Abstract
Insulin resistance (IR) links hypertension (HTN) and coronary heart disease (CHD). This study investigated the link between the triglyceride-glucose (TyG) index, a marker for detecting IR, and the prevalence and severity of CHD in patients with HTN. Limited evidence exists on this relationship. This cross-sectional study included 1,432 hospitalized patients with HTN who underwent coronary angiography between January 1, 2019, and December 31, 2023. The study cohort was predominantly male, with a median age of 65 years. Common comorbidities included type 2 diabetes mellitus, smoking history, and alcohol consumption. Patients were excluded if they lacked complete clinical data, had a history of coronary artery bypass grafting (CABG) or other severe heart diseases, or had malignant tumors, acute or chronic infections, severe cerebrovascular disease, end-stage renal disease, or severe hepatic dysfunction. Multivariate logistic regression analysis was used to assess the association between the TyG index and the prevalence of CHD. The TyG index was categorized into tertiles and also treated as a continuous variable in the analysis. Among HTN-CHD groups, the relationship between the Gensini score (GS), an indicator of CHD severity, and the TyG index was assessed using multivariate linear regression analysis. After adjusting for confounding variables, multivariate logistic regression analysis indicated that compared to tertile 1 of the TyG index, the odds ratio for the prevalence of CHD in patients with TyG index in tertile 2 was 2.01 (95% CI, 1.47–2.73; p < 0.001), and in tertile 3 was 3.86 (95% CI, 2.71–5.50; p < 0.001). Additionally, a raised positive correlation was shown by multivariate linear regression analysis between the GS and the TyG index. In the HTN-CHD group, each 1-unit increase in the TyG index was associated with a 16.777 increase in the GS (β = 16.777; 95% CI, 13.81–19.74; p < 0.001).
Similar content being viewed by others
Introduction
Each year, hypertension (HTN) accounts for over 10 million deaths globally, with China experiencing a particularly high prevalence. The incidence of HTN in China has increased dramatically from 5.1% in 1958 to 27.9% in 20151. HTN and coronary heart disease (CHD) often coexist, serving as key risks for the process of atherosclerosis. Studies on the Chinese population indicate that over 60% of patients with stable CHD also have HTN2. A study revealed that high blood pressure is a major contributor to cardiovascular diseases (CVDs), including CHD. Hypertensive individuals have approximately twice the risk of developing CVDs compared to those with normal blood pressure levels3. The incidence and mortality rates of CHD are increasing, posing a serious public health problem globally4.
Insulin resistance (IR) and HTN, both components of metabolic syndrome, are linked through common pathophysiological mechanisms, including endothelial dysfunction, oxidative stress, and chronic inflammation. These factors significantly influence the onset and progression of CHD5,6,7. A prospective study indicated that lipoprotein insulin resistance, a novel biomarker of IR, is associated with a 600% increase in the risk of early-onset CHD8.
HTN and CHD are major global health concerns that often coexist and exacerbate each other. IR is a key mechanism linking HTN and CHD, with the TyG index emerging as a reliable IR marker. While the TyG index has been associated with metabolic and CVDs, its relationship with CHD prevalence and severity in hypertensive patients remains unclear.
There is growing evidence that the triglyceride-glucose (TyG) index and the insulin-glucose clamp technique, regarded as the most reliable method for diagnosing IR, have a strong association9. Although both TyG and HOMA-IR are indicators of IR, TyG is preferred due to its simplicity and cost-effectiveness. In contrast, the calculation of HOMA-IR requires fasting insulin levels, which are more expensive and may not be readily available in many clinical settings, particularly in resource-limited environments.
This study investigates the association between the TyG index and CHD in hypertensive patients. It examines the link between the TyG index and CHD prevalence, explores its correlation with CHD severity, assessed by the Gensini score, and evaluates its potential as a biomarker for CHD risk. This study explores the hypothesis that a higher TyG index is associated with increased CHD prevalence and severity, as reflected by higher Gensini scores.
Methods
Ethical approval
All experiments and methods were performed in accordance with the Declaration of Helsinki. This research approved by the Ethics Committee of Drum Tower Hospital Affiliated to School of Medicine of Nanjing University. Due to the retrospective nature of the study, the Nanjing Drum Tower Hospital’s ethical committee waived the need of obtaining the informed consent. The approval number was 2024-113-01.
Study population
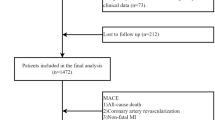
1432 hypertensive patients who had underwent coronary angiography at Nanjing Drum Tower Hospital between January 1, 2019, and December 31, 2023 were included in this cross-sectional research (Fig. 1). The sample size was sufficient in accordance with a power analysis calculated by G*power; The power was 100% with effect size 0.25 and a 5% alpha margin error.
Of the 1972 patients, 540 were excluded for meeting the exclusion criteria (Table 1), i.e. (1) lack of data at admission, no imputation methods were applied to missing data. (2) a previous history of coronary artery bypass grafting or other heart disease such as rheumatic heart disease, valvular heart disease, severe congenital heart disease, cardiomyopathy, etc. (3) malignant tumors, acute or chronic infectious diseases, or serious cerebrovascular accidents. (4) severe kidney disease. (5) severe hepatic dysfunction, characterized by transaminase levels exceeding three times the upper threshold of normal.
Clinical information
Through the assessment of electronic medical records, general clinical information about the patients was gathered, including their sex, age, height, weight, diastolic (DBP) and systolic blood pressures (SBP), diabetes mellitus (DM), history of smoking, and history of drinking.
All patients had their body mass index (BMI) (weight [kg]/height squared [m2]) calculated. Individuals with fasting blood glucose levels of ≥ 126 mg/dL (7.0 mmol/L) or those utilizing insulin injections or oral anti-diabetic medications were classified as having DM. The diagnosis of hypertension was determined via repeated blood pressure measures (at least twice in various contexts) with a SBP of at least 140 mmHg and/or a DBP of at least 90 mmHg, or with the use of antihypertensive medications.
The morning following admission, 3 mL of fasting cubital vein blood was drawn and put in an anticoagulant tube for analysis. Using an enzyme-linked immunosorbent test, biochemical markers such as ALT, AST, Scr, TC, TG, HDL-C, LDL-C, HbA1c, FPG, eGFR, and CRP were assessed. The formula ln [TG (mg/dL) × FBG (mg/dL)/2] was used to calculate the TyG index.
Angiographic examinations
All the patients included underwent selective invasive coronary angiography. Based on the Gensini Score (GS)10, the severity of CHD was evaluated. Two interventional cardiologists who were blind to the clinical and laboratory data examined the coronary angiography images and calculated the GS. If there was a disagreement, the coronary angiograms were assessed and the GS was determined by a third interventional cardiologist who was not aware of the nature of the study or the laboratory data.
Statistical analysis
All data were analyzed using IBM SPSS Statistics version 26.0 (Chicago, IL, USA) and GraphPad Prism 8.0(San Diego, California, USA). The TyG index was categorized into tertiles and also treated as a continuous variable in the analysis. Categorical variables were expressed as number (percentage). The normality of the distribution of continuous variables was assessed using the Shapiro-Wilks test. Data following a normal distribution are presented as mean ± standard deviation, whereas non-normally distributed data are expressed as median with interquartile range. One-way analysis of variance or the Kruskal–Wallis test was employed to compare baseline variables across TyG index tertiles, as appropriate. Categorical variables among groups were compared using the chi-square test. Univariate and multivariate logistic regression analyses were performed to assess the association between TyG levels and the prevalence of CHD. To evaluate multicollinearity, the variance inflation factor (VIF) was computed for each variable. Variables with a VIF value exceeding 10 were considered to exhibit substantial multicollinearity and were excluded from the multivariate regression model. The discriminative capacity of the TyG index for CHD evaluated using receiver operating characteristic curve (ROC) analysis and area under the curve (AUC) values. Additionally, correlation and multiple linear regression analyses were performed to assess the link between GS and TyG in patients with both HTN and CHD.
Results
Clinical and biochemical characteristics by TyG index
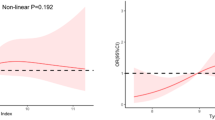
The average TyG index in the TyG tertiles was 7.4(7.2–7.6), 8.1(7.9–8.2), and 8.8(8.7–9.2). Age, BMI, DM, ALT, AST, TC, TG, HDL-C, LDL-C, HbA1c, FPG, CRP, TyG, and CHD rates were shown to differ statistically significantly across the three groups. (all p < 0.05) (Table 2). Restricted cubic splines (RCS) with 3 knots were applied to model the TyG index as a continuous variable. The P-values for testing the nonlinear relationship between the TyG index and CHD risk were all greater than 0.05(Supplementary Figure S1), indicating no significant nonlinear association. However, an elevated TyG index was significantly associated with an increased risk of CHD (p < 0.001) (Supplementary Figure S2).
Uni‑ and multivariate logistic analyses of CHD by TyG group
The association between the TyG index and the prevalence of CHD was examined using logistic regression analysis. The potential confounding variables considered in this study included age, sex, hypertension, diabetes mellitus, smoking history, drinking history, ALT, AST, TC, HDL-C, LDL-C, HbA1c, and CRP. No multicollinearity was detected among the indicators, as determined by the Variance Inflation Factor (VIF) analysis. The results revealed that the adjusted odds ratio for the prevalence of CHD in patients with TyG index in tertile 2 was 2.01 (95% CI, 1.47–2.73; p < 0.001), and in tertile 3 was 3.86 (95% CI, 2.71–5.50; p < 0.001) (Table 3).
ROC analyses of TyG index
Table 3 provides a demonstration of the TyG index ROC analysis results. At a 95% confidence interval of 0.687 to 0.740, the AUC was 0.714. As far as differentiating individuals with and without CHD goes, the TyG index has a rather good AUC value. (Fig. 2; Table 4). To evaluate whether TyG could enhance the discriminatory ability of the traditional risk model (Model1), we compared model performance with and without TyG inclusion. The addition of TyG significantly improved discriminability for CHD (AUC: 0.677 vs. 0.769, P < 0.001) (Supplementary Fig S3). Moreover, integrating TyG into Model1 substantially enhanced both risk reclassification and overall discriminatory capacity (NRI: 0.234, P < 0.001; IDI: 0.119, P < 0.001) (Supplementary Figure S4&Table S5).
Association between TyG index and coronary Gensini score
According to Spearman’s correlation analysis, Weak associations were found between Gensini Score and HbA1c (r = 0.155 p < 0.001), TG (r = 0.350, p < 0.001), and CRP (r = 0.187, p < 0.001). A moderate association was found between the GS and the TyG index (r = 0.420, p < 0.001), as shown in Table 4. Figure 3 displays the GS categorized by TyG group among patients with HTN-CHD, with each group consisting of 277 patients. Table 5 exhibits the findings of the multivariate linear regression analysis. There was a significant positive association between the TyG index and the GS. Each 1-unit increase in TyG in the HTN-CHD group was associated with an increase of 16.777 in GS. (β = 16.777; 95% CI, 13.81–19.74; p < 0.001).
Discussion
This study demonstrated that elevated TyG index values were associated with an increased incidence of CHD in hypertensive patients, even after adjusting for conventional CHD risk factors including age, gender, smoking, alcohol intake, and DM. Furthermore, a positive correlation was observed between TyG index levels and CHD severity in patients with both HTN and CHD. These findings underscore the critical role of IR in CHD pathogenesis among hypertensive populations and propose the TyG index as a novel tool for assessing CHD prevalence and severity in this cohort. The study aims to refine CHD risk stratification in hypertensive patients and explore potential therapeutic interventions. The TyG index may serve as a cost-effective associational marker for CHD risk stratification in hypertensive populations; however, its predictive accuracy and clinical applicability require prospective validation.
Previous studies have consistently shown a strong association between elevated TyG index levels and severity of CHD11. After a median follow-up of 98.2 months, the TyG index was associated with a 0.1-fold increase in the risk of all-cause mortality and a 0.29-fold increase in cardiovascular mortality in the general population12. A cohort study of 410,515 UK Biobank participants revealed that each 1-unit TyG index increase elevated all-cause mortality risk by 3.7% (HR = 1.037, 95% CI 1.016–1.059), supporting its role as an independent predictor of insulin resistance-related mortality13. It is crucial to note, nevertheless, that much earlier research has mostly focused on the general population and has paid little attention to certain high-risk populations. HTN is a prevalent and often disregarded illness that has a mortality rate greater than the whole population. Cardiovascular events are among the most frequent consequences that HTN patients face14. In the Chinese older population, the TyG index shows higher discriminative power for predicting HTN compared to standard lipid and glycemic indicators. It is also strongly related with HTN, according to a cross-sectional research encompassing 47,808 individuals15. Although the TyG index can predict both HTN and CHD, and considering that HTN is associated with an increased risk of CHD, few studies have specifically examined the relationship between TyG and CHD among hypertensive patients.
A recent study underscore TyG’s clinical value in hypertensive populations, albeit in complementary contexts: Liu emphasized prognostic stratification, whereas our work identifies TyG as a marker for early CHD detection and severity assessment9. This study aims to explore this specific association.
The exact mechanism linking the TyG index to HTN and CHD remains poorly understood. However, the TyG index has a strong association with IR, a key contributor to the development and progression of both HTN and CHD. The mechanisms underlying these relationships may include endothelial dysfunction16, inflammation16, dysregulation of glucose and lipid metabolism17 and thrombosis18. These mechanisms may explain why many HTN patients with elevated TyG index levels develop CHD.
Endothelial dysfunction serves as a critical pathological indicator of hypertension and is marked by diminished activity of endothelial nitric oxide synthase (eNOS) and a reduction in nitric oxide (NO) bioavailability19. IR exacerbates endothelial dysfunction by reducing NO production through the PI3K/Akt pathway while simultaneously increasing reactive oxygen species (ROS), prothrombotic, and proinflammatory markers via the MAPK/ERK pathway, thereby escalating cardiovascular risk. Additionally, elevated ROS levels impair insulin-PI3K signaling by phosphorylating IRS-1, which aggravates IR. Modulation of eNOS activity could enhance endothelial function and decelerate the progression of hypertension20. Hypertensive patients with CHD can activate eNOS by taking statin medications, thereby increasing the bioavailability of NO, which contributes to the maintenance of cardiovascular homeostasis21. A recent study involving 840 participants demonstrated a negative correlation between each unit increase in the TyG index and a decrease in FMD, used as an indicator of endothelial dysfunction. The elevated TyG index may serve as a significant independent predictor of endothelial dysfunction22.
The TyG index integrates triglyceride and glucose metabolism. Elevated ALT/AST in higher TyG tertiles likely mirrors hepatic lipid accumulation and IR-driven lipotoxicity, promoting liver injury and cardiovascular risk. It is common for HTN and lipid abnormalities to coexist, with studies finding that many newly diagnosed hypertensive patients have at least one lipid abnormality23. This suggests that the coexistence of dyslipidemia and hypertension may synergistically amplify atherosclerotic events. IR in adipose tissue leads to high free fatty acid levels24, visceral fat accumulation, elevated plasminogen activator inhibitor 1 and blood pressure, and lipotoxicity in blood vessels, which disrupts cellular signaling and cardiac structure25. IR-induced dyslipidemia, characterized by elevated TC, LDL-C, TG, and reduced HDL-C, increases CHD risk by 32% in men and 76% in women26. Insulin facilitates glucose absorption in insulin-sensitive tissues to control glucose metabolism under physiological settings. On the other hand, diminished tissue sensitivity to insulin results in IR, which impairs glucose metabolism. Overall, IR significantly raises CHD risk. Identifying novel indicators of IR in hypertensive patients could facilitate earlier prevention of CHD.
IR-driven metabolic disturbances activate immune responses, fostering a chronic low-grade inflammatory state. Elevated CRP levels in high TyG groups highlight the connection between IR and chronic inflammation. IR triggers pro-inflammatory pathways, such as NF-κB, leading to increased cytokines like IL-6 and TNF-α, which further damage vascular health and drive the progression of CHD. Hypertensive patients often exhibit a systemic inflammatory response, with angiotensin II recognized as a pro-inflammatory mediator27. Angiotensin II’s effects on vascular tone and growth are countered by NO, which also down-regulates ACE and angiotensin II type 1 receptors. The renin-angiotensin system (RAS) amplifies inflammation in atherosclerosis by affecting adhesion molecules and growth factors, facilitating inflammatory cell migration into the subendothelial space. Angiotensin II promotes ROS production and activates inflammatory pathways via its type 1 receptor. Systemic inflammation in hypertension is closely linked to CHD, as HTN disrupts endothelial tissue, generating ROS that activate pro-inflammatory pathways, and insulin resistance induced by inflammation further increases CHD risk.
Hypertensive individuals display abnormalities in vascular walls, blood flow and blood components, including aberrant coagulation levels28. These findings suggest that HTN indeed leads to thrombus formation or a hypercoagulable state. IR results in severe endothelial damage, abnormal states such as inflammation, coagulation, and low levels of fibrinogen, leading to imbalance between bleeding and clotting and promoting thrombus formation. Acute platelet thrombosis and acute coronary syndrome in the coronary arteries are closely associated conditions that frequently result in adverse outcomes29.
Recent studies have highlighted the superior predictive value of the TyG index compared to traditional IR markers such as HOMA-IR. For example, the TyG index demonstrated superior predictive accuracy for incident metabolic syndrome compared to HOMA-IR, and exhibited a linear dose-response relationship30. Additionally, the TyG index has been shown to correlate more strongly with coronary artery calcium scores, a marker of subclinical atherosclerosis, than fasting glucose or TG alone31.
Furthermore, emerging evidence suggests that interventions targeting IR, such as pharmacological agents (e.g., GLP-1 receptor agonists32 and SGLT2 inhibitors33, can significantly reduce TyG index levels and improve cardiovascular outcomes. These findings underscore the clinical relevance of the TyG index as a therapeutic target in hypertensive patients at risk for CHD.
Machine-learnning models such as Random Forest, SVM and Neural Networks integrate TyG index, CRP, HDL-C, and Gensini Score for CHD risk prediction, improving accuracy and enabling automated risk stratification34. Current work prioritizes high-risk patients via resource optimization35. Future systems using reinforcement learning and multi-objective optimization could balance risks, resources, and outcomes.
In conclusion, IR contributes significantly to the risk of cardiovascular events in individuals with HTN. Concurrently, endothelial damage, inflammation, disturbances in glucose and lipid metabolism, all contribute to this process. Triglyceride and glucose levels are linked to the TyG index, and variables that cause excessive triglyceride and glucose levels also cause raised TyG levels.
This study underscores the TyG index as a cost-effective, non-invasive marker for stratifying CHD risk in hypertensive populations. The TyG index can augment routine metabolic assessments, particularly in resource-limited settings. Hypertensive patients with higher TyG index should undergo intensified cardiovascular surveillance. Elevated TyG values indicate the possibility of IR, supporting early interventions, including lifestyle modifications and pharmacotherapy. These strategies help attenuate endothelial dysfunction and inflammation, thus mitigating the progression of atherosclerosis. Furthermore, serial measurements of the TyG index can dynamically track the efficacy of interventions, with reductions in TyG post-treatment correlating with improved vascular health and plaque stabilization.
This study also has its limitations. Firstly, it is single-center and only includes individuals of Asian descent. Further validation is required to ensure the external validity of the results, as the inclusion of patients from a single center may result in selection bias. Additionally, although key confounders such as DM and lipid profiles were adjusted through multivariate regression, residual confounding persists from unmeasured variables including dietary patterns, physical activity levels, and medication adherence. Critical pharmacological data, including the timing and dosage of antiplatelet, lipid-lowering, and antihypertensive therapies, were not systematically recorded, potentially introducing measurement bias. Due to the inherent limitations of cross-sectional studies, while our findings indicate a positive correlation between the TyG index and the occurrence of CHD in hypertensive individuals, causality cannot be established. To confirm the prognostic value of the TyG index for CHD risk in individuals with hypertension, large-scale, multicenter prospective studies are necessary.
Conclusions
Our study found that the TyG index was significantly higher in hypertensive patients with CHD compared to those with hypertension alone, and it positively correlated with CHD severity. As a novel and simple indicator of insulin resistance, the TyG index is affordable, reliable, and promising for evaluating CHD severity in hypertensive patients. It can complement classical cardiovascular risk factors, particularly in high-risk populations. While it reflects disease severity to some extent, further prospective, multicenter studies are needed to confirm its utility.
Data availability
Data and materials are available from the corresponding author on reasonable request.
Abbreviations
- TyG:
-
Triglyceride-glucose
- FPG:
-
Fasting plasma glucose
- TGs:
-
Triglyceride levels
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- DM:
-
Diabetes mellitus
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- CRP:
-
C-creative protein
- WBC:
-
White blood cell
- ALT:
-
Aspartate transaminase
- AST:
-
Alanine aminotransferase
- PCI:
-
Percutaneous coronary intervention
References
Wang, Z. et al. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation 137, 2344–2356 (2018).
Liu, H. H. et al. Lipoprotein (a), hypertension, and cardiovascular outcomes: a prospective study of patients with stable coronary artery disease. Hypertens. Res. 44, 1158–1167 (2021).
Cleven, L., Krell-Roesch, J., Nigg, C. R. & Woll, A. The association between physical activity with incident obesity, coronary heart disease, diabetes and hypertension in adults: a systematic review of longitudinal studies published after 2012. BMC Public. Health. 20, 726 (2020).
Virani, S. S. et al. Heart disease and stroke Statistics-2021 update: A report from the American heart association. Circulation 143, e254–e743 (2021).
Aravani, D., Kassi, E., Chatzigeorgiou, A. & Vakrou, S. Cardiometabolic syndrome: an update on available mouse models. Thromb. Haemost. 121, 703–715 (2021).
Akhigbe, R. & Ajayi, A. The impact of reactive oxygen species in the development of cardiometabolic disorders: a review. Lipids Health Dis. 20, 23 (2021).
Yang, T. et al. Insulin resistance and coronary inflammation in patients with coronary artery disease: a cross-sectional study. Cardiovasc. Diabetol. 23, 79 (2024).
Dugani, S. B. et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. 6, 437–447 (2021).
Liu, Y. et al. Triglyceride–glucose index as a marker of adverse cardiovascular prognosis in patients with coronary heart disease and hypertension. Cardiovasc. Diabetol. 22, 133 (2023).
Gensini, G. G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 51, 606 (1983).
Hu, C. et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc. Diabetol. 19, 116 (2020).
Liu, X., He, G., Lo, K., Huang, Y. & Feng, Y. The Triglyceride-Glucose index, an insulin resistance marker, was Non-linear associated with All-Cause and cardiovascular mortality in the general population. Front. Cardiovasc. Med. 7, 628109 (2021).
Zhang, Z. et al. Associations of modified triglyceride-glucose indices and the triglyceride/high-density lipoprotein ratio with all-cause and cause-specific mortality in the general population: an analysis of the UK biobank database. Lipids Health Dis. 24, 126 (2025).
GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1223–1249 (2020).
Zhu, B. et al. A high triglyceride glucose index is more closely associated with hypertension than lipid or glycemic parameters in elderly individuals: a cross-sectional survey from the reaction study. Cardiovasc. Diabetol. 19, 112 (2020).
Li, M. et al. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Sig Transduct. Target. Ther. 7, 216 (2022).
Isidor, M. S. et al. Insulin resistance rewires the metabolic gene program and glucose utilization in human white adipocytes. Int. J. Obes. 46, 535–543 (2022).
Van Schouwenburg, I. M. et al. Insulin resistance and risk of venous thromboembolism: results of a population-based cohort study. J. Thromb. Haemost. 10, 1012–1018 (2012).
Janaszak-Jasiecka, A., Płoska, A., Wierońska, J. M., Dobrucki, L. W. & Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 28, 21 (2023).
Chalasani, N., Deeg, M. A. & Crabb, D. W. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 99, 1497–1502 (2004).
Chen, W. H. et al. Advances in the molecular mechanisms of Statins in regulating endothelial nitric oxide bioavailability: interlocking biology between eNOS activity and L-arginine metabolism. Biomedicine Pharmacotherapy. 171, 116192 (2024).
Li, Y. et al. Association between triglyceride-glucose index and endothelial dysfunction. Endocrine 85, 717–723 (2024).
Borghi, C., Fogacci, F., Agnoletti, D. & Cicero, A. F. G. Hypertension and dyslipidemia combined therapeutic approaches. High. Blood Press. Cardiovasc. Prev. 29, 221–230 (2022).
Elkanawati, R., Sumiwi, S. & Levita, J. Impact of lipids on insulin resistance: insights from human and animal studies. DDDT 18, 3337–3360 (2024).
Bertero, E. & Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 15, 457–470 (2018).
Hedayatnia, M. et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 19, 42 (2020).
Cantero-Navarro, E. et al. Renin-angiotensin system and inflammation update. Mol. Cell. Endocrinol. 529, 111254 (2021).
Rafaqat, S., Khalid, A., Riaz, S. & Rafaqat, S. Irregularities of coagulation in hypertension. Curr. Hypertens. Rep. 25, 271–286 (2023).
Koupenova, M., Kehrel, B. E., Corkrey, H. A. & Freedman, J. E. Thrombosis and platelets: an update. Eur. Heart J. 38, 785–791 (2017).
Son, D. H., Lee, H. S., Lee, Y. J., Lee, J. H. & Han, J. H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 32, 596–604 (2022).
Park, K. et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 42, 1569–1573 (2019).
Denimal, D. et al. Liraglutide reduces plasma dihydroceramide levels in patients with type 2 diabetes. Cardiovasc. Diabetol. 22, 104 (2023).
Imre, E., Gunhan, H. G., Erel, P. & Ustay, O. SGLT2 inhibitors improve plasma atherogenic biomarkers in patients with type 2 diabetes: A real-world retrospective observational study. Minerva Endocrinol. (Torino). 48, 295–304 (2023).
Maadi, M., Akbarzadeh Khorshidi, H. & Aickelin, U. A review on human-AI interaction in machine learning and insights for medical applications. Int. J. Environ. Res. Public. Health. 18, 2121 (2021).
Sharifi, S. Enhancing kidney transplantation through multi-agent kidney exchange programs: A comprehensive review and optimization models. ijiec https://doi.org/10.5267/j.ijiec.2024.12.002
Acknowledgements
We thank the associate editor and the reviewers for their useful feedback that improved this paper.
Funding
This project was supported by the Jiangsu Provincial Medical Key Discipline (ZDXK202208).
Author information
Authors and Affiliations
Contributions
Lingling Wu and Xue Bao wrote the manuscript text. Ji Xu and Lingyu Ma collected the data together. Lina Kang and Ronglin Zhang administrated the project. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experiments and methods were performed in accordance with the Declaration of Helsinki. This research approved by the Ethics Committee of Drum Tower Hospital Affiliated to School of Medicine of Nanjing University. Due to the retrospective nature of the study, the Nanjing Drum Tower Hospital’s ethical committee waived the need of obtaining the informed consent. The approval number was 2024-113-01.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, L., Bao, X., Xu, J. et al. The triglyceride-glucose index positively associates with the prevalence and severity of coronary heart disease in patients among hypertension. Sci Rep 15, 19571 (2025). https://doi.org/10.1038/s41598-025-03948-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03948-y