Abstract
The advancement of nanotechnology and the growing demand for environmentally sustainable processes have fueled interest in green synthesis methods. In this research, copper-doped zinc oxide nanoparticles (Cu: ZnO NPs) were synthesized using a microwave-assisted approach, employing a bio-extract derived from Pistia Stratiotes (PS) leaves as a reducing agent. Comprehensive characterization through UV-Visible spectroscopy, PL, FTIR, SEM with EDS, TEM, DLS, XRD and XPS confirmed the formation, optical and structural features of the synthesized NPs. SEM and TEM images revealed spherical and nanorod-like morphologies, with particle sizes ranging from 15 nm to 65 nm and a tendency to agglomerate. Density Functional Theory (DFT) simulations using Quantum Espresso indicated a band gap narrowing to 3.0 eV after copper doping. Biologically, the Cu: ZnO NPs exhibited strong antibacterial activity against Candida albicans (16.3–17.5 mm), Staphylococcus aureus (18.4–21.5 mm), and Escherichia coli (19–21.6 mm). Additionally, the NPs showed promising anticancer potential against SK-MEL-28 melanoma cells, with an IC50 value of 30.53 µg/mL. Overall, this research demonstrates an eco-friendly and efficient route for fabricating Cu: ZnO NPs with significant antimicrobial and anticancer properties, emphasizing their potential for future biomedical applications.
Similar content being viewed by others
Introduction
The multidisciplinary study of materials at the nanoscale, which usually lies between 1 and 100 nm in size, is known as nanotechnology, and it is an area that is expanding at a rapid pace. The unique solutions provided by this field have revolutionized numerous industries, including energy, agriculture, biomedical, and industrial processes1,2,3. The distinctive characteristics of nanoparticles (NPs) have led to their widespread application in various fields3. The NPs are crucial to nanotechnology because of their distinctive qualities, which encompass a high surface-to-volume ratio, increased reactivity, and size-dependent characteristics4,5,6.
Green synthesis methods utilize natural resources such as plant extracts have emerged as a promising alternative for the production of NPs7,8,9,10. These methods minimize the environmental impact typically associated with conventional synthesis techniques by eliminating toxic chemicals and reducing waste11,12. Plant extracts not only provide a safe and sustainable source of reducing agent but also act as stabilizers during the synthesis process and helping to control particle size and increase surface area11,12. Importantly, the use of plant extracts contributes significantly to the biocompatibility of the resulting NPs, making them particularly suitable for applications in medicine and healthcare12,13,14. This biocompatibility ensures minimal toxicity and enhances the potential for clinical applications such as drug delivery, tissue engineering, and wound healing12,13,14. The resulting green synthesised NPs are also likely to have enhanced photocatalytic and photovoltaic properties15,16,17. These environmentally friendly techniques make NPs attractive for use in medicine, pharmaceutical and agriculture18,19.
The Copper doped Zinc Oxide (Cu: ZnO) NPs have attracted considerable interest due to their notable antibacterial and anticancer capabilities20,21. The integration of Cu into the Zinc Oxide (ZnO) crystal lattice modifies the energy levels and electronic characteristics of ZnO, hence improving its capacity for light absorption and charge separation. These modifications boost photocatalytic activity, which is crucial for processes such as chemical breakdown under light exposure20,22. The doping of Cu into ZnO NPs increases the production of Reactive Oxygen Species (ROS) which are toxic to microbial cells and contribute their enhanced antibacterial activity. Cu: ZnO NPs have demonstrated strong antibacterial effects against a wide range of pathogens, making them highly suitable for biomedical applications such as wound healing, infection control and medical device coatings23.
The microwave-assisted synthesis of NPs has emerged as a viable technique for improving both the efficiency and quality of NPs manufacturing24,25. This method dramatically affects the structural and optical characteristics of the NPs, offering benefits compared to traditional techniques24,26. Conventional synthesis processes sometimes necessitate hazardous chemicals, elevated temperatures, and prolonged reaction durations, posing risks to both human health and the environment12,27. Microwave-assisted synthesis offers an alternative by using less energy, shorter processing time and fewer toxic reagents24,28. The combination of Cu doping and microwave-assisted synthesis enhances the material properties providing better control over particle size, morphology and uniformity which are crucial for optimizing performance in various applications29,30,31.
Recent studies further reinforce the significance of doped metal oxide NPs synthesized via green and hybrid approaches. For example, Co/Ni-doped hematite NPs synthesized using Azadirachta indica exhibited superior photocatalytic and antioxidant activities compared to their chemically synthesized counterparts32. Similarly, green-synthesized Ni-ZnO and Nd-ZnO NPs using Vitex negundo extract showed notable antibacterial and photocatalytic efficiencies7. Cu/Zn-doped hematite NPs also demonstrated magnetic recoverability and enhanced biological and photocatalytic activities33. Moreover, Fe and Al co-doped ZnO NPs synthesized via a green microwave-assisted route displayed exceptional antibacterial and dye degradation properties8. The biological capabilities of Zn and Gd-doped MnO NPs, synthesized using Pinus roxburghii, underscore their relevance for antifungal and antioxidant applications9. Khan et al.20 synthesized Cu-doped ZnO NPs using aqueous extracts of Abutilon indicum, Clerodendrum infortunatum, and Clerodendrum inerme, which exhibited significant antimicrobial, antioxidant, anticancer, and photocatalytic properties. The Cu-doped ZnO NPs, particularly those derived from C. inerme, demonstrated superior biological activity against various bacterial and fungal strains, and effectively degraded organic dyes like Acid Black 234 under sunlight, owing to their high crystallinity, small particle size, and enhanced surface area. Karthik et al.21 developed Cu-doped ZnO NPs using Synadium grantii leaf extract and tested their photocatalytic ability against a range of hazardous organic dyes including Methylene Blue (MB), Indigo Carmine (IC), and Rhodamine B (RhB). Their results highlighted that Cu doping (particularly at 3–5%) significantly enhanced degradation efficiency, attributed to improved charge separation and defect-induced optical properties. Further evidence of multifunctionality in doped metal oxide NPs is provided by Zn-Co doped TiO2 NPs encapsulated chemically and greenly using Tinospora cordifolia, which achieved up to 99% dye degradation and exhibited remarkable antioxidant performance, highlighting their dual-functionality in environmental and biomedical domains34. Co/Zn-doped α-Fe2O3 NPs synthesized with polyvinylpyrrolidone (PVP) and Azadirachta indica leaf extract achieved dye degradation rates exceeding 98% and strong antioxidant capabilities, emphasizing their potential in wastewater treatment and biological applications35. Cu-Ce dual-doped ZnO NPs synthesized via microwave-assisted green methods using Colocasia esculenta extract displayed excellent antioxidant and antibacterial efficacy, reinforcing the synergy of green synthesis and co-doping for biomedical use36. Similarly, Cu-doped ZnO and ZnO–CuO composite NPs exhibited tunable optical properties and efficient visible-light absorption, making them suitable for photodegradation applications37. Cu doping was also found to significantly improve the photocatalytic efficiency and specific capacitance of ZnO NPs, showcasing their dual utility in environmental remediation and energy storage38. Investigations into optoelectronic behaviour revealed that increased Cu content in ZnO led to enhanced electric field intensities and dye degradation efficiency, with 10% Cu-doped ZnO achieving the highest surface area and photocatalytic activity39. Cu-doped ZnO NPs synthesized via a gliding arc discharge plasma technique demonstrated superior antioxidant activity and promising photocatalytic and antibacterial performance, although efficacy varied with Cu concentration40. These collective findings underscore the transformative potential of doped metal oxide NPs synthesized through green and hybrid approaches for multifunctional environmental and biomedical applications.
Pistia stratiotes (PS), commonly known as water lettuce, was selected as the green reducing and stabilizing agent due to its rich phytochemical composition, which includes flavonoids, phenolics, and other antioxidants known to facilitate NP synthesis41,42,43. Its abundant availability and fast growth, often leading it to be classified as an invasive species, make it a sustainable and eco-friendly choice for NP synthesis43,44. Previous studies have highlighted the antioxidant and antimicrobial potential of PS extracts, supporting its suitability for biosynthesis of metal oxide NPs41,43.
Despite the growing interest in Cu: ZnO NPs and green synthesis techniques, there remains a lack of comprehensive studies combining microwave-assisted synthesis with plant-based green methods, particularly using PS as a biogenic precursor. While several plants have been investigated for their ability to mediate NP synthesis, the unique phytochemical composition of PS, known for its antioxidant properties, has not been fully utilized in this context. Moreover, few studies integrate experimental characterization with DFT based theoretical analysis to understand the influence of Cu doping on ZnO at the electronic level. This study aims to bridge this gap by synthesizing Cu: ZnO NPs using PS extract under microwave irradiation and investigating their structural, optical, and biomedical properties through both experimental and computational approaches.
This research presents a novel and sustainable approach for synthesizing Cu: ZnO NPs by combining green and microwave-assisted methods using PS extract, an area that remains underexplored. The approach integrates experimental characterization with DFT simulations to investigate the impact of Cu doping on ZnO’s structural and electronic properties. The synthesis method leverages microwave irradiation to reduce reaction time and energy consumption, while plant extracts eliminate the need for toxic chemicals, enhancing environmental sustainability. The synthesized NPs will be characterized using UV-Visible spectroscopy (UV-Vis), photoluminescence (PL), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS), transmission electron microscopy (TEM), dynamic light scattering (DLS), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS)., with crystalline size and strain evaluated via Williamson–Hall (W-H) plot and the Size-Strain Plot (SSP) method. Additionally, their biological activities such as antibacterial, antifungal, and anticancer effects are also evaluated, given their relevance in modern biomedical science. The focus on these specific biological applications stems from the increasing demand for novel, efficient antimicrobial agents and alternative anticancer strategies that offer fewer side effects than traditional chemotherapeutics. The selection of antibacterial and antifungal testing addresses the urgent global challenge of antimicrobial resistance, while the anticancer evaluation explores the growing interest in metal oxide NPs for targeted cancer therapy.
Materials and methods
Materials and equipment
The precursors used in the synthesis of Cu: ZnO NPs were of analytical grade and sourced from reputed suppliers to ensure high purity. The detailed information regarding the chemicals, including grade, purity, supplier, and brand, is summarized in Table 1.
The bio-reducing agent Pistia Stratiotes (PS) leaves were harvested from the AVM Canal area in Colachel, Tamil Nadu, India (Latitude: 8.1753°N, Longitude: 77.2476°E) in July 2024. Only healthy and undamaged leaves were selected to ensure optimal phytochemical content. All glassware and instruments, including magnetic stirrers, digital thermometers, and pipettes, were sterilized prior to use. The synthesis was performed using a domestic microwave oven (IBF 20 L-20PM-MEC2) operating at 2450 MHz with adjustable power settings to facilitate uniform heating.
Methods
Extraction of bioactive compounds from leaves
The harvested PS leaves were thoroughly cleansed to eliminate any surface imperfections, including dust, dirt, or other pollutants. The cleaning process included an initial washing with water, repeated thrice, succeeded by an extensive rinsing with double-distilled water to guarantee additional purity. This step was essential to remove any remaining particles or possible interfering chemicals. Post-washing, the leaves were dehydrated in shaded sunlight inorder to prevent the breakdown of bioactive components from direct Ultra Violet (UV) radiation exposure. During the initial seven days, the leaves displayed a colour transition from dark green to light green, signifying moisture depletion and chlorophyll degradation. The drying process was prolonged by three weeks to guarantee the thorough elimination of remaining moisture. Upon the complete dehydration, the leaves were precisely pulverized into a homogeneous powder utilizing a mortar and pestle, guaranteeing uniformity in particle size. The powdered substance was subsequently preserved in a sterile, airtight glass vessel to ensure its stability and shield it from external influences such as moisture and light.
To create the plant extract, 5 g of the finely ground PS leaf powder was precisely measured and combined with 100 ml of double distilled water in a clean glass beaker. The mixture was subjected to heating on a hot plate while being continually agitated at a regulated temperature of around 80 °C for one hour. This approach enabled the extraction of bioactive chemicals that function as reducing and stabilizing agents in NP synthesis. Following heating, the solution was allowed to cool naturally to ambient temperature to avoid thermal damage of the extract. The mixture was subsequently filtered through Whatman No.1 filter paper to eliminate solid residues, resulting in a clear, pale-coloured extract. The filtrate, abundant in phytochemicals, was gathered in a sterile glass vessel and preserved under refrigeration (about 4 °C) for the application in the green synthesis of NPs.
Microwave assisted NP synthesis
The synthesis protocol was designed for reproducibility and optimal yield. In a 250 mL glass beaker, 50 mL of 0.1 M Zn(NO3)2·6H2O solution was prepared using double-distilled water. To this, 5 mL of 0.1 M Cu(NO3)2·3H2O was added dropwise using a calibrated pipette to avoid rapid precipitation and ensure homogenous mixing. The solution was stirred for 5 min to achieve uniform ion dispersion. Then, 25 mL of freshly prepared PS leaf extract was introduced into the reaction mixture. The combined solution was stirred continuously for 30 min at room temperature to initiate the interaction of metal ions with the plant-derived phytochemicals. The mixture was then exposed to microwave irradiation operating at 2450 MHz for 10 min in intermittent cycles (2 min on, 1 min off) to avoid overheating and ensure uniform energy distribution. The microwave-assisted heating promoted rapid nucleation and crystalline growth of the NPs. After irradiation, the reaction mixture was allowed to cool to room temperature. The formed precipitate was separated by centrifugation at 1400 rpm for 10 min, and the pellet was washed multiple times with ethanol and double-distilled water to eliminate unbound phytochemicals and ionic residues. The washed pellet was then dried in a hot air oven at 80 °C for 12 h, followed by calcination at 450 °C in a muffle furnace for 2 h to improve crystallinity and remove organic matter. The resulting dark brownish powder of Cu: ZnO NPs was stored in an airtight container for further characterization and applications. To ensure the reproducibility of the synthesized NPs, the synthesis procedure was performed in triplicate under identical conditions. The characterized results were found to be consistent, confirming the reproducible nature of the synthesis protocol.
To evaluate the efficiency of the green synthesis process, the yield of the obtained Cu: ZnO NPs was calculated based on the total mass of metal precursors used. Specifically, 1.48749 g of Zn(NO3)2·6H2O and 0.1208 g of Cu(NO3)2·3H2O were employed, amounting to a total precursor mass of 1.60829 g. After completion of the synthesis, including the microwave-assisted reaction, purification, and calcination at 450 °C, the final yield of the synthesized Cu: ZnO NPs was found to be 1.04105 g. This corresponds to a synthesis efficiency of 64.73%. The undoped ZnO was also synthesized using the same method, except that Copper (II) nitrate trihydrate was not added. A schematic illustration of the synthesis procedure is provided in Fig. 1.
Characterization
The techniques employed for characterization are essential for understanding the physical and chemical properties of the synthesized NPs, as these characteristics intensely affect their functionality in various applications45,46. For Cu: ZnO NPs, advanced methodologies were utilized to obtain comprehensive insights into their optical, structural, and morphological characteristics. The optical analysis was performed utilizing the JASCO V-730 UV-Visible spectrophotometer, covering a wavelength spectrum from 200 nm to 1000 nm. This analysis facilitated the identification of the absorption spectrum, which was then employed to compute the band gap energy of NPs. The energy associated with the band gap provides essential insights into the electronic structure and prospective uses in the fields of optoelectronics and photocatalysis. A Quanta 200 F-IITM SEM, integrated with an EDS system, was employed to investigate surface morphology and elemental composition. The microscope functioned at an acceleration voltage of 30 kV. The JEOL JEM 2100 h-TEM was utilized for high-resolution structural analysis. This instrument yielded intricate images at the nanoscale, facilitating a comprehensive analysis of the structure, particle size, and morphology. The diameter of NPs was determined using Image J software. The DLS measurement of particle size of synthesized Cu: ZnO NPs in distilled water were performed using Malvern DLS equipment. The surface elemental composition and binding energy were analysed using XPS. The PL measurement was carried out using Cary Eclipse Fluorescence Spectrometer. The investigation utilized a JASCO FT/IR-4600 spectrometer for FTIR spectroscopy, achieving a resolution of 4 cm− 1, which enabled the discernment of functional groups and chemical bonds within the Cu: ZnO NPs. In order to evaluate the structural integrity and crystallinity of the synthesized NPs, a powder XRD analysis was conducted. This method validated the sample’s purity and facilitated the estimation of crystallite dimensions. The XRD measurement was conducted utilizing an advanced XRD functioning at a voltage of 40 kV and a current of 30 mA. The apparatus employed Cu-Kα radiation, characterized by a wavelength of 1.5406Å. The scanning was conducted over a range of 5° to 80°, facilitating a thorough assessment of the crystal structure and phase composition of Cu: ZnO NPs. The size of crystal (D) as given in Eq. 147,48, lattice parameters (a, b,c) as given in Eq. 2, interplanar spacing (d) as given in Eq. 349, lattice strain (\(\:{\upepsilon\:}\)) as given in Eq. 448, dislocation density(\(\:{\updelta\:}\)) as given in Eq. 548, bond length (L) as given in Eq. 650 and volume (V) as given in Eq. 750 was calculated using the following formulas.
Where,
Computational method
The study further examined the structural and electrical characteristics of Cu: ZnO NPs using computational analysis utilizing DFT calculations. VESTA software was used to create a hexagonal wurtzite ZnO supercell (3 × 2 × 1) and copper atoms were added to the lattice to examine their effects on the behaviour of the material. In order to facilitate setup and visualisation, the Quantum Expresso software with the BURAI interface was used to perform the calculations. The PBE exchange-correlation functional and ultrasoft pseudopotentials were used to optimize the structure. The energy convergence criteria of 10− 5 eV was used for SCF iterations. The primary computational parameters such as wavefunction cutoff of 45 Ry, charge density cutoff of 300 Ry and 4 × 8 × 8 Monkhorst-pack K-points grid was used to ensure precise Brillouin zone sampling. Fermi-Dirac smearing was applied to manage partial electronic occupations during SCF calculations.
Antimicrobial efficacy
The antibacterial efficacy of Cu: ZnO NPs has attracted considerable interest owing to their capacity to address antibiotic resistant against infections and various other microbial challenges. This study employed the disc diffusion method to assess the antifungal and antibacterial properties of synthesized Cu: ZnO NPs against both gram-negative and gram-positive bacterial strains, in addition to the fungal pathogen Candida albicans. The antibacterial assays were conducted utilizing nutrient agar medium, whereas the antifungal experiments were carried out using SDA medium. The bacterial and fungal strains were meticulously distributed across the surface of the agar plates utilizing sterile cotton swabs. Discs with a diameter of 2 mm, devoid of bacteria or other contaminants, were infused with both a control substance and solutions of Cu: ZnO NPs, which were prepared in solvents including ethanol, methanol, and chloroform. The meticulously prepared discs were systematically labelled and positioned on the sterilized agar plates. The plates were maintained at a temperature of 37 °C for a duration of 18 h to facilitate microbial interaction. Upon completion of the incubation period, the plates were scrutinized for the presence of zones of inhibition, which signify the suppression of microbial proliferation. This procedure yielded compelling evidence regarding the antimicrobial capabilities of Cu: ZnO NPs, illuminating their potential applications.
Cytotoxic effect of Cu: ZnO NPs
Cell culture methodology
The cell line employed in this investigation was obtained from NCCS Pune, India. A systematic methodology was employed to guarantee the most favourable conditions for cellular proliferation and experimental procedures. The cells were cultivated in DMEM-Himedia, a widely acknowledged medium esteemed for its effectiveness in promoting the proliferation of diverse cell types. To promote cellular growth and viability, the medium was enriched with 10% heat-inactivated FBS, supplying crucial nutrients and growth factors. Moreover, a 1% antibiotic solution was integrated to prevent contamination. The process of cell cultivation was conducted utilizing TC flasks with a surface area of 25 cm2, selected for their capacity to facilitate optimal cell adhesion and offer ample growth space. The conditions for incubation were meticulously regulated to sustain an environment conducive to cellular proliferation. The flasks were positioned within a 37 °C cell culture incubator, thereby facilitating optimal metabolic activity. The incubator upheld a steady 5% CO2 concentration, essential for the stabilization of the medium’s pH levels. Moreover, humidity levels were meticulously controlled to avoid dehydration and maintain the viability of the cultured cells during the entirety of the experimental period. This thorough protocol guaranteed a stable and contamination-free environment for the cell line throughout the study.
Cell viability analysis
The experimental procedure entailed the careful seeding of 2500 cells per well into 96 well plates, followed by incubation under precisely controlled conditions of 37 °C and 5% CO2 for a duration of 24 h. Test samples were meticulously prepared in DMEM at a concentration of 10 mg/mL and subjected to sterilization via a 0.2 μm Millipore syringe filter. The samples were subsequently subjected to serial dilution in DMEM, resulting in final concentrations of 6.25, 12.5, 25, 50, and 100 µg/ml. Untreated wells were preserved as controls to guarantee precision in comparison. All experiments were conducted in triplicate, and the results were averaged to reduce experimental variability. Following the introduction of the test samples into the wells, the plates underwent an incubation period of an additional 24 h. Upon conclusion of the incubation period, the media within the well were aspirated and subsequently discarded. In order to evaluate cell viability, 100 µL of a 0.5 mg/ml MTT solution, meticulously prepared in PBS, was introduced to each well. The plates underwent an additional incubation period of 2 h to facilitate the development of formazan crystals. Upon completion of the incubation period, the supernatant was meticulously extracted, followed by the addition of 100 µl of 100% DMSO to each well to facilitate the dissolution of the crystals. The measurement of absorbance was conducted at a wavelength of 570 nm utilizing a microplate reader. Cell viability was calculated using the Eq. 8.
The IC50 value, indicative of the concentration at which 50% inhibition of cell viability is achieved, was ascertained through a slope equation “y = mx + c”. This was accomplished by plotting the average absorbance values of the test concentrations ranging from 6.25 to 100 µg/ml in Microsoft Excel. Photomicrographs representing cells from each experimental group were obtained using an inverted phase-contrast microscope (LABOMED, TCM 400, USA) to visually substantiate the findings.
Results and discussion
Optical properties and band gap analysis
UV-Vis analysis
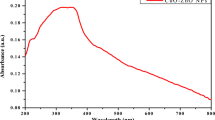
The UV-Vis spectrum of Cu: ZnO NPs was conducted to elucidate their optical properties and band gap energy (E). To achieve this, a suitable amount (0.01 g) of NPs was precisely dispersed in 10 ml of distilled water. The spectrum revealed a significant absorption peak at 398 nm, thereby confirming the existence of Cu: ZnO NPs as illustrated in Fig. 2. The energy band gap of the synthesized NPs was determined utilizing Eq. (9)
The value of ‘h’ is 6.626 × 10− 34Js, while ‘c’ is 2.99 × 108m/s, and ‘λ’ is 398 nm. The energy of the band gap for Cu: ZnO NPs was determined to be approximately 3.11 eV.
The optical band gap of Cu: ZnO NPs is determined using the classical Tauc relation, given by the Eq. (10)51.
In Eq. (10), ‘D’ is a constant, α is the absorption coefficient, Eg is the optical band gap, ‘hν’ signifies the energy associated with a photon and n signifies the electronic transition type with values of 1/2, 3/2, 2, or 3 depending on whether the transition is allowed or forbidden, direct or indirect. For Cu: ZnO NPs, a graph of (αhν)2 versus photon energy (hν) is constructed, assuming an allowed direct transition depicted in Fig. 3. The band gap was determined to be 3.12 eV through the extrapolation of the linear segment of the plot to the hν axis. This value aligns closely with the band gap energy of 3.11 eV calculated directly, thereby affirming the reliability and accuracy of the measurements.
The alignment of values derived from both direct calculation and the Tauc plot method underscores the accurate optical properties of the Cu: ZnO NPs. This discovery highlights the material’s significant potential for utilization in optoelectronics and various other technological domains.
Photoluminescence analysis
Photoluminescence (PL) spectroscopy is a vital tool for probing the electronic and defect states in semiconductor materials51,52. The PL spectrum of Cu: ZnO NPs, recorded under an excitation wavelength of 320 nm which is shown in Fig. 4 that reveals both near-band-edge (NBE) and deep-level emissions (DLE), which are characteristic of ZnO-based systems.
The spectrum exhibits a dominant emission peak in the UV region around 378 nm, which corresponds to the NBE emission arising from the radiative recombination of free excitons near the conduction and valence band edges. This emission is indicative of the high crystallinity and good optical quality of the Cu: ZnO nanostructures. In addition to the UV emission, a prominent broad band appears in the blue-green visible region, centered around 490 nm, which is attributed to deep-level emissions. These emissions are commonly associated with various intrinsic defects, such as oxygen vacancies and zinc interstitials52. Such defects introduce localized mid-gap states that facilitate radiative transitions, especially the recombination of a photogenerated hole with a singly ionized oxygen vacancy. The intensity of this emission band suggests a significant presence of such structural defects, which may be influenced or enhanced by Cu doping. The presence of a minor peak near 430–440 nm may correspond to transitions involving shallow donor levels, often attributed to zinc interstitials.
The observed PL profile indicates that Cu doping modifies the native defect landscape of ZnO, potentially altering the charge carrier dynamics and enhancing defect-related recombination pathways. Such defect states not only influence the optical properties but may also play a role in the observed biological activities of the NPs. The PL data supports the successful synthesis of Cu: ZnO NPs with optical properties influenced by both crystallinity and defect chemistry, reinforcing their potential for optoelectronic and biomedical applications.
SEM with EDS analysis
An examination of the morphology and composition of Cu: ZnO NPs was performed utilizing SEM and EDS. The characteristics of these features are fundamental in determining the properties of material surfaces and their possible applications. The SEM images illustrated in Fig. 5 (a, b) demonstrated that the synthesized NPs displayed densely packed clusters characterized by irregular, rounded forms. The particles exhibited a notable level of agglomeration, a phenomenon commonly observed in NP systems as a result of their elevated surface energy. Moreover, certain particles displayed a morphology similar to nanorods. The observed aggregation and distinctive morphology further suggested the effective integration of Cu into the ZnO matrix. This structural characteristic can be attributed to the doping process, which apparently affected the growth patterns and interactions among particles. EDS analysis was employed to determine the elemental composition of the synthesized NPs and to verify the successful incorporation of Cu as a dopant53, as illustrated in Figs. 5(c). The EDS spectra revealed distinct peaks corresponding to Zn, Cu and O in the Cu doped ZnO NPs confirming the effective doping of Cu into the ZnO lattice. Quantitative analysis showed that the doped samples contained 76.28 wt % Zn, 14.93 wt % O, and 8.79 wt % Cu. In terms of atomic percentages, the composition was approximately 51.81 at% Zn, 6.17 at% Cu, and the remainder Oxygen, corresponding to an estimated atomic ratio of Cu: Zn ≈ 1:8. This supports the successful incorporation of copper as a dopant and aligns with the intended composition in the synthesis strategy.
The findings derived from SEM and EDS analyses indicate that the Cu: ZnO NPs exhibit the requisite structural and compositional characteristics, rendering them appropriate for sophisticated applications in fields such as optoelectronics, photocatalysis, and environmental remediation.
X-Ray photoelectron spectroscopy (XPS) analysis
The XPS was employed to analyze the surface elemental composition and chemical states of the Cu: ZnO NPs54. The XPS survey spectra and high-resolution scans of individual elements, as shown in Fig. 6, confirm the presence of Zn, Cu, O, and C in the synthesized NPs, validating the successful incorporation of copper into the ZnO matrix.
The high-resolution spectrum of Zn (2p), depicted in Fig. 6(a), shows two distinct peaks located at binding energies of 1022 eV and 1045 eV, which are attributed to Zn 2p3/2 and Zn 2p1/2, respectively21. These values are consistent with the characteristic binding energies of Zn2+ in the ZnO lattice, confirming the oxidation state of Zn and its integration in the wurtzite structure of ZnO without significant alteration due to copper doping.
Figure 6(b) presents the Cu 2p spectrum, which shows multiple peaks, indicating the presence of copper in the doped ZnO structure. The primary peaks observed at 933.5 eV and 953.98 eV correspond to Cu 2p3/2 and Cu 2p1/2, respectively, suggesting the presence of Cu2+ ions. Additionally, satellite peaks at 942.2 eV and 963.8 eV further confirm the existence of Cu in the + 2 oxidation state. These satellite features are characteristic of Cu2+ and serve as a fingerprint for distinguishing it from Cu0 or Cu+, thereby affirming the successful doping of divalent copper ions into the ZnO framework.
The O (1s) spectrum, shown in Fig. 6(c), reveals a prominent peak centered at 531.88 eV. This binding energy is attributed to oxygen species in the Zn-O lattice as well as surface-adsorbed oxygen or hydroxyl groups. The slight asymmetry of the peak may also suggest the coexistence of lattice oxygen and chemisorbed oxygen species, which play crucial roles in determining the photocatalytic and antimicrobial properties of the NPs. A distinct C (1s) peak appears at 284.78 eV, as shown in Fig. 6(d). This is typically associated with adventitious carbon contamination from the environment or carbon-containing residues from the plant extract used during the green synthesis process. While not directly involved in the crystal lattice, the presence of carbon indicates organic moieties adhering to the NP surface, likely serving as capping agents and providing colloidal stability. The XPS analysis confirms the successful synthesis of Cu: ZnO NPs through green methods using PS extract. The detected elements and their chemical states provide evidence for the substitutional incorporation of Cu2+ into the ZnO structure and the presence of surface-bound oxygen and organic components, which may influence the physicochemical and functional behavior of the NPs in prospective applications.
HR-TEM analysis
The HR-TEM analysis offers essential insights into the dimensions, morphology, and structural consistency of Cu: ZnO NPs. This method facilitates complex visualization of NPs at atomic and nanometer resolutions, providing profound insights into their physical characteristics and synthesis results. The HR-TEM at elevated magnification disclosed that the particle displayed a distinctive dual shadow characteristic. The observed dark spots within the transparent regions correspond to areas of elevated electron density, likely indicative of the presence of metallic nanostructures and the incorporation of the Cu dopant into the ZnO lattice. This observation provides compelling evidence for the efficacy of the doping process and the structural robustness of the synthesized NPs. The HR-TEM images elucidated variations in particle size, suggesting a certain level of heterogeneity in the synthesized Cu: ZnO NPs. The analysis revealed that the size of the particles ranging from 15 nm to 65 nm. Moreover, certain particles displayed a rod-like and spherical morphology, indicating that the doping process may affect not only the particle size but also their growth patterns and resultant shapes. The HR-TEM images illustrating Cu: ZnO NPs are depicted in Fig. 7, accompanied by a histogram of the particles in Fig. 8. This data highlights the existence of a notable proportion of NPs with dimensions in the lower range, which are expected to demonstrate increased surface area and distinctive nanoscale characteristics.
Dynamic light scattering (DLS)
The DLS analysis of Cu: ZnO NPs, as illustrated in Fig. 9, reveals a polydisperse size distribution ranging from approximately 15 nm to 65 nm, with dominant peaks centered around 20–25 nm. These peaks correspond to the highest intensity of scattered light, indicating that the majority of the NP population falls within this narrow size range. The distribution tailing toward larger sizes suggests the presence of a smaller fraction of agglomerated or larger particles, which is typical in green-synthesized nanoparticles due to bio-organic capping agents from plant extracts. The relatively low polydispersity in the dominant range signifies a moderately uniform synthesis, which is essential for consistent biological and physicochemical behaviour. The DLS results are in good agreement with the nanometric scale observed in TEM analysis and support the potential of the synthesized Cu: ZnO NPs for biomedical applications requiring controlled NPs dimensions.
FTIR analysis
The FTIR spectroscopy was employed to investigate the functional groups present in both the PS plant extract and the synthesized Cu: ZnO NPs. The spectra, shown in Fig. 10, offer critical insights into the organic constituents of the plant extract and the chemical interactions occurring during the nanoparticle formation process.
The FTIR spectrum of the PS extract exhibits characteristic absorption bands at 3454 cm− 1, 2079 cm− 1, 1639 cm− 1, and 673 cm− 1. The broad absorption band observed at 3454 cm− 1 corresponds to O–H stretching vibrations, which are typically associated with hydroxyl groups present in water, alcohols, and phenolic compounds. This confirms the presence of hydrophilic groups in the extract, which are essential for reducing and stabilizing metal ions during nanoparticle synthesis. The absorption peak at 2079 cm− 1 may be attributed to stretching vibrations of alkynes (C = C) or nitrile groups (C = N), which are often present in various phytochemicals. A significant peak at 1639 cm− 1 is indicative of C = O stretching of carbonyl compounds, possibly amides or ketones, or C = C stretching vibrations from aromatic rings. These functional groups suggest the presence of polyphenolic or flavonoid compounds within the extract. The band at 673 cm− 1 is assigned to out-of-plane bending vibrations of aromatic C–H, further supporting the presence of aromatic structures in the plant matrix.
The FTIR spectrum of the ZnO and Cu: ZnO NPs reveals several notable features. A broad band centered around 3430 cm⁻¹ for ZnO and 3435 cm⁻¹ for Cu: ZnO corresponds to O–H stretching vibrations, signifying the presence of surface-adsorbed hydroxyl groups or moisture55. A medium intensity peak observed at 2917 cm− 1 for ZnO and 2925 cm− 1 for Cu: ZnO can be attributed to the C–H stretching vibrations of aliphatic groups, likely originating from residual organic compounds derived from the plant extract53. The absorption band at 1649 cm− 1 for ZnO and 1627 cm− 1 for Cu: ZnO is related to C = O or aromatic C = C stretching vibrations, indicating the adsorption of bio-organic molecules on the NP surface. These organic functionalities suggest that the plant extract acts not only as a reducing agent but also as a capping and stabilizing agent for the synthesized NPs. The absorption band observed at 1389 cm− 1 for ZnO and 1379 cm− 1 for Cu: ZnO corresponds to the C-H bending vibrations, thereby affirming the existence of organic functionalities within the NPs. Crucially, the low-frequency region of the spectrum shows bands at 596 and 434 cm− 1, which are characteristic of metal-oxygen stretching vibrations. The peak at 608 cm− 1 and 596 cm− 1 corresponds to Zn–O stretching, while the 434 cm− 1 band is indicative of Cu–O bond vibrations56. These findings confirm the successful doping of Cu into the ZnO lattice, thereby altering the structural framework of the host material. Such modifications are significant as they can enhance the optical, catalytic, and electronic properties of the NPs.
The FTIR analysis reveals the co-existence of organic and inorganic functional groups in the Cu: ZnO NPs, evidencing the successful incorporation of plant-derived phytochemicals onto the NP surface and the formation of metal-oxygen bonds. These interactions underscore the role of PS extract in facilitating a green and efficient synthesis route and suggest the potential of the synthesized NPs for applications in photocatalysis, antimicrobial formulations, and environmental remediation.
Structural analysis of Cu: ZnO NPs
Crystalline size and strain Estimation
The crystalline size and strain are the factors that affect the chemical, physical, and mechanical properties of NPs. The accurate assessment of these parameters yields significant understanding of the structural attributes of the synthesized NPs, facilitating the enhancement of their functional properties for a range of applications. The analysis of X-ray peak broadening has become a prevalent and dependable method for assessing crystalline size and strain57.
XRD for crystallite analysis
The FWHM of the diffraction peaks derived from X-ray diffraction patterns is examined. The FWHM indicates the extent of peak broadening, a phenomenon that can be ascribed to variations in crystalline size as well as the influence of strain effects. Figure 11 presents the XRD patterns of Cu: ZnO NPs, showcasing sharp and distinctly defined peaks that affirm the crystalline characteristics of the material. The diffraction peaks exhibit a close alignment with the wurtzite hexagonal phase of ZnO, reflecting the crystal structure of ZnO54. The introduction of Cu ions into the ZnO lattice maintains the integrity of the primary wurtzite structure, as demonstrated by the stable peak positions observed. The XRD patterns reveal distinct diffraction peaks at precise 2θ angles of 31.72, 34.36, 36.17, 47.49, 56.55, 62.78, 66.28, 67.88, and 68.96. The specified angles are associated with the crystallographic planes designated as (100), (002), (101), (102), (110), (103), (200), (112), and (201) in a systematic manner54.
The XRD analysis of Cu: ZnO NPs provided profound insights into the structural transformations prompted by the incorporation of copper doping. The introduction of Cu modifies the crystal structure, thereby affecting essential parameters including lattice constants, interplanar spacing, lattice strain, and dislocation density. The XRD analysis substantiated that the incorporation of Cu influences the lattice constant, a critical parameter delineating the intercellular distances within the crystal structure. The alterations in the lattice constant result from the integration of Cu ions into the ZnO lattice, which induces a slight distortion due to the disparity in ionic radii between Cu2+ and Zn2+. The lattice parameters of Cu: ZnO NPs were determined through the application of Eq. (2), yielding a = 3.2636 Å and c = 5.2226 Å58. The values underscore the subtle yet impactful alterations induced by doping, which can influence the characteristics of the NPs, including band gap energy and electronic behaviour.
Interplanar spacing, distance between adjacent crystallographic planes, is another parameter obtained from XRD analysis. This spacing influence the morphology, size and overall crystalline characteristics of the NPs. Equation (3) was employed to compute the interplanar spacing of Cu: ZnO NPs which reflects the material’s characteristics and confirmed its crystalline integrity. Lattice strain is a significant factor in nanomaterials which is a measure of distortion in the crystal lattice. In Cu: ZnO NPs, lattice strain arises due to factors like reduced particle size, increased surface energy and introduction of defects during synthesis. The strain was determined to be 1.467 × 10− 3 lines2/m4 using Eq. (4). The presence of lattice strain indicates the defects contribute to structural modifications impacting properties such as thermal stability and electronic behaviour.
Dislocation density is the indicative of imperfections within the crystal lattice significantly influences material properties like conductivity, mechanical strength and thermal resistance. The average dislocation density of Cu: ZnO NPs was calculated using Eq. (5), gives a value of 1.7977 × 1015 lines/m2. It reflects the synthesis-induced disruptions in the orderly atomic arrangement which can also contribute to unique material properties such as enhanced catalytic or optical activity. Table 2 summarizes the structural parameters of synthesized Cu: ZnO NPs including crystallite size, interplanar spacing, lattice strain and dislocation density.
Scherrer method
The Scherrer method serves as a fundamental approach for estimating crystallite size. The phenomenon is contingent upon the widening of XRD peaks, which exhibits an inverse relationship with the size of the crystallites. The FWHM of diffraction peaks serves as a crucial parameter for the Debye-Scherrer Eq. (1)59. The average crystallite size of the synthesized Cu: ZnO NPs was determined to be roughly 23.6 nm. This outcome reflects the nanoscale characteristics that are crucial for applications necessitating a high surface area.
Williamson–Hall (W–H) method
The W-H method offers a thorough framework for assessing crystallite size alongside lattice strain. The W-H plot is generated by graphing β cos θ on the vertical axis and 4 sin θ on the horizontal axis, utilizing the specified Eq. (11)60. The W-H plot of Cu: ZnO NPs shown in Fig. 12.
From the plot the intercept proves the crystalline size and slope gives the micro strain. The result shows that the crystalline size and strain values are consistent with nanoscale characteristics, reflecting high surface energy.
The Size-Strain plot (SSP) method
The SSP method is an alternate strategy to analyse the XRD data in order to distinguish size and strain effects61. The SSP constructed by plotting (d β cos θ)2 against (d2 β cos θ) as shown in Fig. 13 using the Eq. (12).
The intercept is indicative of crystallite size, while the slope serves to represent strain. Table 3 delineates the crystalline dimensions and strain characteristics of the synthesized Cu: ZnO NPs.
Analysis of bond length, volume and lattice parameters
An evaluation of the structural features of Cu: ZnO NPs was conducted to gain insights into the bond length, volume, and lattice parameters. The characteristics of these properties are essential in elucidating the physical, chemical, and functional behaviour of the material for applications within the realm of nanotechnology. The bond length serves as a defining characteristic, representing the average distance that separates the nuclei of two atoms that are chemically bonded within a molecule. This parameter has a direct impact on the molecular arrangement, morphology, and the overall behaviour of the material. The length of the bond may fluctuate as a result of various influences such as temperature, pressure, and the existence of supplementary molecules. The bond length of Cu: ZnO NPs was determined through Eq. (6) that yields an average value of 1.98547 Å. This measurement substantiates the structural integrity of the Cu: ZnO NPs lattice and lays the groundwork for understanding its electronic and optical characteristics. The NPs display distinctive characteristics as a result of their elevated surface-to-volume ratio, which is inherently affected by their volume. The calculated volume of synthesized Cu: ZnO NPs, as derived from Eq. 8, is determined to be 48.17475 Å3. The reduced volume and heightened surface energy significantly affect the interaction of NPs with biological systems, thereby impacting their efficacy in drug delivery and biosensing applications.
The lattice parameters and c/a ratio are essential for comprehending the crystal geometry and anisotropic behaviour of the synthesized material. The c/a ratio, calculated as 1.600252, serves as a defining characteristic of geometry, indicating minimal anisotropic distortion within the crystal lattice as a result of Cu incorporation. Table 4 presents a detailed summary of structural parameters, encompassing the lattice parameter, c/a ratio, volume, and bond length.
These findings emphasize the material’s nanoscale dimensions and high surface energy which are vital for catalytic, optical and biological applications.
Computational studies
Electronic structure of Cu: ZnO NPs
The optimized structure of Cu: ZnO denotes the most stable arrangement of Cu: ZnO super cell, as calculated using computational techniques like DFT. This structure is important for understanding the characteristics and behaviour of Cu doped ZnO at the nanoscale, which can differ from the bulk form. These structure act as benchmarks for theoretical models and computational methods, allowing researchers to test and evaluate DFT methodologies using experimental or higher-level theoretical data. The optimized structure of Cu: ZnO super cell given in the Fig. 14.
Electronic band structure and density of state
The electronic characteristics of Cu: ZnO NPs were evaluated using band structure and DOS calculations. The introduction of new energy level proximate to the fermi level in the band structure attributed to copper doping significantly amplifies the material’s optical absorption within the visible spectrum. The band gap was determined to be 3 eV, showing a potential reduction from intrinsic band gap of pure ZnO. The HOMO and LUMO energies correspond to the valance band and conduction band respectively and the energy gap aligned with experimental results. This shows that copper doping narrows the band gap which could improve the optical and electrical properties of the nanoparticles, making it suitable for photovoltaics and optoelectronics applications. The band structure and density of state calculations for Cu: ZnO NPs depicted in Fig. 15 and 16.
The Ionization Energy (IE) and Electron Energy (EA) are directly correlated with energy values. The chemical reactivity of substances is significantly influenced by energy gap. The global softness, absolute softness and chemical hardness values are significant indicators of chemical stability. A more stable system is indicated by higher hardness and lower softness level. The quantum chemical parameters are calculated using Eq. (13) to Eq. (22) and tabulated in Table 5.
Antibacterial activity
The antibacterial activity of Cu: ZnO NPs was meticulously examined, uncovering their potential efficacy against bacterial strains including Staphylococcus aureus and Escherichia coli. This examination highlights the significance of ROS, structural morphology, and surface area in augmenting antibacterial efficacy. Cu: ZnO NPs have demonstrated a marked ability to augment the generation of ROS, including hydroxyl radicals (.OH), superoxide anions (O2.−), and hydrogen peroxide (H2O2). The ROS engage with bacterial cellular constituents such as proteins, lipids, and DNA, resulting in oxidative stress and subsequent cellular dysfunction. It undermines the structural integrity of the bacterial cell membrane, leading to the leakage of intracellular contents and ultimately resulting in cell death. This oxidative mechanism underscores the essential function of Cu: ZnO NPs in suppressing bacterial proliferation and significant cell mortality. The assessment of antibacterial activity involved the preparation of suspensions of Cu: ZnO NPs in a range of solvents. This investigation assessed the inhibition zone to quantify the antibacterial efficacy. The results depicted in Fig. 17, demonstrate significant antibacterial activity with clear zone of inhibition around Cu-doped ZnO NP suspensions. Table 6 provides the measurements of inhibition zones for both bacterial strains reflecting superior antibacterial performance of the synthesized NPs.
The maximum inhibition zones recorded for Cu: ZnO NPs dispersed in ethanol, methanol and chloroform were 18.4 mm, 21 mm and 21.5 mm respectively, while the control maintained a consistent zone of 16 mm against S.aureus. Similarly, the inhibition zones measured 21.6 mm, 19 mm and 20.5 mm for the same solvent against E.coli. These results highlight the significant role of solvent in enhancing the dispersion and activity of NPs. The studies on Microwave assisted green synthesis of ZnO NPs using PS reported maximum inhibition zone of 20.4 mm for S. aureus and 21.3 mm for E. coli showing that the current Cu: ZnO NPs demonstrate superior antibacterial activity43. Additional comparison with Cu-doped ZnO synthesized using Stachyarpheta Jamaicensis showed smaller inhibition zones of 14.97 mm and 13.42 mm for B. subtilis and S. aureus respectively54. Research by Raju et al.62 revealed inhibition zone of 20 mm and 18 mm for E. coli and S. aureus respectively when using 1% Cu doping and slightly improved zones of 21 mm and 20 mm at 3% Cu doping. The enhanced antibacterial activity of Cu: ZnO NPs of current study with maximum inhibition zones of 21.5 mm and 21.6 mm for S. aureus and E. coli respectively indicates superior performance due to their optimized process using PS plant extract.
The finding emphasizes the significance of particle size and surface area in improving antibacterial properties as smaller particle size facilitate better interaction with bacterial cell membranes leading to effective disruption and eradication. The study underscores the potential of Cu: ZnO NPs synthesized through green method for developing advanced antibacterial materials. These materials could be pivotal in addressing growing concerns over bacterial resistance and are promising candidates for innovative therapeutic applications. The demonstrated antibacterial efficacy of Cu: ZnO NPs promising agents for various applications such as coating surgical instruments, implant to prevent infections, eliminating bacterial contaminant in water system, food packing, pharmaceuticals, especially against antibiotic-resistant bacteria.
Antifungal activity
The antifungal efficacy of synthesized Cu: ZnO NPs was assessed against the pathogen Candida albicans through various solvents, highlighting their potential as effective antifungal agents. This investigation revealed that the inhibition zone of Cu: ZnO NPs fluctuated based on the solvent employed for dispersion. The largest inhibition zones were recorded in methanol (17.5 mm), succeeded by ethanol (17 mm) and chloroform (16.3 mm). These results are detailed in Fig. 18 with corresponding data summarized in Table 7.
Comparative analysis with previous literature highlights that microwave-assisted green synthesized ZnO NPs utilizing the same plant for this study, PS exhibited an inhibition zone of 17.2 mm against C. albicans43. The superior performance of Cu: ZnO NPs in methanol with a slightly larger inhibition zone of 17.5 mm underscores the enhancement achieved through copper doping. The radical growth inhibition of C. albicans observed in this study is indicative of the ability of the NPs to effectively hinder fungal development by interacting with pathogen’s cell membrane, leading to structural damage and eventual cell death.
The finding emphasizes the important of solvent choice maximizing antifungal activity and demonstrate that green synthesis using PS offers an eco-friendly and efficient method for producing Cu: ZnO NPs with enhanced antifungal properties. These results show the synthesized Cu: ZnO NPs as promising candidates for applications in antifungal treatments and the development of sustainable biocidal agents to combat fungal infections.
Anticancer activity
The evaluation of the anticancer potential of Cu: ZnO NPs on SK-MEL-28 melanoma cells revealed a dose-dependent decrease in cell viability across varying concentrations. The findings demonstrate a notable decline in the viability percentage of the treated cells as the concentrations of NPs increased, as illustrated in Fig. 19. The half-maximal inhibitory concentration (IC50) of the synthesized NPs was established at 30.53 µg/mL, indicating a significant level of cytotoxic activity. The IC50 value is significantly reduced in comparison to earlier documented Cu: ZnO NPs, which demonstrated IC50 values of 219.56 µg/mL for MCF7 breast cancer cells and 137.27 µg/mL for HeLa cervical cancer cells, highlighting the superior efficacy of the PS-mediated synthesis63.
The morphological changes noted in SK-MEL-28 human melanoma cells substantiate the significant cytotoxic effects of green synthesized Cu: ZnO NPs. The observed effects were apparent within a concentration spectrum of 6.25 to 100 µg/mL, with peak cytotoxicity manifesting at the uppermost dosage of 100 µg/mL. Table 8 elucidates the results of the MTT assay, while Fig. 20 illustrates the notable morphological alterations observed in melanoma cells subsequent to exposure to different concentrations of Cu: ZnO NPs.
The cytotoxic mechanism of Cu: ZnO NPs involves enhanced generation of ROS which disrupts cellular integrity, induce apoptosis and arrest the cell cycle, collectively lading to high cancer cell mortality. Copper doping not only amplifies the therapeutic efficiency of ZnO NPs but also offers precision in targeting cancer cells. This targeted cytotoxic effect minimizes damage to healthy tissues and enhances treatment specifically making these NPs a promising candidate for melanoma therapy.
This study reveals that Cu: ZnO NPs enhances their cytotoxic efficacy and establishing them as a promising candidate for targeted cancer therapies. The adoption of green synthesis using PS plant extract not only ensure eco-friendly production process but also reduces the environmental impact associated with NPs manufacturing. These finding highlight Cu: ZnO NPs is a sustainable and effective platform for development of innovative anticancer drugs with potential for further optimization and application across various cancer cell lines.
Conclusion
This research successfully developed an eco-friendly strategy for synthesizing Cu: ZnO NPs using a microwave-assisted method and Pistia stratiotes leaf extract. Our results demonstrate that the use of plant-derived bioactive compounds not only facilitated NPs formation but also contributed to their enhanced stability and biological activity. Structural and morphological analyses confirmed the formation of spherical and nanorod-like particles prone to slight agglomeration, while DFT studies revealed that copper doping effectively narrowed the ZnO band gap to 3.0 eV, suggesting altered electronic properties. Importantly, the synthesized Cu: ZnO NPs showed strong antibacterial and antifungal activities, alongside notable cytotoxic effects against melanoma cells, highlighting their dual potential for antimicrobial and anticancer therapies. This research emphasizes that the microwave-assisted green synthesis method offers a sustainable pathway for developing multifunctional NPs. Future investigations should explore their detailed mechanism of action, biocompatibility across a wider range of biological systems, and performance in preclinical models to pave the way for real-world therapeutic applications.
Data availability
All relevant data supporting the findings of this study are provided within this paper, including experimental measurements, characterization results, and computational data. Additional datasets analyzed during the study are available upon request from the corresponding author.
Abbreviations
- nm:
-
Nanometres
- g:
-
Gram
- ml:
-
Millilitre
- oC:
-
Degree celsius
- mA:
-
Milli Ampere
- λ:
-
Wavelength of the absorption peak
- E:
-
Band gap energy
- rpm:
-
Revolutions per minute
- c:
-
Speed of light
- MHz:
-
Mega Hertz
- h:
-
Planck’s constant
- O-H:
-
Hydroxyl groups
- C=C:
-
Carbon-carbon double bonds
- Eg :
-
Band gap of the material
- D:
-
Crystalline size
- k:
-
Scherrer constant (k=0.9)
- λ:
-
X-ray wavelength
- β:
-
Line broadening at FWHM
- FWHM:
-
Full Width Half Maximum
- h, k, and l:
-
Miller indices
- a, c:
-
Lattice constants
- θ:
-
Peak position/Bragg angle
- d:
-
Interplanar spacing
- n:
-
Diffraction order
- ε:
-
Lattice strain
- δ:
-
Dislocation density
- L:
-
Bond length
- u:
-
Distance travelled by an atom
- V:
-
Volume
- SEM:
-
Scanning electron microscope
- HR-TEM:
-
High-resolution transmission electron microscopy
- EDX:
-
Energy dispersive X-ray
- XRD:
-
X-ray powder diffraction
- FTIR:
-
Fourier transform infrared spectroscopy
- UV-Visible:
-
Ultraviolet–visible
- ROS:
-
Reactive oxygen species
- Cu:
-
Copper
- ZnO:
-
Zinc Oxide
- NCCS:
-
National centre for cell sciences
- NPs:
-
Nanoparticles
- FBS:
-
Fetal bovine serum
- DMEM:
-
Dulbecco’s modified eagle’s medium
- TC:
-
Tissue culture
- CO2 :
-
Carbon dioxide
- PS:
-
Pistia stratiotes
- W-H:
-
Williamson-Hall
- SSP:
-
Size strain plot
- TDS:
-
Total dissolved solids
- pH:
-
Potential of hydrogen
- SDA:
-
Sabouraud dextrose agar
- DMSO:
-
Dimethyl sulfoxide
- PBS:
-
Phosphate-buffered saline
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide
- DFT:
-
Density functional theory
- PBE:
-
PERdew-Burke-Ernzerhof
- SCF:
-
Self-consistence field
- DOS:
-
Density of states
- HOMO:
-
Highest occupied molecular orbital
- LUMO:
-
Lowest unoccupied molecular orbital
References
Anjum, A., Das, M. & Garg, R. Introduction to Nanotechnology: Transformative Frontier, in: R. Garg, A. Anjum (Eds.), Advances in Chemical and Materials Engineering, IGI Global, : pp. 1–35. (2024). https://doi.org/10.4018/979-8-3693-1094-6.ch001
Singh, N. B., Kumar, B., Usman, U. L. & Susan, M. A. B. H. Nano revolution: exploring the frontiers of nanomaterials in science, technology, and society. Nano-Structures Nano-Objects. 39, 101299. https://doi.org/10.1016/j.nanoso.2024.101299 (2024).
Malik, S., Muhammad, K., Waheed, Y. & Nanotechnology A revolution in modern industry. Molecules 28, 661. https://doi.org/10.3390/molecules28020661 (2023).
Szczyglewska, P., Feliczak-Guzik, A. & Nowak, I. Nanotechnology–General aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules 28, 4932. https://doi.org/10.3390/molecules28134932 (2023).
Ahmed, S. F. et al. Green approaches in synthesising nanomaterials for environmental nanobioremediation: technological advancements, applications, benefits and challenges. Environ. Res. 204, 111967. https://doi.org/10.1016/j.envres.2021.111967 (2022).
Joseph, T. et al. Nanoparticles: taking a unique position in medicine. Nanomaterials 13, 574. https://doi.org/10.3390/nano13030574 (2023).
Sharma, S. & Thakur Devi,Meena, Kumar, Pankaj, Thakur, Nikesh, Kumar, Kuldeep, Jeet, Kamal, Sharma, Arvind, Kumar, Sunil, Kumar, Ashwani, N. and Photocatalytic, antibacterial and antioxidant study of Vitex negundo mediated green synthesized nickel and neodymium doped zinc oxide nanoparticles, Toxicological & Environmental Chemistry 107 178–206. (2025). https://doi.org/10.1080/02772248.2024.2448952
Verma, N. et al. Photocatalytic, antibacterial and antioxidant capabilities of (Fe, Al) double doped ZnO nanoparticles with Murraya Koenigii leaf extract synthesized by using microwave assisted technique. Mater. Chem. Phys. 333, 130422. https://doi.org/10.1016/j.matchemphys.2025.130422 (2025).
Devi, M. et al. Antifungal, antibacterial and antioxidant activity of Pinus Roxburghii mediated green synthesized zinc and gadolinium doped manganese oxide nanoparticles. Colloids Surf. C: Environ. Aspects. 2, 100046. https://doi.org/10.1016/j.colsuc.2024.100046 (2024).
Balkrishna, A. et al. Synthesis, characterization and antibacterial efficacy of Catharanthus roseus and Ocimum tenuiflorum-Mediated silver nanoparticles: phytonanotechnology in disease management. Processes 11, 1479. https://doi.org/10.3390/pr11051479 (2023).
Osman, A. I. et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 22, 841–887. https://doi.org/10.1007/s10311-023-01682-3 (2024).
Afonso, I. S. et al. Green synthesis of nanoparticles from Olive oil waste for environmental and health applications: A review. J. Environ. Chem. Eng. 12, 114022. https://doi.org/10.1016/j.jece.2024.114022 (2024).
Karnwal, A. et al. Nanotechnology for healthcare: Plant-Derived nanoparticles in disease treatment and regenerative medicine. Pharmaceuticals 17, 1711. https://doi.org/10.3390/ph17121711 (2024).
Devi, L., Kushwaha, P., Ansari, T. M., Kumar, A. & Rao, A. Recent trends in biologically synthesized metal nanoparticles and their biomedical applications: a review. Biol. Trace Elem. Res. 202, 3383–3399. https://doi.org/10.1007/s12011-023-03920-9 (2024).
Ali, J. et al. Green synthesized zinc oxide nanostructures and their applications in dye-sensitized solar cells and photocatalysis: A review. Mater. Today Commun. 36, 106840. https://doi.org/10.1016/j.mtcomm.2023.106840 (2023).
Helmy, E. T., Abouellef, E. M., Soliman, U. A. & Pan, J. H. Novel green synthesis of S-doped TiO2 nanoparticles using Malva parviflora plant extract and their photocatalytic, antimicrobial and antioxidant activities under sunlight illumination. Chemosphere 271, 129524. https://doi.org/10.1016/j.chemosphere.2020.129524 (2021).
Thakur, N. & Kumar, P. Effect of shape and size on synthesized triple (Co/Ni/Zn)-Doped α-Fe2O3 nanoparticles on their photocatalytic and scavenging properties. Int. J. Nanosci. 23, 2450010. https://doi.org/10.1142/S0219581X24500108 (2024).
Degefa, M., Muleta, G. & Teshome, K. Synthesis of Cu-doped ZnO nanoparticles using Aloe vera leaf Extractfor antibacterial and photocatalytic activities evaluation. CNM 08 https://doi.org/10.2174/2405461508666230905115443 (2024).
Ekinci, A. et al. Designing copper-doped zinc oxide nanoparticle by tobacco stem extract-mediated green synthesis for solar cell efficiency and photocatalytic degradation of methylene blue. Int. J. Phytoremediation. 1–11. https://doi.org/10.1080/15226514.2024.2379605 (2024).
Khan, S. A., Noreen, F., Kanwal, S., Iqbal, A. & Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon Indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Engineering: C. 82, 46–59. https://doi.org/10.1016/j.msec.2017.08.071 (2018).
Karthik, K. V. et al. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 287, 132081. https://doi.org/10.1016/j.chemosphere.2021.132081 (2022).
Lemecho, B. A. et al. Biogenic synthesis of Cu-Doped ZnO photocatalyst for the removal of organic dye. Bioinorg. Chem. Appl. 2022 (8081494). https://doi.org/10.1155/2022/8081494 (2022).
Al-Rajhi, A. M. H., Yahya, R., Bakri, M. M., Yahya, R. & Abdelghany, T. M. In situ green synthesis of Cu-doped ZnO based polymers nanocomposite with studying antimicrobial, antioxidant and anti-inflammatory activities. Appl. Biol. Chem. 65, 35. https://doi.org/10.1186/s13765-022-00702-0 (2022).
Adeola, A. O., Duarte, M. P. & Naccache, R. Microwave-assisted synthesis of carbon-based nanomaterials from biobased resources for water treatment applications: emerging trends and prospects. Front. Carbon. 2 https://doi.org/10.3389/frcrb.2023.1220021 (2023).
Nandhini, J. et al. Optimization of Microwave-Assisted green synthesis of zinc oxide nanoparticles using Ocimum americanum and Euphorbia hirta extracts: in vitro evaluation of antioxidant, Anti-inflammatory, antibacterial, cytotoxicity, and wound healing properties. Intell. Pharm. https://doi.org/10.1016/j.ipha.2024.09.003 (2024).
Abbas, R. et al. Silver nanoparticles: synthesis, structure, properties and applications. Nanomaterials 14, 1425. https://doi.org/10.3390/nano14171425 (2024).
Gupta, D., Boora, A., Thakur, A. & Gupta, T. K. Green and sustainable synthesis of nanomaterials: recent advancements and limitations. Environ. Res. 231, 116316. https://doi.org/10.1016/j.envres.2023.116316 (2023).
Serdar, G. & Kılınç, G. G. Microwave assisted production and characterization of gold nanoparticles using green tea and Catechin extracts obtained by supercritical extraction method. Chem. Pap. 77, 5155–5167. https://doi.org/10.1007/s11696-023-02851-y (2023).
Rajalakshmi, P., Vijayan, S. & Amirtharaj, M. A study on microwave synthesis of unique Zn1-3xCuxCexCrxO nanoparticles with efficient photocatalytic reactivity under visible sunlight irradiation. J. Mol. Struct. 1307, 137992. https://doi.org/10.1016/j.molstruc.2024.137992 (2024).
Harish, V. et al. Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: A review. J. Taiwan Inst. Chem. Eng. 149, 105010. https://doi.org/10.1016/j.jtice.2023.105010 (2023).
Cai, J. et al. Exploring advanced microwave strategy for the synthesis of two-dimensional energy materials. Appl. Phys. Reviews. 11, 041320. https://doi.org/10.1063/5.0231081 (2024).
Kumar, P., Kaushik, A., Kumar, S. & Thakur, N. Chemical and green synthesized Co/Ni-doped hematite nanoparticles for enhancing the photocatalytic and antioxidant properties. Phys. Scr. 99, 105960. https://doi.org/10.1088/1402-4896/ad7329 (2024).
Kumar, P., Arya, V., Kumar, A. & Thakur, N. Polymer/phytochemical mediated eco-friendly synthesis of Cu/Zn doped hematite nanoparticles revealing biological properties and photocatalytic activity. Int. J. Mater. Res. 116, 30–49. https://doi.org/10.1515/ijmr-2023-0343 (2025).
Thakur, N. & Thakur N. and Photocatalytic adsorption and scavenging potential of chemical and green encapsulated anatase phase of coupled doped Zn-Co TiO2 nanoparticles. J. Dispers. Sci. Technol. 0 (n.d.) 1–16. https://doi.org/10.1080/01932691.2024.2312841
Kumar, P., Kumar, S., Tapwal, A., Nimesh, S. & Thakur, N. Water purification and biological efficacy of green synthesized Co/Zn-Doped α-Fe2O3 nanoparticles. Sustainable Chem. Environ. 8, 100160. https://doi.org/10.1016/j.scenv.2024.100160 (2024).
Verma, N., Pathak, D. & Thakur, N. Eco-friendly green synthesis of (Cu, Ce) dual-doped ZnO nanoparticles with Colocasia esculenta plant extract using microwave assisted technique for antioxidant and antibacterial activity. Next Mater. 5, 100271. https://doi.org/10.1016/j.nxmate.2024.100271 (2024).
Morales-Mendoza, J. E. et al. Synthesis, structural and optical properties of Cu doped ZnO and CuO–ZnO composite nanoparticles. Nano-Structures Nano-Objects. 34, 100967. https://doi.org/10.1016/j.nanoso.2023.100967 (2023).
Sivakumar, S., Robinson, Y. & Mala, N. A. Studies on photocatalytic performance and supercapacitor applications of undoped and Cu-doped ZnO nanoparticles. Appl. Surf. Sci. Adv. 12, 100344. https://doi.org/10.1016/j.apsadv.2022.100344 (2022).
Sonkar, R., Mondal, N. J., Boro, B., Ghosh, M. P. & Chowdhury, D. Cu doped ZnO nanoparticles: correlations between tuneable optoelectronic, antioxidant and photocatalytic activities. J. Phys. Chem. Solids. 185, 111715. https://doi.org/10.1016/j.jpcs.2023.111715 (2024).
Benkhira, L. et al. Multifunctional assessment of copper-doped ZnO nanoparticles synthesized via gliding Arc discharge plasma technique: antioxidant, antibacterial, and photocatalytic performance. Environ. Sci. Pollut Res. 31, 43743–43756. https://doi.org/10.1007/s11356-024-34054-7 (2024).
Gautam, A. K. & Pandey, G. Green synthesis of Pistia stratiotes Ag/AgCl nanomaterials and their Anti-Bacterial activity. Chem. Proc. 14, 88. https://doi.org/10.3390/ecsoc-27-16054 (2023).
Nair, V. A. et al. Nano-phytoremediation approach using Pistia stratiotes: biosynthesized copper nanoparticles for textile wastewater treatment and toxicity analysis. Waste Biomass Valor. 15, 6465–6477. https://doi.org/10.1007/s12649-024-02560-x (2024).
M, A. M., A. B, U. D. & M Microwave-assisted green synthesis of zinc oxide nanoparticles using pistia stratiotes for anticancer and antibacterial applications. Mater. Res. Express. 11, 085004. https://doi.org/10.1088/2053-1591/ad6d34 (2024).
Wasagu, R. S., Lawal, M., Shehu, S., Alfa, H. H. & Muhammad, C. Nutritive values, mineral and antioxidant properties of Pistia stratiotes (Water lettuce). Nig J. Bas App Sci. 21, 253. https://doi.org/10.4314/njbas.v21i4.2 (2014).
Aminzai, M. T., Yildirim, M. & Yabalak, E. Metallic nanoparticles unveiled: synthesis, characterization, and their environmental, medicinal, and agricultural applications. Talanta 280, 126790. https://doi.org/10.1016/j.talanta.2024.126790 (2024).
Goswami, S. et al. Recent trends in the synthesis, characterization and commercial applications of zinc oxide nanoparticles- a review. Inorg. Chim. Acta. 573, 122350. https://doi.org/10.1016/j.ica.2024.122350 (2024).
Ahammed, K. R. et al. Microwave assisted synthesis of zinc oxide (ZnO) nanoparticles in a noble approach: utilization for antibacterial and photocatalytic activity. SN Appl. Sci. 2, 955. https://doi.org/10.1007/s42452-020-2762-8 (2020).
Rajan, M. et al. Comparative study of biological (Phoenix loureiroi Fruit) and chemical synthesis of Chitosan-Encapsulated zinc oxide nanoparticles and their biological properties. Arab. J. Sci. Eng. 45, 15–28. https://doi.org/10.1007/s13369-019-04174-1 (2020).
Mallikarjunaswamy, C., Lakshmi Ranganatha, V., Ramu, R., Udayabhanu, G. & Nagaraju Facile microwave-assisted green synthesis of ZnO nanoparticles: application to photodegradation, antibacterial and antioxidant. J. Mater. Sci: Mater. Electron. 31, 1004–1021. https://doi.org/10.1007/s10854-019-02612-2 (2020).
Karam, S. T. & Abdulrahman, A. F. Green synthesis and characterization of ZnO nanoparticles by using thyme plant leaf extract. Photonics 9, 594. https://doi.org/10.3390/photonics9080594 (2022).
Geetha Devi, P. & Sakthi Velu, A. Structural, optical and photoluminescence properties of copper and iron doped nanoparticles prepared by co-precipitation method. J. Mater. Sci: Mater. Electron. 27, 10833–10840. https://doi.org/10.1007/s10854-016-5190-1 (2016).
Kumar, S. et al. Structural, morphological, optical and magnetic studies of Cu-Doped ZnO nanostructures. Materials 15, 8184. https://doi.org/10.3390/ma15228184 (2022).
Srivastava, V. & Choubey, A. K. Kinetic and isothermal study of effect of transition metal doping on adsorptive property of zinc oxide nanoparticles synthesized via green route using Moringa oleifera leaf extract. Mater. Res. Express. 6, 1250i7. https://doi.org/10.1088/2053-1591/ab7158 (2020).
Khan, M. M. et al. Antibacterial studies of ZnO and Cu-Doped ZnO nanoparticles synthesized using aqueous leaf extract of Stachytarpheta jamaicensis. BioNanoSci 10, 1037–1048. https://doi.org/10.1007/s12668-020-00775-5 (2020).
Naik, E. I. et al. Influence of Cu doping on ZnO nanoparticles for improved structural, optical, electrochemical properties and their applications in efficient detection of latent fingerprints. Chem. Data Collections. 33, 100671. https://doi.org/10.1016/j.cdc.2021.100671 (2021).
Pashchanka, M. et al. A molecular approach to Cu doped ZnO nanorods with tunable Dopant content. Dalton Trans. 40, 4307. https://doi.org/10.1039/c0dt01567a (2011).
Suryanarayana, C. & Norton, M. G. Determination of crystallite size and lattice strain, in: X-Ray Diffraction, Springer US, Boston, MA, : 207–221. https://doi.org/10.1007/978-1-4899-0148-4_9. (1998).
Rojas-Michea, C., Morel, M., Gracia, F., Morell, G. & Mosquera, E. Influence of copper doping on structural, morphological, optical, and vibrational properties of ZnO nanoparticles synthesized by Sol gel method. Surf. Interfaces. 21, 100700. https://doi.org/10.1016/j.surfin.2020.100700 (2020).
Hassanzadeh-Tabrizi, S. A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 968, 171914. https://doi.org/10.1016/j.jallcom.2023.171914 (2023).
Devesa, S., Rooney, A. P., Graça, M. P., Cooper, D. & Costa, L. C. Williamson-hall analysis in Estimation of crystallite size and lattice strain in Bi1.34Fe0.66Nb1.34O6.35 prepared by the sol-gel method. Mater. Sci. Engineering: B. 263, 114830. https://doi.org/10.1016/j.mseb.2020.114830 (2021).
Md. Kawsar, M. S., Hossain, N. M., Bahadur, S. & Ahmed Synthesis of nano-crystallite hydroxyapatites in different media and a comparative study for Estimation of crystallite size using scherrer method, Halder-Wagner method size-strain plot, and Williamson-Hall model. Heliyon 10, e25347. https://doi.org/10.1016/j.heliyon.2024.e25347 (2024).
Raju, P., Deivatamil, D., Martin Mark, J. A. & Jesuraj, J. P. Antibacterial and catalytic activity of Cu doped ZnO nanoparticles: structural, optical, and morphological study. J. Iran. CHEM. SOC. 19, 861–872. https://doi.org/10.1007/s13738-021-02352-3 (2022).
Rishikesan, S. & Basha, M. A. M. Synthesis, characterization and evaluation of antimicrobial, antioxidant & anticancer activities of copper doped zinc oxide nanoparticles. ACSi 67, 235–245. https://doi.org/10.17344/acsi.2019.5379 (2020).
Author information
Authors and Affiliations
Contributions
Abisha Meji M: Conceptualization, Validation, Formal analysis, Methodology, Investigation, Writing – original draft. Usha D: Project Administration, Data curation, Resources, Supervision, Writing – review & editing. Ashwin B M: Data curation, Writing – review & editing. Milon Selvam Dennison: Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Plant Guidelines
The authors confirm that the use of plant in the present study complies with international, national and/or institutional guidelines.
Permissions to collect the plants/plant parts
The plant specimen used in this research was collected with proper permissions.
Source of the plant used in your study
The name of the plant and its source are mentioned in the Materials and Methods section.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
M, A.M., D, U., B.M, A. et al. Integrating microwave-assisted green synthesis, DFT simulations, and biological activity evaluation of copper-doped zinc oxide nanoparticles. Sci Rep 15, 19348 (2025). https://doi.org/10.1038/s41598-025-03922-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03922-8