Abstract
The ASC-1 complex, consisting of TRIP4, ASCC1, ASCC2, and ASCC3, plays a pivotal role in regulating nuclear receptors and transcription factors that influence immunity, cellular proliferation, and apoptosis, and a multiomic analysis of ASCC1/2/3 was therefore performed to investigate its potential role on a pan-cancer scale. Although it has been implicated in DNA repair mechanisms and neurodegenerative diseases, its specific function within cancer biology remains poorly understood. This study conducted a pan-cancer analysis of ASCC1/2/3’s roles in the tumorigenesis process and explored their regulatory effects on lung adenocarcinoma. We conducted a comprehensive analysis of 10,967 tumor samples across 30 distinct cancer types. It was found that alterations in ASCC1/2/3 occurred in 754 cases (7% of the patients analyzed), with ASCC3 being the most frequently altered at 4%. Furthermore, elevated expression levels of ASCC1/2/3 were observed in the majority of cancer types examined. Notably, the expression levels of ASCC1 and ASCC3 demonstrated a correlation with CD4-positive T cell infiltration. In vitro experiments demonstrated that the ASCC family effectively inhibited tumor proliferation, migration, and invasion in lung adenocarcinoma cell line. Our findings indicate a significant association between the ASCC family and tumors while providing a foundation for further investigation into its role in cancer biology and potential clinical applications.
Similar content being viewed by others
Background
Transcriptional co-regulators are currently reported to play a pivotal role in the regulation of nuclear receptors (NRs) and transcription factors (TFs)1. The activating signal co-integrator 1 (ASC-1, also called TRIP4), together with 3 subunits including ASCC1, ASCC2, and ASCC3, constitute the ASC-1 complex, interacting directly with TFs including AP-1, NF-kappa-B involved in immunity, proliferation, and apoptosis2. Moreover, the complex has been implicated in DNA alkylation repair and cancer cell proliferation, functioning as a helicase3. Overall, according to the American Cancer Society, cancer incidence spiked in the early 1990 s and generally declined until 2013, when it stabilized4. Among them, the incidence of lung cancer has steadily declined since 20064. In terms of cancer survival, the five-year survival rate for all cancers was around 70% between 2013 and 2019, compared to 49% in the 1970 s4. Lung cancer survival gains mostly limited to nonsmall cell lung cancer4. Although surgery has always been the cornerstone of cancer treatment, since tamoxifen entered clinical trials in 1970, systemic therapies, especially targeted drugs, have significantly prolonged patient survival, highlighting the importance of novel biomarkers5. While the structure and function of ASC-1 have not been fully understood, many studies suggested it was also involved in the process of neurodegenerative diseases6,7,8. The mutation of its encoding genes can result in a wide range of serious neuromuscular diseases including progressive scoliosis, dilated cardiomyopathy, early-onset respiratory failure, etc9.
Given its interactions with TFs and links to DNA repair deficiency diseases, ASC-1 may play a key regulatory role. We were therefore keen to investigate whether it also contributes to tumor biology. Furthermore, the complex has been reported to participate in the dynamic balance between cellular differentiation and proliferation, a key feature of tumor genesis9. Although originally identified as a proliferation suppressor in non-neoplastic cells9, knockdown of its encoding genes in cancer cell lines enhanced both proliferation and migration10,11. Moreover, they also find the high expression of its encoding gene TRIP4 in these cell lines. Considering the diverse regulatory effects of transcription co-regulators across different cells in varying temporal and spatial contexts, the contrasting roles of ASC-1 in cancer mentioned above and normal cells appear to be elucidated. However, we were still curious whether this conclusion could apply to the pan-cancer field. In other words, there is still a need for a comprehensive characterization of the ASC-1 complex, its encoding genes, and their mechanisms within tumor cells.
In both neuromuscular diseases and human cancers, researchers predominantly focus on the role of TRIP4. For instance, in human melanoma11, TRIP4 exhibits higher expression levels than normal tissue. Knockdown of TRIP4 can lead to the suppression of both cell proliferation and migration and therefore induce the sensitivity of targeted therapy in vitro and vivo11. Similar results were also identified in human cervical cancer cells10. It induces tumor growth and metastasis through multiple cancer-related pathways including MAPK, PI3 K, etc10. Orloff, Mohammed, et al. found that ASCC1 was associated with Barrett Esophagus, a precursor of esophageal adenocarcinoma12. Nevertheless, this study primarily identified the association between ASCC1 and precancerous lesions through a prospective cohort approach. The potential molecular mechanisms behind the association remain to be elucidated. In the past decade, no study has focused on the function of the other 3 subunits of the ASC-1 complex (ASCC1, ASCC2, ASCC3). Given that the knockdown of each member significantly diminishes the levels of others, thereby impacting the translation of related genes, it appears imperative to consolidate these genes rather than analyze them independently.
Taken together, considering the lack of comprehensive understanding of the function of ASCC1, 2, and 3 in cancer biology, a pan-cancer analysis was performed based on the public multimers database including The Cancer Genome Atlas (TCGA), Clinical Proteomic Tumor Analysis Consortium (CPTAC), etc. Besides multiple bioinformatic tools were applied for the initial exploration, and our biobank was supplemented for further study and validation. Hence, in this study, we aim to perform a comprehensive pan-cancer investigation of ASCC1, 2, and 3, aiming to enhance our understanding of their functions and mechanisms for subsequent studies.
Results
Common mutations of the ASCC family on a pan-cancer scale
Given the lack of knowledge about ASCC1/2/3 in cancer research, alterations were first summarized based on the mutational profile in 10,967 tumor samples from 30 different cancer types (Fig. 1). Overall, ASCC1/2/3 are altered in 754 (7%) of the included patients: ASCC3 is the most common (4%), followed by ASCC2 (1.8%) and ASCC1 (1.4%), highlighting a clinically relevant subgroup that may benefit from risk stratification and personalized therapies. This mutation rate also indicates their clinical potential as biomarkers for patient monitoring or as therapeutic targets. The predominant genetic alteration of ASCC1 and ASCC2 is amplification followed by deep deletion, whereas ASCC3 shows the opposite (Supplementary Fig. S1). Regarding cancer types, ASCC1/2/3 genes are most frequently altered in endometrial cancer in the TCGA cohort (> 15%). In addition, melanoma, bladder cancer, esophagogastric cancer, mature B-cell neoplasms, prostate cancer and cholangiocarcinoma also show a high alteration frequency of more than 10% (Fig. 1A). Separately, mutated ASCC1 is mostly found in cholangiocarcinoma (> 5%, Supplementary Fig. S2) and mutated ASCC2 in endometrial cancer (> 5%, Supplementary Fig. S3), while mature large B-cell neoplasia has the most alterations in ASCC3 (> 10%, Supplementary Fig. S4).
The number and types of alterations and the simplified structure of ASCC1/2/3 were also depicted: among these 3 genes, missense mutation is the most common type (Fig. 1B). As shown in Fig. 1B, the mutation sites of ASCC1, ASCC2, and ASCC3 were predominantly missense mutations (green), followed by truncating (grey) and splice-site mutations (orange), while inframe mutations (brown) were not detected in any of the three genes. On the structure of ASCC1, the alterations are mainly located in a type 1 KH domain and AKAP7 2’5’ RNA ligase-like domain (Fig. 1B). The CUE domain can also be found on the structure of ASCC2, while 2 DEAD domains, 2 Sect. 63 Brl domains and 2 helicase conserved C-terminal domains are restricted to the DEAD domains (Fig. 1B). Although the role of most of these domains has not been elucidated, mutations in the DEAD domain have been reported to have a strong association with several cancers, and they may serve as an attractive target for cancer treatment13.
Genetic alternation of the ASCC1/2/3 on a pan-cancer scale. (A) Pan-cancer analyses of genomic changes in ASCC 1/2/3 in the TCGA database were conducted, including analyses of mutations, structural variants, and CNA. (B) The mutation types, sites and case number of ASCC 1/2/3 genetic alternation were display.
Widespread transcriptomic and proteomic upregulation of the ASCC family in cancers
To investigate the RNA expression level of ASCC1/2/3, the Wilcoxon test was applied to compared the differential expression between tumor and normal tissues by the TIMER platform. The RNA expression level of ASCC1/2/3 was then visualized in different tumors and paired normal tissues14 (Fig. 2). Taken together, ASCC1/2/3 shared a similar RNA expression pattern in these tumors. In most of the included cancer types, the expression level of ASCC1/2/3 is upregulated. Compared with normal tissues, the expression of ASCC1/2/3 is statistically higher in CHOL, ESCA, HNSC, LIHC, LUAD, LUSC. And in BRCA, CESC, GBM, KIRC, KIPR, THCA, UCEC, they also showed the same pattern, although some of them are not statistically different. Only in PRAD, ASCC1/2/3 is downregulated in tumors compared to adjacent normal tissues.
Given the similar transcriptomic pattern in cancer and normal tissues, we examine the expression pattern of proteins in these tumors (Supplementary Fig.S5-7). Compared to normal tissues, the expression of ASCC1/2/3 proteins is statistically higher in breast cancer, clear cell renal cell carcinoma, glioblastoma, head and neck squamous cell carcinoma, hepatocellular carcinoma, lung adenocarcinoma, uterine corpus endometrial carcinoma. And the protein level of ASCC1/3 is also statistically higher in colon cancer. The only exception is pancreatic adenocarcinoma. The expression level of ASCC2/3 proteins is downregulated in pancreatic adenocarcinoma compared to normal tissue. Therefore, these data indicate that the transcriptomic and proteomic expression level of the ASCC family is commonly upregulated in different types of cancer.
The RNA expression level of ASCC1/2/3 in different tumors and paired normal tissues. ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, low-grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, thymoma and uveal melanoma. *p < 0.05; **p < 0.01; ***p < 0.001.
ASCC family can predict the patient survival in multiple cancers
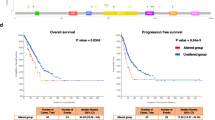
Given their widespread upregulation and heterogeneity among cancers, we were then interested in the intertumoral heterogeneity of survival associated with their expression pattern. Based on the RNA expression level of these genes, patients of each cancer were divided into high and low-expression groups. In ACC, LIHC, LUAD, PAAD, the overall survival rate of the high ASCC1 expression group is significantly lower than that of the low expression group with p values of 0.0026, 0.028, 0.03 and 0.0066, respectively, indicating that the expression of ASCC1 is associated with an unfavorable prognosis (Fig. 3A). On the contrary, in GBM, KIRC and LGG, the high expression of ASCC1 indicates a favorable prognosis with p values of 0.036, < 0.001 and < 0.001, respectively (Fig. 3A). For ASCC2, the only cohort with statistical significance is ACC with a p value of 0.016, where ASCC2 is an unfavorable factor (Fig. 3A). And for ASCC3, the overall survival of the high-expression group is significantly lower in HNSC, LUAD and SARC with p values of 0.013, 0.011 and 0.031, respectively, while no cohort has a high-expression group with a significantly better prognosis (Fig. 3A). However, despite the CESC p-value of 0.054 approximating the critical value, the trend in the survival curves still suggests a relatively poor prognosis for the high-expression group (Fig. 3A).
Since the lifespan of different cancers can vary widely, overall survival is not always the best indicator of a patient’s prognosis. Disease-free survival (DFS) was used to assess the prognostic difference between the high and low-expression groups. Similarly, while ASCC1 is the favorable factor in LGG (p value = 0.009), ASCC1 and ASCC2 is still the risk factor in ACC (p value < 0.01, p value = 0.016) and ASCC3 is the risk factor in LUAD (p value = 0.013) (Fig. 3B). This indicates that high expression of these genes is associated with significantly worse survival. In addition, high expression of these genes is also significantly correlated with poor survival in BLCA (p value = 0.017), PAAD (p values = 0.0062)and STAD (p value = 0.048) (Fig. 3B). Although the cancer types with statistical differences have changed due to the different indicators of DRS and OS, the pattern and tendency of these prognostic data remain similar among different cancer types.
Correlation between ASCC1/2/3 expression levels and survival prognosis of tumors in TCGA dataset. (A) The correlation between the expression of ASCC1/2/3 and the OS of patients with different tumors. The Logrank p-values of the correlation between ASCC3 expression and OS in CESC, HNSC, LUAD, and SARC patients were 0.054, 0.013, 0.011, and 0.031, respectively; the Logrank p-value of the correlation between ASCC2 expression and OS in ACC patients was 0.016; the Logrank p-values of the correlation between ASCC1 expression and OS in ACC, GBM, KIRC, LGG, LIHC, LUAD, and PAAD patients were 0.0026, 0.036, 3.2e-05, 1.1e-06, 0.028, 0.03, and 0.0066, respectively. (B) The correlation between ASCC1/2/3 and DFS of different tumor patients. The Logrank p-value of the correlation between ASCC3 expression and DFS in LUAD patients was 0.013; the Logrank p-values of the correlation between ASCC2 expression and DFS in ACC, STAD, and THYM patients were 0.016,0.048 and 0.027, respectively; the Logrank p-values of the correlation between ASCC1 expression and DFS in ACC, BLCA, LGG, and PAAD patients were 8e-07, 0.017, 0.0009, and 0.0062, respectively. OS, overall survival; DFS, disease-free survival.
Epigenetic modification pattern and downstream protein phosphorylation
To better characterize the epigenetic modification in these cancers, DNA methylome data and other clinical information were analyzed together with the transcriptomic expression level of ASCC1/2/3. For example, in HNSC, the DNA methylation level of ASCC1 negatively correlates with its RNA expression at various probes in the non-promoter and promoter regions (Fig. 4A). A similar association was also found for ASCC2 in HNSC (Supplementary Table S2). On the contrary, the DNA methylation level of ASCC3 correlates positively with the RNA expression of ASCC3 itself (Supplementary Table S3). The correlations with other clinical parameters include tumor stages, histology type, lymphovascular invasion, etc. The correlation between ASCC1/2/3 in other cancers was also shown in Supplementary Table S 1–3.
Based on the comprehensive characterization of protein phosphorylation, only ASCC2 and ASCC3 were identified to be significantly different between normal and tumor tissues (Fig. 4B). Among them, the T157 site of ASCC2 protein was more phosphorylated in tumors than in normal tissue in clear cell renal cell carcinoma, glioblastoma multiforme, head and neck squamous cell carcinoma, and pancreatic adenocarcinoma (Fig. 4B). Phosphorylation of S630 and Y626 sites was also found to be higher in head and neck squamous cell carcinoma than in paired normal tissues. In hepatocellular carcinoma, the S707 site is also significantly more phosphorylated in the primary tumor (Fig. 4B). Regarding ASCC3, the S2195 site was found to be significantly more phosphorylated in primary tumor tissue than in paired normal tissue in breast cancer, clear cell renal cell carcinoma, hepatocellular carcinoma and lung adenocarcinoma. Instead, also in lung adenocarcinoma, the phosphorylation level of the S297 site in the primary tumor was significantly lower than that in normal tissue (Fig. 4B).
DNA methylation analysis and protein phosphorylation analysis of ASCC family in pan-cancer. (A) Association between ASCC1 DNA methylation and gene expression for the HNSC cases of TCGA. (B) The comprehensive characterization of protein phosphorylation of ASCC2 and ASCC3 between normal and tumor tissues. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
ASCC family correlates positively with the T cell infiltration
Based on the pan-cancer transcriptomic data, immune cell infiltration was assessed using the deconvolution algorithms. Furthermore, their correlation with the expression of ASCC1/2/3 was calculated to explore the potential regulatory pattern. CD4-positive immune cells were found to be largely positively associated with the expression of the ASCC family (Fig. 5A). Interestingly, despite their positive correlation, CD4-positive th1 cells were significantly negatively correlated, especially with the expression of ASCC3 (Fig. 5A). Although CD4-positive th2 cells were also positively correlated with ASCC1/2/3 expression, given their distinct function and differentiation pathway, ASCC3 appears to upregulate CD4-positive T cells while inhibiting their differentiation into CD4-positive th1 cells and inducing their differentiation into CD4-positive th2 cells, which may explain why no cohort with high ASCC3 expression has a significantly better prognosis (Fig. 3A). CD8-positive T cells were also found to be highly positively correlated with ASCC1/2 expression (Fig. 5A). In addition to the infiltration pattern of CD4-positive and CD8-positive T cells, the association between ASCC1/3 and Treg cells was also examined using these deconvolution algorithms (Fig. 5B and C). However, no broadly significant correlations were found between ASCC2 and T-cell infiltration, which is consistent with the heterogeneity of its correlation with patient survival (Supplementary Fig. S8). To experimentally verify these results, multiple immunofluorescences staining in lung adenocarcinoma and normal tissue adjacent to the tumor (NAT) ware conducted for these different marker proteins in a range of paraffin-embedded cancer sections. The expression of ASCC1/2/3, CD4 and CD8 was significantly increased in tumor tissues, which was consistent with the analysis results, suggesting that ASCC family is positively correlated with T cell infiltration (Fig. 6C).
Analysis of the correlation between immune cell infiltration and ASCC1/2/3 expression based on transcriptomic data. (A) The heatmap shows the values of Spearman’s correlation coefficient for the correlations between ASCC1/2/3 and CD4, CD8, as well as specific differentiated CD4-positive Th1 and Th2 cells. (B, C) The display of the correlation between ASCC1/3 and infiltrating CD4, CD8 and T reg cells. Significant correlations are shown in color, while non-significant correlations are marked with a cross.
ASCC family affect lung adenocarcinoma cancer cell growth and malignant phenotype
The impact of the ASCC family on the malignant characteristics of lung adenocarcinoma cells was assessed to further clarify the aforementioned findings using A549 and H1975 cell lines. Both qPCR and Western blotting techniques were utilized to confirm successful knockdown of the ASCC family in these cells. As depicted in the figure, the siRNAs employed were all capable of effectively knocking down ASCC1/2/3 in both of these cell lines (Fig. 6A and B). This knockdown significantly suppressed the proliferative activity of both cell lines (Fig. 7A and B), while also inhibiting their migratory and invasive abilities as demonstrated by wound healing and Transwell assays (Fig. 7C-E). Collectively, these results provide evidence that the ASCC family can effectively impede proliferation, migration, and invasion in patients with LUAD.
To evaluate the effect of ASCC1/2/3 on malignant characteristics of lung adenocarcinoma cells. (A, B) In the A549 and H1975 cell lines, different siRNAs were utilized to knockdown the expression of ASCC1/2/3, and qPCR and Western blot experiment assays were employed to determine the expression at the mRNA and protein levels, respectively (original Western blots are presented in Supplementary Figure S9). Non-targeting siRNA control was transfected in an identical manner to control for transfection mediated toxicity. Viability was measured at each timepoint. (C) Multiple immunofluorescence staining was carried out for the expression levels of ASCC1/2/3 and CD4/CD8 in lung adenocarcinoma and adjacent tissues. Merged staining of anti-ASCC1/2/3(Yellow), anti-CD8(Cyan), anti-CD4(Brown), and DAPI (blue). (Scale bar: 50 μm). NC, negative control; NAT, normal tissue adjacent to the tumor. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Evaluation of the ability of ASCC 1/2/3 to regulate LUAD tumor cell malignancy. (A-B) The impact of ASCC1/2/3 on the proliferation of tumor cells was assessed through CCK-8, colony formation. (C) Tumor cell migration was assessed with a Transwell assay approach (Scale bar: 100 μm). (D-E) Tumor cell migration following knockdown-ASCC1/2/3 was assessed through wound healing assay approach. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
Previous studies have identified the role of the ASC-1 complex in human neuromuscular diseases and cancers6,10,11. It acts as a suppressor of proliferation in human fibroblasts and myogenic cells. Deficiency of the ASC-1 complex contributes to the dysregulation of the cell cycle, which may be the cause of some mild or severe hereditary myopathies9. Interestingly, the ASC-1 complex appears to play a completely different role in human cancers. Its encoding gene, TRIP4, has been found to be overexpressed in human melanoma cells and may therefore promote cancer cell proliferation11. Similarly, high expression of TRIP4 has also been identified in cervical cancer, where inhibition of TRIP4 can suppress cancer progression in vitro and in vivo, and overexpression promotes proliferation and invasion10. Considering the potential role of ASC-1 complex in immunity, proliferation and apoptosis and the reported function of TRIP4 in tumorigenesis2,10,11, we are quite interested in the function of the other 3 subunits ASCC1/2/3 in tumor biology. To comprehensively understand their potential functions, a pan-cancer analysis was performed on the multi-omic data of different cancer cohorts consisting of more than 10,000 samples using different algorithms. Based on these results, the widespread transcriptomic and epigenetic dysregulation, the survival correlation and their role in the dysregulation of infiltrating immune cells in a wide range of cancers were first outlined.
The widespread overexpression of ASCC1/2/3 in various cancers, as we have previously shown, is either the cause of tumorigenesis or the result of tumor progression. If the former is true, then these overexpressed genes and their encoding protein may provide potential targets for antitumor drugs. Given their association with survival in various cancers, they may also serve as a prognostic tool for risk stratification and personalized treatment, although their upregulation may predict varying clinical outcomes in different tumor entities as demonstrated previously. Because only a few studies have reported the function of ASC-1 complex in neuromuscular diseases6, many questions about their function remain to be answered.
Despite the strengths of bioinformatics analyses, several limitations should be acknowledged. Public datasets, such as those from TCGA or GEO, may introduce inherent biases due to sample heterogeneity, batch effects, and uneven representation of clinical subtypes or populations15. Liu et al. investigated the aberrant expression of voltage-gated sodium channels (VGSCs) in cancers, highlighting their potential as diagnostic and therapeutic targets15. By comparing the results generated by TCGA data and previously published studies, they’ve identified inconsistencies between the published studies and TCGA, highlighting the limitations of bioinformatic approaches15.
Nevertheless, these multiomic analysis at the pan-cancer scale is still essential for identifying shared and cancer-type-specific molecular features, enabling a deeper understanding of tumor biology and the discovery of broadly applicable diagnostic and therapeutic targets. Several recent pan-cancer studies have leveraged large-scale genomic data to identify potential diagnostic and therapeutic biomarkers. Genetic analyses of disulfidptosis- and cuproptosis-related gene sets have revealed dysregulation across multiple tumor types, underscoring their potential involvement in cancer-specific cell death mechanisms16,17. Similarly, investigations into copper metabolism genes and mitochondrial DNA repair pathways have highlighted their roles in tumor progression and metabolic vulnerability18,19. Additional studies have pointed to mitotic intra-S DNA damage checkpoint genes and protein kinases involved in phagocytosis as promising biomarker candidates, reflecting the broad utility of functional gene sets in uncovering novel targets for cancer diagnosis and therapy20,21. Focusing solely on highly expressed genes may overlook key contributors to tumorigenesis22. Single-cell and spatial transcriptomics now help resolve cell-specific signals obscured by bulk data, while CRISPR screens and integrative multi-omics approaches improve identification of functionally relevant genes, enhancing the precision of cancer diagnostics and therapies22. While transcriptomic data from resources such as TCGA have advanced cancer research, technical and biological biases, as well as misinterpretation of correlational and mass data, highlight the need for careful analysis to ensure reliable clinical insights23.
Thanks to the deconvolution algorithms, the immune infiltration pattern of the analyzed cancers was depicted using the transcriptomic data to explore the potential regulatory function of ASCC1/2/3 in immunity. Initially, although there is no specific correlation between the expression of ASCC2 and infiltrated T cells, ASCC1/3 was identified to be correlated positively with the infiltrated CD4-positive T cells. CD-positive T cells usually support the antitumor immunity through multiple approaches. They have been reported not only to produce cytokines to stimulate the function of B cells and CD8-positive T cells24, but also to kill cancer cells directly by secreting tumor necrosis factor (TNF)-α, interferon (IFN)-γ, granzyme B, and perforin25. In addition, multiple pathways are also regulated by CD4-positive T cells to induce apoptosis in the tumor microenvironment26,27. These findings seem to contradict the results of Kaplan-Meier analysis, as high expression of ASCC1/2/3 correlates with poor prognosis while associated with a highly infiltrated CD4-positive T cell. Overall, CD4-positive T cells are closely associated with anti-tumor immunity in humans28: not only do they directly enhance the anti-tumor effects of tumor-killing cells29,30,31, but they have also been reported to have a direct killing effect on the tumor itself32,33. The adoptive transfer of CD4-positive T cells has even shown very promising efficacy in tumor patients34. In addition to immune cell interactions, other components of the tumor microenvironment, such as the extracellular matrix, fibroblasts and the microbiome, can also influence immune cell infiltration and efficacy35. Considering the plasticity of immune cells in the tumor microenvironment, the development of new biomarkers based on immune cell infiltration characteristics likewise shows broad promise36. For example, biomarkers and prognostic models developed based on depleted CD8-positive T-cell infiltration characteristics have shown impressive robustness in breast cancer prognosis prediction36.
However, further results helped to explain the contradiction, as infiltrating CD4-positive th1 T cells correlate negatively with the expression of ASCC1/3, while infiltrating CD4-positive th2 T cells correlate positively. Th1 and Th2 cells are two important but distinct types of CD4-positive T cells: the former exert anti-tumour effects by activating tumor effectors and inhibiting angiogenesis, while the latter inhibit the function of dendritic cells and anti-tumor T cells, thus helping tumour cells to evade immune recognition28,32,37. Th2 T cells have been reported to correlate with a poor prognosis in different cancers as their produced cytokines involve in cancer immune escape while detailed mechanism remains to be elucidated38,39,40,41,42,43,44. In our study, ASCC1/3 seems to play a role in the irreversible differentiation of CD4-positive th1 cells to CD4-positive th2 cells, which may explain the poorer prognosis in patients with high expression of ASCC1/3. To validate this hypothesis, future studies could assess Th1 and Th2 cytokine profiles—such as IFN-γ and IL-12 for Th1, and IL-4 and IL-10 for Th2—using ELISA or multiplex cytokine assays28,45. Additionally, flow cytometry or single-cell RNA sequencing could be employed to characterize the functional states of tumor-infiltrating CD4⁺ T cells, providing direct insight into Th1/Th2 polarization within the tumor microenvironment28. These approaches would clarify the immunological mechanisms underlying ASCC3-associated immune modulation and support its potential role as a therapeutic target or prognostic biomarker. While our in vitro findings confirmed by the immunofluorescence staining provide some evidence for the role of ASCC3 in LUAD, these results may not be generalizable to other cancers where ASCC family genes are dysregulated. Given the heterogeneity of ASCC1/2/3 expression and function across cancers, further studies are needed to determine whether similar mechanisms operate in other tumor contexts. Future work involving additional cancer models, such as patient-derived xenograft, or larger cohorts will be essential to validate the broader relevance of our findings46.
Conclusions
In conclusion, the ASC-1 complex, including ASCC1/2/3, played a critical role in both neuromuscular health and cancer progression. Our pan-cancer analysis indicated that these subunits were overexpressed in various cancers, potentially serving as biomarkers for prognosis and as targets for therapy. The ASCC family influences CD4-positive T cell differentiation, particularly the transition from Th1 to Th2 cells, providing new insights into its role in tumor immunity and underscoring the necessity for further research to leverage this knowledge for cancer treatment.
Methods
The genetic landscape of TCGA pan-cancer datasets
To have an overview of the expression of the ASCC family, structural variant, mutation, and copy number alteration (CNA) data were analyzed and therefore summarized in a bar chart by the corporeal for Cancer Genomics platform (https://www.cbioportal.org/)47–49. Datasets of TCGA pan-cancer atlas were picked in the platform for the overview, and ‘Cancer Types Summary’ module was then selected to depict the genetic landscape of ASCC family across 30 different types of cancers. All the pan-cancer datasets available on the cBioportal platform were selected for the analysis of ASCC1/2/3. Additionally, specific mutational sites of each gene were also illustrated by the function in module ‘Mutations’.
Integrative omics analysis of ASCC family
To locate the ASCC proteins in the scale of cancer tissues and cells, the Human Protein Atlas (HPA, https://www.proteinatlas.org/humanproteome/pathology) was applied to visualize the distribution50,51,52,53,54,55,56,57. Normalized expression (NX) ≥ 1 was chosen as the cutoff value to define ‘low specificity’.
The cohorts with primary tumor and paired normal tissue in TCGA database were analyzed by TIMER platform (http://timer.cistrome.org/) to visualize the expression of ASCC1/2/314[,58[,59. The remaining types of tumors were then analyzed by GEPIA platform (http://gepia2.cancer-pku.cn/) to have a comprehensive insight across cancers with different transcriptomic and biological background60. Expression of ASCC1/2/3 in these cases was therefore compared among different pathological stages by the function ‘Expression DIY’ in the GEPIA platform.
The pan-cancer proteomic data was also analyzed by the University of ALabama at Birmingham Cancer data analysis Portal (UALCAN, https://ualcan.path.uab.edu/analysis-prot.html)61[,62. Proteomic data across 14 types of solid tumors were obtained from Clinical Proteomic Tumor Analysis Consortium (CPTAC) and the International Cancer Proteogenome Consortium (ICPC) datasets. Cohorts with primary tumors and matched normal tissue were selected for our study. Additionally, the phosphoprotein of ASCC1/2/3 was also compared between normal and tumor tissue in available cohorts.
Epigenetic data of the TCGA pan-cancer cohorts was obtained and then visualized by the MEXPRESS platform (https://mexpress.ugent.be/index.html)6364. Pearson correlation coefficient, t-test, ANOVA, and chi-square test were applied and calculated for the statistical analysis among numeric and categorical variables including transcriptomic and DNA methylation data, tumor stage, gender, and other clinical information. P values used for the statistical test are Benjamini-Hochberg-adjusted p values. They were adjusted for multiple hypothesis testing.
Survival analysis
By using the GEPIA platform, follow-up data of the TCGA pan-cancer cohorts was analyzed. Overall survival (OS) and Disease-free survival (DFS) was applied as two parameters to assess the prognosis of the patients. The median value of the expression level of each gene was used as the cut-off value to divide the patients into high and low expression group. Kaplan-Meier curves and parameters including hazard ratio and log-ranked p value were generated by the function ‘survival analysis’ of GEPIA platform to visualize and assess the correlation between the transcriptome and patients’ prognosis.
Protein-protein interaction analysis and gene annotation
Known and predicted interactions of ASCC1/2/3 related proteins were initially analyzed and downloaded from the STRING database (https://string-db.org/)47. Bioinformatic prediction, high-throughput sequencing, co-expression experiments, published results and background knowledge from databases were used to predict physical and functional interactions between proteins. In total, top 50 ASCC1/2/3-interacted proteins were included for further visualization. Primarily handled data was then reorganized and visualized by Cytoscape platform65,66. ASCC1/2/3 related proteins were reordered and the protein-protein interaction network was built based on their incidence of interaction by the ‘Cytohubba’ ’plugin67. Based on the transcriptomic expression, top correlated genes in the TCGA pan-cancer cohort were obtained by the function ‘Similar Genes Detection’ function of GEPIA platform. 5 correlated genes were therefore identified and depicted in scatter plot. P value and Peason correlation coefficient was calculated simultaneously to assess the correlation between these genes and ASCC1/2/3.
To annotated the related genes, Gene enrichment analysis was performed in R software by the package ‘clusterProfiler’68,69. Kyoto Encyclopedia of Genes and Genomes (KEGG)70 and Gene Ontology Resource (GO)13,71 were applied as reference information for the gene annotation. Benjamini-Hochberg-adjusted p value was used for the multiple hypothesis testing. Annotations with a p value < 0.05 were selected for the visualization.
Estimation of immune cells infiltration
The infiltration level of immune cells was predicted for expression profile of the TCGA pan-cancer cohorts by TIMER, CIBERSORT, quanTIseq, xCell, MCP-counter and EPIC algorithms separately58,72,73,74,75,76,77. The correlation between the estimated immune infiltration and transcriptomic expression of ASCC 1/2/3 was calculated and visualized as heatmaps using TIMER platform. Our interested immune cells were further explored by the function ‘immune’ of the platform. Additionally, the correlation between immune checkpoint inhibitor-related genes and ASCC1/2/3 in the TCGA cohorts was also analyzed by the web tool Sangerbox (http://sangerbox.com/Tool). MSI information of the TCGA pan-cancer cohorts were also analyzed by Sangerbox. Pearson correlation coefficient was applied to assess the correlation between MSI and the expression level of ASCC1/2/378.
Experimental methods
Multiple fluorescence staining of Pan-Cancer tissue chip
The paraffin-embedded sections used in this study were prepared and provided by the Department of Pathology at Xiangya Hospital. Multiple fluorescence staining was performed on paraffin sections of lung adenocarcinoma and adjacent tissue to verify the ability of ASCC1/2/3 as a tumor biomarker and to explore their association with T cell infiltration. The sections were dewaxed and sealed with 5% bovine serum albumin (BSA). Subsequently, they were incubated sequentially with the primary antibodies, ASCC1(human,1:30, Invitrogen PA5-50355), ASCC2(human,1:150, Proteintech 11529-1-AP), ASCC3(human,1:150, Novus NBP1-88854), CD4(human, 1:500, Abcam ab133616), CD8(human, 1:200, CST 85336 T). After primary antibody labeling, the sections were incubated by secondary antibody (RecordBio, RC0086Plus-45RM). Next, 4′,6- diamidino-2-phenylindole dihydrochloride (DAPI) counterstaining of nuclei, the antifade mounting medium was applied, and an Automatic Multi-label Multi-spectral Quantitative Pathological Imaging System (AKOYA BIOSCIENCES, VECTRA POLARIS) was used to obtain multispectral images of the stained sections. For fluorescence spectra, the excitation wavelength and emission wavelength for different fluorescence dyes are listed respectively as follows: DAPI (Blue, 330–380 and 420 nm), ASCC1/2/3 (Yellow, 550 and 570 nm), and CD8 (Cyan, 590 and 620 nm), CD4 (Brown, 630 and 690 nm). Finally, Phenochart (C.V 1.0) image analysis software was used to display the cells with positive staining at single-cell levels in the multispectral images.
Cell lines and culture
Human lung adenocarcinoma cell lines (A549 and H1975) were purchased from the American Type Culture Collection (ATCC). Cells were maintained in DMEM containing 10% fetal bovine serum-premium (NEWZERUM Ltd.) and 1% penicillin/streptomycin (Invitrogen, USA) in a humidified incubator at 37 °C with 5% CO2.
Cell transfection
The cells were cultured in 6-well plates until the cell confluence reached 60%−70%, and siRNA transfection was performed using the RNA transfection reagent RNATransMate (Sangon Biotech E607402) (the specific procedure should be carried out according to the reagent instructions). The knockdown efficiency of siRNA was measured 48 hours after transfection. The siRNAs used in this study were all designed and produced by Sangon Biotech (Shanghai) Co., Ltd. The siRNA sequences of negative control were (sense) 5’-UUCUCCGAACGUGUCACGUTT-3’ and (antisense) 5’-ACGUGACACGUUCGGAGAATT-3’. The siRNA sequences of human ASCC1 were (sense) 5’-GCUUCAUCUAACUAUUGGGAUTT-3’ and (antisense) 5’-AUCCCAAUAGUUAGAUGAAGCTT-3’. The siRNA sequences of human ASCC2 were (sense) 5’-CGAUGACACAUACGAUGGCAATT-3’ and (antisense) 5’-UUGCCAUCGUAUGUGUCAUCGTT-3’. The siRNA sequences of human ASCC3 were (sense) 5’-CCCAGUAGAAUCAAGUUUAUUTT-3’ and (antisense) 5’-AAUAAACUUGAUUCUACUGGGTT-3’.
RNA isolation and qRT-PCR
Total RNA was extracted from transfected cells with TRIzol (Thermo Fisher Scientific) according to the provided directions. Gene expression relative to RNA was measured by qRT-PCR using an IQ5 Multicolour Real-Time PCR Detection system (Bio-Rad Laboratories, CA, USA). PCR detection included reaction at 95 °C (5 min), 95 °C (30 s) for 40 cycles, 56.5 °C (5 s), and 72 °C (30 s). The sequences of human ASCC1 primer were (forward) 5’-AGAATTGATGGCCGGAATTACAG-3’ and (reverse) 5’-TGGAGCCTTGATAGAAGTCCTC-3’. The sequences of human ASCC2 primer were (forward) 5’-GTTCCTCCGGGACTATGATGC-3’ and (reverse) 5’-GCGTCTCGTCCAAGACTGATG-3’. The sequences of human ASCC3 primer were (forward) 5’-ATTTGGACCACTTTGACGAACT-3’ and (reverse) 5’-TCTACTCTTGGCACTTCAGTCAT-3’.
Western blotting
Proteins were extracted from cells in RIPA buffer with a protease and phosphatase inhibitor cocktail (Abiowell, Changsha, China). After determination of concentrations using BCA, the proteins were separated on SDS-PAGE and transferred to nitrocellulose. The blots were treated with anti-ASCC1 (1:2000) (TA503721, Origene, China), anti-ASCC2 (1:500) (11529-1-AP, Proteintech, China), anti-ASCC3 (1:400) (17627-1-AP, Proteintech, China) and anti-GAPDH (1:5000) (ab181602, Abcam, UK) antibodies, the bands were visualized using enhanced chemiluminescence(AMERSHAM ImageQuant 800) and Adobe Photoshop 2021 software was used for image processing and assembly. The loading control was GAPDH.
Cell proliferation assays
CCK-8 assays were performed by seeding and maintaining 2000 cells in 96-well plates for 1, 2 or 3 days. Fresh medium was replaced with 100µL, and CCK-8 (10µL, Sigma) was added. Cells were then incubated for 2 h for OD 450 measurement. In colony formation assays, 700 cells were seeded and cultured for 12 days in 6-well plates. Next, they were washed with PBS and fixed with 4% paraformaldehyde for 30 min. Cells were stained with 0.5% crystal violet for 0.5 h. at room temperature, and colonies were counted with clone-counter program.
Wound-healing assay
106 cells were seeded per well in a six-well plate after being trypsinized. Micropipettes were scraped with sterile tips after overnight incubation. The width of the gap was photographed at 0-, 12-, 36-, and 48-hours post-scratching.
Transwell assays
Migration assays were carried out in Transwell chambers 8 mm in diameter, Corning. Suspensions of lung adenocarcinoma cells (A549 or H1975) in 200 µl serum-free DMEM medium were added to the upper chamber, and 20% fetal bovine serum was added to the bottom chamber. After 24 h of culture, cells in the chamber were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet. We viewed cells under an inverted light microscope in three random fields of view that had migrated or invaded to the lower surface.
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- ACC:
-
Adrenocortical carcinoma
- ATCC:
-
American Type Culture Collection
- BLCA:
-
Bladder urothelial carcinoma
- BRCA:
-
Breast invasive carcinoma
- BSA:
-
Bovine serum albumin
- CESC:
-
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL:
-
Cholangiocarcinoma
- CNA:
-
Copy number alteration
- COAD:
-
Colon adenocarcinoma
- CPTAC:
-
Clinical Proteomic Tumor Analysis Consortium
- DAPI:
-
4′,6- diamidino-2-phenylindole dihydrochloride
- DFS:
-
Disease-free survival
- DLBC:
-
Diffuse large B-cell lymphoma
- ESCA:
-
Esophageal carcinoma
- GBM:
-
Glioblastoma multiforme
- GO:
-
Gene Ontology Resource
- HNSC:
-
Head and neck squamous cell carcinoma
- ICPC:
-
The International Cancer Proteogenome Consortium
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRP:
-
Kidney renal papillary cell carcinoma
- LGG:
-
Low-grade glioma
- LIHC:
-
Liver hepatocellular carcinoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- MESO:
-
Mesothelioma
- NAT:
-
Normal tissue adjacent to the tumor
- NRs:
-
Nuclear receptors
- NX:
-
Normalized expression
- OS:
-
Overall survival
- OV:
-
Ovarian serous cystadenocarcinoma
- PAAD:
-
Pancreatic adenocarcinoma
- PCPG:
-
Pheochromocytoma and paraganglioma
- PRAD:
-
Prostate adenocarcinoma
- READ:
-
Rectum adenocarcinoma
- SARC:
-
Sarcoma
- SKCM:
-
Skin cutaneous melanoma
- STAD:
-
Stomach adenocarcinoma
- TCGA:
-
The Cancer Genome Atlas
- TFs:
-
Transcription factors
- TGCT:
-
Testicular germ cell tumors
- THCA:
-
Thyroid carcinoma
- THYM:
-
Thymoma
- UCEC:
-
Uterine corpus endometrial carcinoma
- UCS:
-
Uterine carcinosarcoma
- UVM:
-
Thymoma and uveal melanoma
References
Dasgupta, S., Lonard, D. M. & O’Malley, B. W. Nuclear receptor coactivators: master regulators of human health and disease. Annu. Rev. Med. 65, 279–292. https://doi.org/10.1146/annurev-med-051812-145316 (2014).
Jung, D. J. et al. Novel transcription coactivator complex containing activating signal cointegrator 1. Mol. Cell. Biol. 22, 5203–5211. https://doi.org/10.1128/mcb.22.14.5203-5211.2002 (2002).
Dango, S. et al. DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol. Cell. 44, 373–384. https://doi.org/10.1016/j.molcel.2011.08.039 (2011).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49. https://doi.org/10.3322/caac.21820 (2024).
Sonkin, D., Thomas, A. & Teicher, B. A. Cancer treatments: past, present, and future. Cancer Genet. 286–287, 18–24. https://doi.org/10.1016/j.cancergen.2024.06.002 (2024).
Chi, B. et al. The neurodegenerative diseases ALS and SMA are linked at the molecular level via the ASC-1 complex. Nucleic Acids Res. 46, 11939–11951. https://doi.org/10.1093/nar/gky1093 (2018).
Oliveira, J., Martins, M., Pinto Leite, R., Sousa, M. & Santos, R. The new neuromuscular disease related with defects in the ASC-1 complex: report of a second case confirms ASCC1 involvement. Clin. Genet. 92, 434–439. https://doi.org/10.1111/cge.12997 (2017).
Knierim, E. et al. Mutations in subunits of the activating signal cointegrator 1 complex are associated with prenatal spinal muscular atrophy and congenital bone fractures. Am. J. Hum. Genet. 98, 473–489. https://doi.org/10.1016/j.ajhg.2016.01.006 (2016).
Villar-Quiles, R. N. et al. ASC-1 is a cell cycle regulator associated with severe and mild forms of myopathy. Ann. Neurol. 87, 217–232. https://doi.org/10.1002/ana.25660 (2020).
Che, Y. et al. TRIP4 promotes tumor growth and metastasis and regulates radiosensitivity of cervical cancer by activating MAPK, PI3K/AKT, and hTERT signaling. Cancer Lett. 452, 1–13. https://doi.org/10.1016/j.canlet.2019.03.017 (2019).
Hao, J. et al. The Tumor-Promoting role of TRIP4 in melanoma progression and its involvement in response to BRAF-Targeted therapy. J. Invest. Dermatol. 138, 159–170. https://doi.org/10.1016/j.jid.2017.07.850 (2018).
Orloff, M. et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. Jama 306, 410–419. https://doi.org/10.1001/jama.2011.1029 (2011).
Aleksander, S. A. et al. The Gene Ontology knowledgebase in 2023. Genetics 224, https://doi.org/10.1093/genetics/iyad031 (2023).
Li, T. et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 48, W509–w514. https://doi.org/10.1093/nar/gkaa407 (2020).
Liu, H., Weng, J., Huang, C. L. & Jackson, A. P. Voltage-gated sodium channels in cancers. Biomark. Res. 12, 70. https://doi.org/10.1186/s40364-024-00620-x (2024).
Liu, H. & Tang, T. Pan-cancer genetic analysis of disulfidptosis-related gene set. Cancer Genet. 278–279, 91–103. https://doi.org/10.1016/j.cancergen.2023.10.001 (2023).
Liu, H. & Tang, T. Pan-cancer genetic analysis of Cuproptosis and copper metabolism-related gene set. Front. Oncol. 12, 952290. https://doi.org/10.3389/fonc.2022.952290 (2022).
Liu, H. Pan-cancer profiles of the Cuproptosis gene set. Am. J. Cancer Res. 12, 4074–4081 (2022).
Dong, A., Rasteh, A. M. & Liu, H. Pan-Cancer Genetic Analysis of Mitochondrial DNA Repair Gene Set. bioRxiv 2024.2009.2014.613048, https://doi.org/10.1101/2024.09.14.613048 (2024).
Agarwal, K. & Liu, H. Potential Cancer Biomarkers: Mitotic Intra-S DNA Damage Checkpoint Genes. bioRxiv 2024.2009.2019.613851, https://doi.org/10.1101/2024.09.19.613851 (2024).
Arumilli, S. & Liu, H. Protein Kinases in Phagocytosis: Promising Genetic Biomarkers for Cancer.. bioRxiv (2024).
Liu, H., Guo, Z. & Wang, P. Genetic expression in cancer research: challenges and complexity. Gene Rep. 37, 102042. https://doi.org/10.1016/j.genrep.2024.102042 (2024).
Liu, H., Li, Y., Karsidag, M., Tu, T. & Wang, P. Technical and biological biases in bulk transcriptomic data mining for Cancer research. J. Cancer. 16, 34–43. https://doi.org/10.7150/jca.100922 (2025).
Kennedy, R. & Celis, E. Multiple roles for CD4 + T cells in anti-tumor immune responses. Immunol. Rev. 222, 129–144. https://doi.org/10.1111/j.1600-065X.2008.00616.x (2008).
Borst, J., Ahrends, T., Bąbała, N., Melief, C. J. M. & Kastenmüller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647. https://doi.org/10.1038/s41577-018-0044-0 (2018).
Schattner, E. J. et al. CD4 + T-cell induction of Fas-mediated apoptosis in Burkitt’s lymphoma B cells. Blood 88, 1375–1382 (1996).
Thomas, W. D. & Hersey, P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J. Immunol. 161, 2195–2200 (1998).
Miggelbrink, A. M. et al. CD4 T-Cell exhaustion: does it exist and what are its roles in cancer?? Clin. Cancer Res. 27, 5742–5752. https://doi.org/10.1158/1078-0432.Ccr-21-0206 (2021).
Ahrends, T. et al. CD4(+) T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 47, 848–861e845. https://doi.org/10.1016/j.immuni.2017.10.009 (2017).
Busselaar, J., Tian, S., van Eenennaam, H. & Borst, J. Helpless priming sends CD8(+) T cells on the road to exhaustion. Front. Immunol. 11, 592569. https://doi.org/10.3389/fimmu.2020.592569 (2020).
Tay, R. E., Richardson, E. K. & Toh, H. C. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 28, 5–17. https://doi.org/10.1038/s41417-020-0183-x (2021).
Quezada, S. A. et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207, 637–650. https://doi.org/10.1084/jem.20091918 (2010).
Takeuchi, A. et al. CRTAM determines the CD4 + cytotoxic T lymphocyte lineage. J. Exp. Med. 213, 123–138. https://doi.org/10.1084/jem.20150519 (2016).
Perez-Diez, A. et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 109, 5346–5354. https://doi.org/10.1182/blood-2006-10-051318 (2007).
Maiorino, L., Daßler-Plenker, J., Sun, L. & Egeblad, M. Innate immunity and Cancer pathophysiology. Annu. Rev. Pathol. 17, 425–457. https://doi.org/10.1146/annurev-pathmechdis-032221-115501 (2022).
Liu, H., Dong, A., Rasteh, A. M., Wang, P. & Weng, J. Identification of the novel exhausted T cell CD8 + markers in breast cancer. Sci. Rep. 14, 19142. https://doi.org/10.1038/s41598-024-70184-1 (2024).
Xie, Y. et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 207, 651–667. https://doi.org/10.1084/jem.20091921 (2010).
De Monte, L. et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 208, 469–478. https://doi.org/10.1084/jem.20101876 (2011).
Ubukata, H. et al. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J. Surg. Oncol. 102, 742–747. https://doi.org/10.1002/jso.21725 (2010).
Kusuda, T. et al. Relative expression levels of Th1 and Th2 cytokine mRNA are independent prognostic factors in patients with ovarian cancer. Oncol. Rep. 13, 1153–1158 (2005).
Sheu, B. C. et al. Predominant Th2/Tc2 Polarity of tumor-infiltrating lymphocytes in human cervical cancer. J. Immunol. 167, 2972–2978. https://doi.org/10.4049/jimmunol.167.5.2972 (2001).
Nevala, W. K. et al. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin. Cancer Res. 15, 1931–1939. https://doi.org/10.1158/1078-0432.Ccr-08-1980 (2009).
Burkholder, B. et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta. 1845, 182–201. https://doi.org/10.1016/j.bbcan.2014.01.004 (2014).
Andreu-Sanz, D. & Kobold, S. Role and Potential of Different T Helper Cell Subsets in Adoptive Cell Therapy. Cancers (Basel) 15. https://doi.org/10.3390/cancers15061650 (2023).
McCaw, T. R. et al. The expression of MHC class II molecules on murine breast tumors delays T-cell exhaustion, expands the T-cell repertoire, and slows tumor growth. Cancer Immunol. Immunother. 68, 175–188. https://doi.org/10.1007/s00262-018-2262-5 (2019).
Li, R., Huang, Y., Liu, H., Dilger, J. P. & Lin, J. Abstract 2162: comparing volatile and intravenous anesthetics in a mouse model of breast cancer metastasis. Cancer Res. 78, 2162–2162. https://doi.org/10.1158/1538-7445.Am2018-2162 (2018).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404. https://doi.org/10.1158/2159-8290.Cd-12-0095 (2012).
de Bruijn, I. et al. Analysis and visualization of longitudinal genomic and clinical data from the AACR project GENIE biopharma collaborative in cBioPortal. Cancer Res. 83, 3861–3867. https://doi.org/10.1158/0008-5472.Can-23-0816 (2023).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6(pl1), https://doi.org/10.1126/scisignal.2004088 (2013).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7, https://doi.org/10.1126/sciadv.abh2169 (2021).
Sjöstedt, E. et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367, https://doi.org/10.1126/science.aay5947 (2020).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, https://doi.org/10.1126/science.aal3321 (2017).
Uhlén, M. et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteom. 4, 1920–1932. https://doi.org/10.1074/mcp.M500279-MCP200 (2005).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419. https://doi.org/10.1126/science.1260419 (2015).
Uhlén, M. et al. The human secretome. Sci. Signal. 12, https://doi.org/10.1126/scisignal.aaz0274 (2019).
Uhlen, M. et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 366, https://doi.org/10.1126/science.aax9198 (2019).
Uhlen, M. et al. A pathology atlas of the human cancer transcriptome. Science 357, https://doi.org/10.1126/science.aan2507 (2017).
Li, B. et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 17, 174. https://doi.org/10.1186/s13059-016-1028-7 (2016).
Li, T. et al. A web server for comprehensive analysis of Tumor-Infiltrating immune cells. Cancer Res. 77, e108–e110. https://doi.org/10.1158/0008-5472.Can-17-0307 (2017).
Tang, Z., Kang, B., Li, C., Chen, T. & Zhang, Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–w560. https://doi.org/10.1093/nar/gkz430 (2019).
Chandrashekar, D. S. et al. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19, 649–658. https://doi.org/10.1016/j.neo.2017.05.002 (2017).
Chandrashekar, D. S. et al. An update to the integrated cancer data analysis platform. Neoplasia 25. UALCAN, 18–27. https://doi.org/10.1016/j.neo.2022.01.001 (2022).
Koch, A., De Meyer, T., Jeschke, J. & Van Criekinge, W. MEXPRESS: visualizing expression, DNA methylation and clinical TCGA data. BMC Genom. 16, 636. https://doi.org/10.1186/s12864-015-1847-z (2015).
Koch, A., Jeschke, J., Van Criekinge, W., van Engeland, M. & De Meyer, T. MEXPRESS update 2019. Nucleic Acids Res. 47, W561–W565. https://doi.org/10.1093/nar/gkz445 (2019).
Cline, M. S. et al. Integration of biological networks and gene expression data using cytoscape. Nat. Protoc. 2, 2366–2382. https://doi.org/10.1038/nprot.2007.324 (2007).
Otasek, D., Morris, J. H., Bouças, J., Pico, A. R. & Demchak, B. Cytoscape automation: empowering workflow-based network analysis. Genome Biol. 20, 185. https://doi.org/10.1186/s13059-019-1758-4 (2019).
Chin, C. H. et al. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8(Suppl 4), https://doi.org/10.1186/1752-0509-8-s4-s11 (2014).
Wu, T. et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. (Camb). 2, 100141. https://doi.org/10.1016/j.xinn.2021.100141 (2021).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16, 284–287. https://doi.org/10.1089/omi.2011.0118 (2012).
Jin, Z., Sato, Y., Kawashima, M. & Kanehisa, M. KEGG tools for classification and analysis of viral proteins. Protein Sci. 32, e4820. https://doi.org/10.1002/pro.4820 (2023).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat. Genet. 25, 25–29. https://doi.org/10.1038/75556 (2000).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220. https://doi.org/10.1186/s13059-017-1349-1 (2017).
Becht, E. et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 17, 218. https://doi.org/10.1186/s13059-016-1070-5 (2016).
Finotello, F. et al. Molecular and Pharmacological modulators of the tumor immune contexture revealed by Deconvolution of RNA-seq data. Genome Med. 11, 34. https://doi.org/10.1186/s13073-019-0638-6 (2019).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 12, 453–457. https://doi.org/10.1038/nmeth.3337 (2015).
Racle, J., de Jonge, K., Baumgaertner, P., Speiser, D. E. & Gfeller, D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. eLife (2017).
Sturm, G. et al. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics (2019).
Bonneville, R. et al. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis Oncol. 2017, https://doi.org/10.1200/po.17.00073 (2017).
Acknowledgements
The results shown here are in part based upon data generated by TCGA database, and HPA database, the STRING database, CPTAC database and the ICPC datasets.
Funding
This study was supported by one grant from the National Key Research and Development Program of China (No. 2022YFE0133400), three grants from National Natural Science Foundation of China (No. 82203397, No. 82303253 and No. 82172834), two grants from the Nature Science Foundation of Hunan Province (No. 2022 JJ40814 and No. 2024 JJ6686), one grant from the Nature Science Foundation of Changsha (No. kq2202375), one grant from the Youth Foundation of Xiangya Hospital (No. 2021Q06), two grants from the China Postdoctoral Science Foundation (No. 2022M723560 and No. 2023M733960).
Author information
Authors and Affiliations
Contributions
Y.P., F.L., Q.L.: conception and design; Y.P., C.W.: bioinformatics data analysis; J.T., C.L., F.L.: other Data analysis and interpretation; Z.C., Y.Z.: Collection and assembly of data; Y.P., F.L., Q.L.: Manuscript writing and revisions. Final approval of manuscript: All authors. Accountable for all aspects of work: All authors. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Clinical Medical Ethics Committee of Xiangya Hospital, Central South University. Ethics Lot number: 202303046. The tissue sections for immunohistochemistry were obtained from the Department of Pathology. No other privacy of the patient is involved, and informed consent of the patient is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
41598_2025_3946_MOESM8_ESM.pdf
Supplementary Material 8 Supplementary Fig.S5 The proteins expression level of ASCC1 in different tumors and paired normal tissues.** p < 0.01;*** p < 0.001
41598_2025_3946_MOESM9_ESM.pdf
Supplementary Material 9 Supplementary Fig.S6 The proteins expression level of ASCC2 in different tumors and paired normal tissues. *** p < 0.001
41598_2025_3946_MOESM10_ESM.pdf
Supplementary Material 10 Supplementary Fig.S7 The proteins expression level of ASCC3 in different tumors and paired normal tissues. ** p < 0.01;*** p < 0.001
41598_2025_3946_MOESM11_ESM.pdf
Supplementary Material 11 Supplementary Fig.S8 Association between ASCC2 and immune infiltration of T cells. Significant correlations are shown in color, while non-significant correlations are marked with a cross
41598_2025_3946_MOESM12_ESM.pdf
Supplementary Material 12 Supplementary Fig.S9 Original Western blots images of the A549 and H1975 cell lines knocking down ASCC1/2/3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, Y., Tan, J., Wu, C. et al. Pan-cancer analysis reveals ASCC family promotes the cancer progression of lung adenocarcinoma. Sci Rep 15, 22799 (2025). https://doi.org/10.1038/s41598-025-03946-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03946-0