCO levels, and have their blood pressure (BP) and heart rate (HR) assessed. Self-reported use of tobacco cigarette and HTPs or ECs since the previous visit was noted in a study diary and recorded in the electronic case report form at each visit (from V2 to V6). Additionally, EC/HTP consumption was verified through checks of product use (by counting the number of used and unused tobacco sticks and liquid refill containers) and calculated as average consumption on a per daily basis. Additionally, the Chester step test was scheduled to be repeated at weeks 4 and 12.
Exhaled carbon monoxide measurements
Measurements of exhaled carbon monoxide (eCO) levels, expressed in parts per million (ppm), were taken using a hand-held CO meter (Micro CO; Micro Medical Ltd, UK). Subjects were instructed to exhale slowly into a disposable mouthpiece connected to the CO meter, following the manufacturer’s recommendations. Expiratory maneuvers were taken late in the morning or early in the afternoon with participants sitting comfortably. Participants were requested to refrain from smoking, vaping, or using heated tobacco products (HTPs) for at least 30 min prior to each measurement. Smoking status was objectively confirmed when eCO levels exceeded 10 ppm.
Chester step test procedure
The Chester Step Test (CST) is a validated test assessing maximal aerobic capacity (maximal oxygen consumption, V̇O2Max) by having subjects step on and off a gym step at gradually increasing stepping rates every two minutes to increase their heart’s rate, which was used to calculate their V̇O2 max29,30. The test provides good test–retest reliability and is an acceptable method for estimating V̇O2max in the general healthy adult population31.
Before the commencing the test, participant’s blood pressure (BP) and resting heart rate (HR) were measured. Participants were then instructed to step in time with the beat of a metronome initially set at 15 beats/minute. Every 2-min, stepping rate was increased by 5 steps/minute (from 15 to 35 bpm, a total of 5 stages). HR and rating of perceived exertion (RPE) were recorded at each stage. The test continues until the participant reaches a specific HR (80% HR max) or a moderately vigorous level of exertion (RPE below 14). Aerobic capacity (V̇O2Max) and fitness rating are determined using a Chester Step Test calculator available at: https://www.brianmac.co.uk/chester.htm.
Smoking phenotypes
The study analyzed the time-course of V̇O2max changes in relation to three different smoking phenotypes: quitters vs. reducers vs. failures.
Smoking abstinence is defined as complete self-reported abstinence from cigarette smoking since the previous study visit, which was biochemically verified by eCO levels of < 10 ppm. Smokers in this category are classified as quitters. Continuous abstinence rate from week 2 to week 4 (CAR 2–4 Weeks) and from week 2 to week 12 (CAR 2–12 Weeks) were used as a robust characterization of smoking abstinence. Among the quitters, we employed a further subclassification to distinguish between individuals who achieved abstinence after switching to ECs and those who quit after switching to HTPs. This allowed for a more granular comparison of outcomes based on the type of study product used.
Smoking reduction is defined as self-reported ≥ 50% reduction in the number of cigarettes smoked per day from baseline (eCO levels were measured to verify smoking status and confirm a reduction compared with baseline). Smokers in this category are classified as reducers. Continuous reduction rates from week 2 to week 4 (CRR 2–4 Weeks) and from week 2 to week 12 (CRR 2–12 Weeks) were used as a robust characterization of smoking reduction.
Smoking failure is the smoking phenotype that excludes smoking abstinence or smoking reduction. Smokers in this category are classified as failures.
Statistical analysis
In the primary analysis, a total of 220 smokers were enrolled, with 110 participants assigned to the EC Study arm and 110 to the HTP Study arm. For the current secondary analysis, subjects’ V̇O2max measurements from both study arms were pooled and evaluated for changes from baseline at the 4-week and 12-week time points, stratified by smoking behavior phenotype: Failures (continued smokers), Reducers, and Quitters (complete abstainers). In addition, this secondary analysis also investigated possible differential impact of exclusive EC use and exclusive HTP use on V̇O2max, providing novel insights into the cardiorespiratory effects of N–C NTPs.
Categorical variables were summarized using counts and percentages, while continuous variables with symmetrical distribution were presented as mean and standard deviations (SD). Skewed continuous data were summarized using median and interquartile ranges (IQR). Comparisons were conducted using the χ2 test for categorical variables, and one-way Analysis of Variance (ANOVA) and Kruskal–Wallis tests for normally and not normally distributed data, respectively. A two-way ANOVA was used to assess the effects of classification and sex on V̇O2 max at baseline. A repeated measures ANOVA model was applied to examine potential effects of classifications and sex on V̇O2 max over time. To evaluate the association between smoking phenotypes and changes in V̇O2 max from baseline to week 4 and week 12, while accounting for potential confounders, a multiple linear regression analysis was performed. The outcome variable was ΔV̇O2 max (i.e., the absolute difference in V̇O2 max from baseline to weeks 4 and 12), with classification, age, sex and BMI changes from baseline included as independent factors. These variables were selected based on existing evidence indicating their potential influence on cardiorespiratory performance.
The analyses were performed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL) for Windows version 20.0 and p values < 0.05 were considered significant.
Results
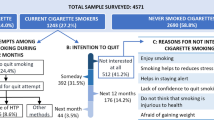
For this secondary analysis, data from 187 subjects were available. This accounted for missing data from subjects who either could not perform the Chester Step Test or did not qualify as continuous smoking phenotypes (Quitters, Reducers, or Failures, based on the provided definitions). Among the 187 evaluated subjects, 32 (17%) were classified as Failures, 88 (47%) as Reducers, and 67 (36%) as Quitters. A description of the evaluated sample is presented in Table 1. Table 2 illustrates the level of cigarette consumption for each smoking phenotype, showing that Reducers had on average a greater than 75% reduction in cigarette consumption from baseline.
At baseline, the frequency distribution of sex among classes (i.e., Failures, Reducers, and Quitters) was significantly different (p = 0.027, χ2 test). Conversely, no significant difference was found for age among classes and sex (Two-way ANOVA). Additionally, no significant difference was found at baseline for cigarettes per day, pack-years, and the number of years of smoking among sex and classes (Two-way ANOVA). As expected, V̇O2max was significantly different between males and females (p < 0.0001) at baseline, while no difference was found among classes (p = 0.147, Two-way ANOVA).
Regarding the effect of individual smoking history on baseline V̇O2max, strong negative correlations were found between V̇O2max and both the natural log of years of smoking (R2 = 0.469, p < 0.0001) and the natural log of pack-years (R2 = 0.389, p < 0.0001). In a multiple linear model, both correlations disappeared when corrected for sex and age, which were the only independent variables correlated to V̇O2max at baseline. However, to limit the obvious effect of age on individual smoking history by evaluating a narrower age window, in a subset with age range between 19 and 30 years, we found that in a multiple regression model for V̇O2max at baseline, sex (B = − 4.834 [95% CI − 6.218/ − 3.451], reference: Males, p < 0.0001) and the natural log of pack-years (B = − 1.269 [95% CI − 2.469/ − 0.069], p = 0.038) showed a significant correlation, but not age (p = 0.820).
The correlation between V̇O2max and the natural log of eCO at baseline was significant (R2 = 0.055, p = 0.0013). Additionally, the correlation between changes in V̇O2max (ΔV̇O2max) at week 12 and at week 4 was also significant (R2 = 0.77, p < 0.0001).
Table 3 and Fig. 1 present the means and standard deviations (SDs) of V̇O2max at baseline, week 4, and week 12, separately for each continuous smoking phenotypes. A Repeated Measures ANOVA model, using time as the within-subject factor and classification and sex as between-subject factors, showed that time (p < 0.0001), phenotype classification (p = 0.004), and sex (p < 0.0001) all had significant effects on V̇O2max changes over time. In the model, no significant effect was produced by the product used (EC or HTP, p = 0.144).
Means ± SDs relevant to the values of V̇O2 max at baseline, week 4 and week 12 separately for smoking phenotype classification. Time (p < 0.0001), smoking phenotype classification (p = 0.01) and sex p < 0.0001 had a significant effect on V̇O2 max (Repeated Measures ANOVA model, adopting time as within factor and classification and sex as between factors).
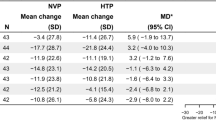
In Fig. 2 and Table 3, the means and SDs of changes in V̇O2max (ΔV̇O2max) at week 4 and week 12 from baseline are shown separately for each continuous smoking phenotypes. A one-way ANOVA model indicated that, among Quitters, ΔV̇O2max was significantly greater at both week 4 (2.4 ± 1.7 mL kg−1 min−1) and week 12 (2.7 ± 1.9 mL kg−1 min−1, p < 0.0001 for both). Statistically significant improvements in ΔV̇O2max were also observed among Reducers at both week 4 and week 12, with increases of 1.3 ± 1.9 and 1.9 ± 1.8 mL kg−1 min−1, respectively (p < 0.0001 for both). In subjects who continued to smoke at the same rate as baseline, the estimated V̇O2max remained unchanged.
Means ± SDs relevant to the changes in V̇O2 (ΔV̇O2) max at week 4 and week 12 separately for phenotype smoking classification. P values were computed by means of one-way ANOVA and Fisher’s protected Least Significant Difference. MCID; the minimum clinically important difference is defined as an improvement in anaerobic threshold of at least 2 ml O2/kg/min.
In Fig. 3 the means and SDs relevant to the comparisons of the changes in ΔV̇O2 max at week 4 and week 12 are shown for Quitters using EC or HTP. Both exclusive EC and HTP use caused a statistically significant and clinically relevant amelioration in V̇O2max. Compared to baseline, V̇O2max values were significantly greater at both week 4 (from 38.4 ± 5.9 mL kg−1 min−1 to 41.0 ± 6.1 mL kg−1 min−1; p < 0.0001, Repeated Measures ANOVA) and week 12 (from 38.4 ± 5.9 mL kg−1 min−1to 41.4 ± 6.3 mL kg−1 min−1; p < 0.0001) for exclusive EC use. Likewise, V̇O2max values were significantly greater than baseline at both week 4 (from 39.2 ± 6.7 mL kg−1 min−1 to 41.4 ± 6.4 mL kg−1 min−1; p < 0.0001) and week 12 (from 39.2 ± 6.7 to 41.6 ± 6.5 mL kg−1 min−1; p < 0.0001) for exclusive HTP use. No significant differences between EC and HTP usage were found by means of one-way ANOVA at both time points.
Means ± SDs relevant to the changes in V̇O2 (ΔV̇O2) max at week 4 and week 12 separately for EC and HTP users, in Quitters only. P values were computed by means of one-way ANOVA. MCID; the minimum clinically important difference is defined as an improvement in anaerobic threshold of at least 2 ml O2/kg/min.
Tables 4 and 5 present the multiple linear regression models for ΔV̇O2max at week 4 and week 12, respectively. At both weeks 4 and 12, V̇O2max significantly increased in both Reducers and Quitters, with no significant effects found for age and sex. Being a Reducer or a Quitter resulted in a significantly greater improvement in V̇O2max. The number of pack-years (as natural log) was positively correlated to ΔV̇O2max, while age was negatively correlated. Changes in BMI from baseline (this information was only available at week 12) were similarly negatively correlated to ΔV̇O2max.
Discussion
Research suggests that abstaining from smoking can improve exercise performance, though the evidence from longitudinal studies is very limited. This 12-week prospective cohort analysis shows significant and clinically relevant improvements in exercise capacity among smokers who were encouraged to quit their cigarette consumption by switching to HTPs or ECs. Improvements were also observed in individuals who reduced their tobacco cigarette consumption. The study specifically shows—for the first time—consistent improvement in V̇O2max, among quitters exclusively using EC or HTP. Notably, there was a significant increase in aerobic capacity (i.e. maximal oxygen consumption, V̇O2max), observable as early as 4 weeks.
The study used the standardized Chester Step Test (CST) to estimate maximal aerobic capacity (i.e., V̇O2max). While the CST may not be as accurate as direct V̇O2max measurements obtained through standard cardiopulmonary exercise testing (which requires gas analyzers and complex procedures), it offers a simpler and valid alternative that could be easily incorporated into routine clinical practice29,30,31. Additionally, the CST, being sub-maximal, is safer than cardiopulmonary exercise testing and is also more rapid, making it a useful tool for assessing large study samples with repeated measures after interventions aimed at improving the O2 transport/utilization pathway. For these reasons, CST was considered the ideal test to examine V̇O2max changes in the context of a large prospective switching trial of ECs and HTPs.
This study demonstrates that abstaining from smoking can improve V̇O2max. Specifically, consistent and clinically relevant improvements were observed among quitters exclusively using EC or HTP. This adds important new information to the current understanding of how stopping smoking and complete substituting tobacco cigarettes with N–C NTPs can reverse the detrimental effects of cigarette smoke exposure on aerobic capacity in relatively young and apparently healthy individuals. The improvement in V̇O2max in our study aligns with the findings that the aerobic capacity of current smokers is significantly lower compared to non-smokers and former smokers32,33.
Our study shows an improvement in V̇O2max of at least 2.2 and 2.6 mL kg−1 min−1 from baseline among HTP and EC users, respectively. These changes are is statistically significant as early as week 4 and exceed the minimum clinically important difference (MCID) for V̇O2max (defined as an increase in anaerobic threshold of at least 2 mL O2 kg−1 min−1). The reported changes were not significantly different between EC and HTP. This suggests that individuals who quit smoking by switching to ECs or HTPs can achieve an early and clinically meaningful improvement in aerobic capacity. An improvement beyond the MCID reflects enhanced cardiorespiratory fitness, which may translate into better daily functioning and physical performance.
An important additional finding of this present study is that we found statistically significant improvements in ΔV̇O2max among reducers, with increases of 1.3 and 1.9 mL kg−1 min−1 at week 4 and week 12, respectively. In this cohort of reducers, daily cigarette consumption was consistently reduced by at least 75% from baseline throughout the entire study duration. By substantially reducing cigarette smoking with N-C NTPs (i.e. HTPs or ECs) use and thereby curtailing exposure to several toxic chemicals, we observed an amelioration in aerobic capacity may have resulted.
The rapid and significant improvements in V̇O2max following smoking cessation after switching to N-C NTPs can be attributed to several potential mechanisms, including a reduction in carbon monoxide (CO) levels. Tobacco smoking increases CO in the bloodstream, which binds to hemoglobin with a much higher affinity than oxygen, leading to elevated carboxyhemoglobin (COHb) levels and reduced oxygen-carrying capacity. This compromises oxygen delivery to muscle mitochondria, affecting V̇O2max by reducing available binding sites on hemoglobin and slowing oxygen unloading at active muscles34. Inhalation of CO sufficient to raise COHb to approximately 4.5% (equivalent to smoking three cigarettes) has been shown to decrease V̇O2max by about 7%35 Consistently, our study found a small but significant correlation between exhaled CO and estimated V̇O2max. The correlation remained modest because smoking influences exercise capacity through multiple mechanisms, including improvements in vascular endothelial function, and reductions in exposure to advanced glycation end products (AGEs) and nitrosamines such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL)36,37. For example, years of smoking – a key predictor of reduced physical fitness—was inversely correlated with baseline V̇O2max in our study.
The marked decline in CO and COHb levels following cigarette substitution with combustion-free alternatives38,39 rapidly restores the oxygen-carrying capacity of the blood, improving oxygen delivery to muscles during exercise. This contributes to the time-dependent improvement in aerobic capacity, with greater improvement observed at 12 weeks due to a higher prevalence of quitters compared to 4 weeks. This aligns with improved exercise tolerance seen in COPD patients who switched to e-cigarettes26 and heated tobacco products40.
The study had both strengths and limitations. The initial RCT provided a large, well-characterized cohort with standardized data collection methods, ensuring careful tracking of physiological changes over time and biochemical verification of smoking status. This approach minimized biases commonly associated with retrospective or cross-sectional studies, enhancing the credibility of our findings, particularly in terms of data quality and consistency. Notably, the use of biochemically verified continuous abstinence rate (CAR) as a measure of abstinence is particularly valuable in this type of study, as continuous abstinence is the key driver of health improvements in a per-protocol analysis. The high CAR in this dataset can be attributed to strong acceptability and effectiveness of the smoking substitution products used in the original study27. This, combined with personalized counseling from psychologists specialized in both smoking cessation and harm reduction, played a crucial role in preventing relapse. While we recognize the importance of evaluating unaided cessation in the broader context of harm reduction, our secondary analysis specifically focused on the pathophysiological impact of smoking cessation/reduction, as reflected in measurable improvements in aerobic capacity. Both ECs and HTPs were effective in promoting smoking cessation and improving V̇O2max. However, it is important to emphasize that the original CEASEFIRE trial was specifically designed to compare ECs and HTPs, and therefore did not include a control group to isolate the individual effects of each product. While the inclusion of an unaided quitter control group would certainly enhance future research, its absence in this study does not compromise the validity of the current findings. Another limitation of this study is the limited generalizability of the findings, as the relatively young population with normal weight and no preexisting diseases may not accurately represent the broader patient population. Additionally, study participants were from urban Sicilian settings, which may limit the generalizability due to unique lifestyle factors such as diet, exercise, and genetics. Furthermore, we used only one exercise modality, namely CST, to assess improvement in aerobic capacity, whereas other types of exercise tests may be more precise for this assessment. However, the Chester Step test has previously been demonstrated as an appropriate tool to track changes over the time, which was the purpose of our study. It also has a high test–retest reliability, which lends itself to repeat testing manoeuvres. We also could not control for changes in exercise habits during the study, which is a known factor influencing improvements in V̇O2max and/or V̇O2peak41 but this is unlikely to have been different between the randomised cohorts to account for the differences seen.
In conclusion, this post-hoc analysis suggests early significant improvements in exercise capacity among healthy smokers who quit smoking by switching to either exclusive ECs or HTPs use Given that V̇O2max is the key indicator of exercise capacity and a strong predictor of cardiovascular morbidity and all-cause mortality7,8, these findings provide valuable insights into the potential benefits of switching from tobacco cigarettes to combustion free nicotine alternatives. However, as this is a post-hoc analysis, causality cannot be established, and further prospective studies are needed to confirm the present findings.
Recognizing changes in V̇O2max as early indicators of health effect will strengthen their value across clinical, regulatory, and research settings. Clinicians can leverage this information to recommend smoking cessation interventions, that may lead to tangible improvements in physical performance and overall well-being. Additionally, researchers may consider including V̇O2max measurements as clinically meaningful outcomes in future studies involving e-cigarettes, heated tobacco products, oral tobacco/nicotine products, smoking cessation medications, and other medicinal products designed to improve physical performance.
Data availability
The de-identified datasets from the trial were sourced from the open science repository maintained by the Center of Excellence for the Acceleration of Harm Reduction (CoEHAR) at the University of Catania, and subsequently utilized for this analysis: https://zenodo.org/records/7941030.
References
Montoye, H. J., Gayle, R. & Higgins, M. Smoking habits, alcohol consumption and maximal oxygen uptake. Med. Sci. Sports Exerc. 12(5), 316–321 (1980).
Sengbusch, J. R., Tiernan, D. L., Tamulevicius, N. & Martinasek, M. P. The impact of smoking on maximum oxygen uptake. Respir. Care 66(5), 857–886 (2021).
Cooper, K. H., Gey, G. O. & Bottenberg, R. A. Effects of cigarette smoking on endurance performance. JAMA 203(3), 189–192 (1968).
Conway, T. L. & Cronan, T. A. Smoking, exercise, and physical fitness. Prev. Med. 21(6), 723–734 (1992).
Suminski, R. R. et al. The effect of habitual smoking on measured and predicted VO2(max). J. Phys. Act Health. 6(5), 667–673. https://doi.org/10.1123/jpah.6.5.667 (2009).
Harber, M. P. et al. Assessing cardiorespiratory fitness in clinical and community settings: Lessons and advancements in the 100th year anniversary of VO2max. Prog. Cardiovasc. Dis. 83, 36–42. https://doi.org/10.1016/j.pcad.2024.02.009 (2024).
Harber, M. P. et al. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog. Cardiovasc. Dis. 60(1), 11–20. https://doi.org/10.1016/j.pcad.2017.03.001 (2017).
Ekblom-Bak, E. et al. Sex- and age-specific associations between cardiorespiratory fitness, CVD morbidity and all-cause mortality in 266.109 adults. Prev. Med. 127, 105799. https://doi.org/10.1016/j.ypmed.2019.105799 (2019).
Albrecht, A. E., Marcus, B. H., Roberts, M., Forman, D. E. & Parisi, A. F. Effect of smoking cessation on exercise performance in female smokers participating in exercise training. Am. J. Cardiol. 82(8), 950–955 (1998).
Berkovitch, A. et al. Time-dependent relation between smoking cessation and improved exercise tolerance in apparently healthy middle-age men and women. Eur. J. Prev. Cardiol. 22(6), 807–814 (2015).
Asthana, A. et al. Long-term effects of smoking and smoking cessation on exercise stress testing: three-year outcomes from a randomized clinical trial. Am. Heart J. 163(1), 81–87 (2012).
George, J. et al. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J. Am. Coll. Cardiol. 74(25), 3112–3120 (2019).
Jerzyński, T., Stimson, G. V., Shapiro, H. & Król, G. Estimation of the global number of e-cigarette users in 2020. Harm Reduct. J. 18(1), 109 (2021).
Hori, A., Tabuchi, T. & Kunugita, N. Rapid increase in heated tobacco product (HTP) use from 2015 to 2019: from the Japan “Society and New Tobacco” Internet Survey (JASTIS). Tob. Control 30(4), 474–475 (2020).
Caruso M, Emma R, Distefano A, Rust S, Poulas K, Zadjali F, Giordano A, Volarevic V, Mesiakaris K, Al Tobi M, Boffo S, Arsenijevic A, Zuccarello P, Giallongo C, Ferrante M, Polosa R, Li Volti G; Replica Project Group. Electronic nicotine delivery systems exhibit reduced bronchial epithelial cells toxicity compared to cigarette: The Replica Project. Sci. Rep. https://doi.org/10.1038/s41598-021-03310-y (2021).
Daynard, R. Public health consequences of e-cigarettes: A consensus study report of the National Academies of Sciences, Engineering, and Medicine. J. Public Health Pol. 39(3), 379–381 (2018).
Farsalinos, K. E. & Polosa, R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther. Adv. Drug Saf. 5(2), 67–863 (2014).
Cullen, J. T., Kärkelä, T. & Tapper, U. Signatures that differentiate thermal degradation and heterogeneous combustion of tobacco products and their respective emissions. J. Anal. Appl. Pyrol. 179, 106478 (2024).
O’Leary, R. & Polosa, R. Tobacco harm reduction in the 21st century. Drugs Alcohol Today 219–234, 2020. https://doi.org/10.1108/DAT-02-2020-0007 (2020).
Royal College of Physicians. E-cigarettes and harm reduction: An evidence review. RCP (2024).
Lindson, N. et al. Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD015226.pub2 (2023).
O’Leary, R., La Rosa, G. R. M. & Polosa, R. Examining e-cigarettes as a smoking cessation treatment: A critical umbrella review analysis. Drug Alcohol Depend 266, 112520 (2025).
Polosa, R. Examining the evidence for the health impact of combustion-free products: Progress and prospects for tobacco harm reversal and reduction. Intern. Emerg. Med. 16(8), 2043–2046 (2021).
La Rosa, G., Vernooij, R., Qureshi, M., Polosa, R. & O’Leary, R. Clinical testing of the cardiovascular effects of e-cigarette substitution for smoking: A living systematic review. Intern. Emerg. Med. 18(3), 917–928 (2023).
Polosa, R. et al. Impact of exclusive e-cigarettes and heated tobacco products use on muco-ciliary clearance. Ther. Adv. Chronic Dis. 12, 20406223211035268 (2021).
Polosa, R. et al. COPD smokers who switched to e-cigarettes: Health outcomes at 5-year follow up. Ther. Adv. Chronic Dis. 11, 2040622320961617 (2020).
Caponnetto, P. et al. Comparing the effectiveness, tolerability, and acceptability of heated tobacco products and refillable electronic cigarettes for cigarette substitution (ceasefire): Randomized controlled trial. JMIR Public Health Surveill 9, e42628 (2023).
Caponnetto, P. et al. Non-inferiority trial comparing cigarette consumption, adoption rates, acceptability, tolerability, and tobacco harm reduction potential in smokers switching to Heated Tobacco Products or electronic cigarettes: Study protocol for a randomized controlled trial. Contemp Clin. Trials Commun. 17, 100518 (2020).
Sykes, K. The Chester Step Test: ASSIST Physiological measurement resources manual version 3. Liverpool: ASSIST Creative Resources Ltd. (1998).
Sykes, K. & Roberts, A. The Chester step test—A simple yet effective tool for aerobic capacity. Physiotherapy 90(4), 183–188 (2004).
Bennett, H., Parfitt, G., Davison, K. & Eston, R. Validity of submaximal step tests to estimate maximal oxygen uptake in healthy adults. Sports Med. 46(5), 737–750. https://doi.org/10.1007/s40279-015-0445-1 (2016).
Kim, D. J. Study on cardiopulmonary function, maximal oxygen uptake, and obesity index according to smoking status in middle-aged and older office workers. Osong Public Health Res. Perspect. 9(3), 95–100 (2018).
Caci, G. et al. Assessment and repeatability of aerobic capacity using the Chester Step Test among current, former, and never smokers. Intern. Emerg. Med. 20(1), 297–305 (2025).
McDonough, P. & Moffatt, R. J. Smoking-induced elevations in blood carboxyhaemoglobin levels. Effect on maximal oxygen uptake. Sports Med. 27, 275–283 (1999).
Klausen, K., Andersen, C. & Nandrup, S. Acute effects of cigarette smoking and inhalation of carbon monoxide during maximal exercise. Eur. J. Appl. Physiol. 51(3), 371–379 (1983).
Bahrke, M. S., Baur, T. S., Poland, D. F. & Connors, D. F. Tobacco use and performance on the U.S. Army physical fitness test. Mil. Med. 153, 229–235. https://doi.org/10.1093/milmed/153.5.229 (1988).
Jacob, P. et al. Biomarkers of exposure for dual use of electronic cigarettes and combustible cigarettes: Nicotelline, NNAL, and total nicotine equivalents. Nicotine Tob. Res. 22(7), 1107–1113 (2020).
Caponnetto, P., Maglia, M., Prosperini, G., Busà, B. & Polosa, R. Carbon monoxide levels after inhalation from new generation heated tobacco products. Respir. Res. https://doi.org/10.1186/s12931-018-0867 (2018).
Beatrice, F. & Massaro, G. Exhaled carbon monoxide levels in forty resistant to cessation male smokers after six months of full switch to electronic cigarettes (e-cigs) or to a tobacco heating systems (THS). Int. J. Environ. Res. Public Health 16(20), 3916 (2019).
Polosa, R. et al. Health outcomes in COPD smokers using heated tobacco products: A 3-year follow-up. Intern. Emerg. Med. 16(3), 687–696 (2021).
Darabseh, M. Z., Selfe, J., Morse, C. I., Aburub, A. & Degens, H. Does aerobic exercise facilitate vaping and smoking cessation: A systematic review of randomized controlled trials with meta-analysis. Int. J. Environ. Res. Public Health 19(21), 14034 (2022).
Funding
The authors received no funding for the secondary analysis using the de-identified datasets sourced for free from an open research repository: https://zenodo.org/records/7941030. Supporting for publication fees of this article was provided by Department of Clinical and Experimental Medicine at the University of Catania (Departmental Fund 6C725202094). The original randomized trial was funded by Philip Morris Products S.A.
Author information
Authors and Affiliations
Contributions
Polosa Riccardo:designed research Davide Campagna: performed research Grazia Caci: collected data, Fabio Cibella, Claudio Saitta , Jacob George: analyzed data and revised themanuscript Lucia Spicuzza: wrote paper. Francesco Pennisi: MS second revision and literature revision Giulio Geraci MS second revision and literature revision Yusuff Adebisi: Ms third revision.
Corresponding author
Ethics declarations
Competing interests
RP is full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. He has received grants from U-BIOPRED and AIR-PROM, Integral Rheumatology & Immunology Specialists Network (IRIS), Foundation for a Smoke Free World, Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, Merk Sharp & Dohme, Boehringer Ingelheim, Novartis, Arbi Group Srl., Duska Therapeutics, Forest Laboratories, Ministero dell Universita’ e della Ricerca (MUR) Bando PNRR 3277/2021 (CUP E63C22000900006) and 341/2022 (CUP E63C22002080006), funded by NextGenerationEU of the European Union (EU), and the ministerial grant PON REACT-EU 2021 GREEN- Bando 3411/2021 by Ministero dell Universita’ e (MUR) – PNRR EU Community. He is founder of the Center for Tobacco Prevention and Treatment (CPCT) at the University of Catania and of the Center of Excellence for the Acceleration of Harm Reduction at the same university. He receives consultancy fees from Pfizer, Boehringer Ingelheim, Duska Therapeutics, Forest Laboratories, CV Therapeutics, Sermo Inc., GRG Health, Clarivate Analytics, Guidepoint Expert Network, and GLG Group. He receives textbooks royalties from Elsevier. He is also involved in a patent application for ECLAT Srl. He is a pro bono scientific advisor for Lega Italiana Anti Fumo (LIAF) and the International Network of Nicotine Consumers Organizations (INNCO); and he is Chair of the European Technical Committee for Standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). All other authors declare no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Spicuzza, L., Pennisi, F., Caci, G. et al. Improved aerobic capacity in a randomized controlled trial of noncombustible nicotine and tobacco products. Sci Rep 15, 19104 (2025). https://doi.org/10.1038/s41598-025-03904-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03904-w