Abstract
Antibubbles are unique bubbles (within a bulk liquid) that comprise liquid core(s). This study focused on creating and evaluating antibubbles with multiple cores using double emulsion (DE) templating. The primary emulsion (PE) was made using high-shear homogenization and then passed through a T-junction to form the DE. These DEs were then freeze-dried and rehydrated to form antibubbles. The study examined the effect of PE parameters (homogenization speed (rpm), internal phase (\({W}_{1}\), %), and interfacial particle concentration (\({PC}_{O}\), %)), second emulsification parameters (flow rate ratio, interfacial particle concentration (\({PC}_{W2}\), %), microchannel size, and additional cryoprotectants in continuous phase), and post-emulsification freezing temperature on DE and the antibubble. The best conditions for yielding small-sized DE and the antibubble with maximum reconstitution coefficient (RC) were selected to evaluate the encapsulation efficiency (EE) of calcein in \({W}_{1}\). The study found that antibubbles could provide better EE than DEs for storage longer than 7 days. Future studies should focus on scaling up production, improving EE during the DE-to-antibubble transition, and evaluating stability and release dynamics under in-vitro gastrointestinal simulations using human digestive fluids and tissues for more accurate in vivo predictions.
Similar content being viewed by others
Introduction
Antibubbles are unique physical entities characterized by liquid core(s) encased in an air film/shell, suspended within a bulk liquid1. Hence, an antibubble is a liquid-in-air-in-liquid (L/A/L) system, which is the opposite of a bubble in the air, forming an air-in-liquid-in-air (A/L/A) system2. Hence, antibubbles may also be defined as “bubbles (within a bulk liquid) that comprise liquid core(s)”, as any gas globule surrounded by a liquid phase (A/L system) can be referred to as a bubble. The occurrence of antibubbles was first reported in 19323, although the term “antibubble” was not used. For some decades, they were called “inverted bubbles” or “inverse bubbles”4,5 until coined “antibubble” in 19746.
Conventionally, antibubbles were prepared by the falling drop method, where a falling drop enclosed in an air film, upon appropriate impact energy (sufficient for penetration without bursting), could penetrate the liquid bath for antibubble formation1,2,7. Various modifications, including the pouring and jetting methods and modifying the liquid bath surface with foams, have been used to achieve antibubble formation1. More recently, antibubbles were prepared by modifying the density of liquids7, and from the secondary drops detached from Worthington jet2. However, such antibubbles were surfactant-stabilized, core-shelled, large (mostly in mm to a few cm), and short-lived8,9.
Particle-stabilized interfacial systems, often referred to as “Pickering”, gained substantial appraisal for their exceptional stability against coalescence and Ostwald ripening compared to surfactant-stabilized interfaces10,11. Although most of those studies may not be true “Pickering”10,12, this led to interest in particle-stabilized antibubble, first reported by Poortinga13. This study laid the foundation for developing long-lived particle-stabilized antibubble. However, these antibubbles were prepared using the falling drop method, characterized by the probability-based formation of large antibubbles, limiting their application potential9,14. A significant advancement came with the introduction of a double emulsion (DE) templated system to prepare small-sized (< 100 µm) stable antibubbles by removing the intermediate volatile oil from a water-in-oil-in-water (W/O/W) DE15. This study demonstrated the feasibility of producing long-lived small-sized antibubbles. Following this, several studies explored the preparation and potential application of DE-templated antibubbles for encapsulation, targeted delivery, and controlled release of active components16,17,18,19.
Despite these developments, most DE-templated antibubbles have been produced from polydisperse DEs generated using techniques such as high-shear homogenization (HSH), high-pressure homogenization (HPH), or ultrasonication. In contrast, microfluidics has emerged as a powerful alternative for producing highly monodisperse DEs20,21. A commonly used microfluidic geometry is the T-junction, where the continuous and dispersed phases meet orthogonally, and droplet formation occurs at the junction22. Several parameters have been shown to influence the droplet size in T-junction devices. Key dimensionless numbers such as the Capillary number (Ca, ratio of viscous to interfacial forces), Weber number (We, ratio of inertial to interfacial forces), and Reynolds number (Re, ratio of inertial to viscous forces) provide insight into the governing mechanisms; higher Ca, We or Re values typically favor small-sized polydisperse droplets, while lower values promote larger, stable and monodispersed droplets22,23,24,25. Additionally, geometrical parameters like width ratio (side channel to main channel) and height ratio (channel height to width) also impact droplet size and dispersity22,23,26. Hence, the droplet size and dispersity in T-junction devices is an interplay of shear forces, interfacial tension, flow rates, channel geometry, and fluid viscosities27,28. Careful tuning of these physical and geometrical parameters is essential to achieve consistent droplet breakup and high monodispersity. Monodispersity is important for the standardization, reproducibility, and predictability of both DEs and antibubbles, directly impacting critical performance attributes, such as encapsulation efficiency (EE) and the release behavior of encapsulated active components28,29.
To our knowledge, only one study reported the preparation of antibubbles from a monodisperse DE30, which were core-shelled and fabricated using X-junction microfluidics. However, the study was only a proof-of-concept without investigation on the effect of process parameters or the encapsulation stability. Hence, this study aimed to prepare DE-templated monodisperse antibubbles by combining HSH for the first emulsification and T-junction microfluidics for the second emulsification. The influence of different primary emulsion (PE) parameters (homogenization speed (rpm), internal aqueous phase (\({W}_{1}\), %), and interfacial particle concentration i.e., in the oil phase (\({PC}_{O}\), %)), second emulsification parameters (flow rate ratio (FRR), interfacial particle concentration i.e., in continuous aqueous phase (\({W}_{2})\) (\({PC}_{W2}\), %), microchannel size (μm), and additional cryoprotectants in \({W}_{2}\)), and post-emulsification freezing temperature (℃) were studied. The best conditions for obtaining small-sized DE and antibubble with the highest reconstitution coefficient (RC) were selected to prepare the final monodisperse antibubble and evaluate EE of a model active component in \({W}_{1}\).
Materials and methods
Materials
The hydrophobized fumed silica particles, AEROSIL® R972 (68611-44-9) and AEROSIL® R816 (199876-45-4) were kindly arranged by Evonik Gulf FZE (Dubai, UAE). Maltodextrin (9050-36-6), D-mannitol (69-65-8), decane (124-18-5), sodium phosphate dibasic heptahydrate (7782-85-6), and sodium phosphate monobasic monohydrate (10049-21-5) were from Sigma-Aldrich (Darmstadt, Germany). Calcein (1461-15-0) was obtained from MP Biomedicals, LLC (Illkirch, France). Isopropanol (67-63-0) was obtained from Honeywell (Riedel-de Haen, Seelze, Germany). Distilled water (refractive index (RI): 1.33) was used throughout the experiment.
Preparation of double emulsion (DE) and antibubbles
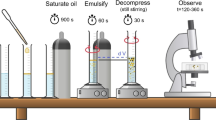
The schematic representation of the preparation process of DE and antibubbles is presented in Fig. 1.
Preparation of primary emulsion (PE)
PE (20 mL) was prepared by HSH using ULTRA-TURRAX® (T25 D with 18 G probe, IKA®-Werke GmbH & Co. KG, Staufen, Germany). In 50 mL centrifuge tubes, the required amount of decane was poured, and the calculated amount of internal aqueous phase (\({W}_{1}\)) was added. The 18 G probe was dipped into the tube without touching the bottom and operated at different homogenization speeds for 1 min to obtain PE.
\({W}_{1}\) comprised of 0.2% calcein and 10% maltodextrin. Calcein served as the active core component for EE evaluation, while maltodextrin prevent structural collapse during lyophilization and osmotic uptake of water during reconstitution. Before emulsification, the oil phase was prepared by dispersing calculated amount of interfacial particle (R972) in decane using a magnetic stirrer (300 rpm, 15 min), followed by 30 s of ultrasonication at 50% amplitude using an ultrasonic processor (750 W, 20 kHz Sonics Vibra-Cell, with CV 334 converter, 13 mm 306-B probe, Sonics and Materials, Inc., Newtown, CT, USA). To evaluate the effect of primary emulsification parameters, three treatments of each factor were evaluated: homogenization speed (6000, 10,000, and 15,000 rpm), \({W}_{1}\) (5, 10, and 15% (v/v)), and interfacial particle (R972) concentration (\({PC}_{O}\), 0.5, 1, and 1.5%). Unless otherwise stated, a homogenization speed of 10,000 rpm, \({W}_{1}\) of 10%, and \({PC}_{O}\) of 1% were used.
Second emulsification
For the second emulsification, a microfluidic apparatus (The Dolomite Centre Ltd., Royston, UK) was used, which comprised of Bambi PT5 compressor (Bambi Air Ltd., Birmingham, UK), flow rate sensors (1–50 µL/min and 30–1000 µL/min) (The Dolomite Centre Ltd., Royston, UK), and MSZ-AP0-V high-speed digital microscope (Seiwa Optical Co. Ltd.), all operated with a flow control software (Dolomite Flow Control Center, version 4.1.9). T-junction microchips (100 µm (3000158), (140 µm (3201045), and 190 µm (3000436) were used (The Dolomite Centre Ltd., Royston, UK). The PE was pumped at a constant flow rate (100 µL/min) with varying flow rates of \({W}_{2}\) to obtain DE. In contrast to normal T-junction flow, herein, the dispersed phase i.e., PE was passed through the main channel, and the continuous phase i.e., \({W}_{2}\) was passed through the perpendicular stem as shown in Fig. 1. This reverse flow configuration reduced clogging and allowed for least fluctuation in pressure and flow rate, thus yielding more reproducible results for parameters evaluated as described in "Determination of size, dispersity index (DI), and reconstitution coefficient (RC)".
Before emulsification, \({W}_{2}\) was prepared by dispersing different concentrations of R816 (1–3%) with maltodextrin (10–20%) and mannitol (0–10%) using a magnetic stirrer (300 rpm, 15 min), followed by 30 s of ultrasonication at 50% amplitude using the same ultrasonic processor. Maltodextrin and mannitol served as cryoprotectants to prevent structural collapse during freeze-drying and to maintain the osmotic balance of the antibubble upon reconstitution15,18,19.
Freeze-drying and reconstitution
The produced DEs were frozen at – 20 °C (Samsung top mount freezer refrigerators, 460L RT66CG6406S9) or at − 80 °C (Ultra-freezer, BINDER GmbH, Germany). They were lyophilized using a freeze dryer (LyoAlfa 15, Telstar, Germany) at − 85 °C and 0.01 mbar vacuum for 24 h to obtain freeze-dried DE (FDDE). FDDE was reconstituted to the original volume by adding distilled water to obtain antibubbles.
Microscopic imaging
Optical and fluorescent images were acquired on a Delphi-X Observer fluorescence microscope (Euromex, Arnhem, The Netherlands) with a 10×, 20×, and 100× objective lens. All samples were observed in a thick cavity slide. PE was diluted 5 times with its dispersing phase solvent (decane). Euromex ImageFocus Alpha software (version 1.3.7.12967.20180920) was used for image acquisition and processing. DE was imaged without dilution. The antibubbles were imaged after 8–10 min of adding distilled water to rehydrate FDDE to the original volume.
Determination of size, dispersity index (DI), and reconstitution coefficient (RC)
The size of emulsions and antibubbles was evaluated by analyzing microscopic images using ImageJ31 software (version 1.54 g). For each sample, 100 measurements were carried out in triplicates. Images obtained using a 100 × objective lens were used for PE, while images obtained using a 20 × objective lens were used for the DE and antibubbles.
The DI was calculated as the square of the ratio of the standard deviation (\(\sigma\)) to the arithmetic mean droplet diameter (\(\mu )\) (Eq. 1). DI values range from 0 to 1, where 0 indicates the uniform size and 1 indicates the \(\sigma\) is equal to \(\mu\). Hence,
The mean droplet diameters were abbreviated as \({D}_{PE}\), \({D}_{DE}\), and \({D}_{AB}\) for PE, DE, and antibubbles respectively. Whilst \({DI}_{PE}\), \({DI}_{DE}\), and \({DI}_{AB}\) is used to indicate the DI of PE, DE, and antibubbles respectively.
For emulsions, a coefficient of variance (CV, i.e., \(\sigma /\mu\)) of < 25% (corresponding to a DI of 0.625) has traditionally been used as a criterion for monodispersity28,32,33. However, some studies have adopted a more stringent criterion, defining monodispersity as a CV of < 10% (DI of < 0.01)34,35. Accordingly, the more stringent criterion of CV < 10% (DI < 0.01) is applied in this study.
The RC is the ratio of the antibubble diameter (\({D}_{AB}\)) to DE diameter (\({D}_{DE})\) and calculated as:
Hence, the RC values < 1 indicate \({D}_{AB}\)< \({D}_{DE}\).
Encapsulation efficiency (EE)
The EE was measured based on the concentration of calcein in \({W}_{2}\) before and after interface disruption. DE was evaluated immediately after preparation and after storage at 4 ± 2 °C for specified durations. For FDDE and antibubbles evaluation, 3 mL of DE were collected in 5 mL Eppendorf tubes, frozen, and lyophilized. FDDE was evaluated at the specified duration of storage after lyophilization and reconstituted to the original volume at the time of evaluation. Meanwhile, antibubbles were evaluated at the specified storage time after reconstitution.
Samples (DE, FDDE, and antibubbles) in each 5 mL tube were centrifuged at 5000 × g for 5 min (Hermle model Z327-K, Hermle Labortechnik GmbH, Wehingen, Germany). For calcein content in \({W}_{2}\), the clear solution in the middle of each tube was carefully drawn with a 5 mL sterile syringe, and 0.5 mL was transferred to 2 mL Eppendorf tubes, avoiding the creamed top layer (DE or antibubbles) and sediment (unadsorbed particles in continuous phase) at the bottom. The remaining sample in the syringe was transferred back to the initial tube for the evaluation of total calcein in samples. In each tube, an equal volume of isopropanol was added and vortexed for 2 min, then centrifuged at 12,000 × g for 5 min. From each, 100 µL was drawn from the top, and appropriate dilution was prepared with phosphate buffer (pH 7.4). 300 µL of two dilutions were pipetted in duplicates to 96-well fluorescence plates for the detection of fluorescence intensity by excitation at 485 nm and measuring the emission at 520 nm using a Fluorescent Microplate Reader (Synergy™2, BioTek Instrument Inc., Winooski, VT, USA). EE during storage was calculated as:
where \({C}_{b}\) and \({C}_{max}\) are calcein concentrations in the continuous phase before interfacial disruption and maximum calcein concentration in DE (or antibubble), respectively. \({C}_{max}\) was calculated as:
where \({C}_{b}\) and \({C}_{a}\) are the calcein concentrations in \({W}_{2}\) (before interfacial disruption) and in the remaining sample (after interfacial disruption), respectively, and \({V}_{b}\) and \({V}_{a}\) are the volume of samples used for calcein concentration before and after interfacial disruption, respectively. In this study, fixed values of 0.5 mL and 2.5 mL were used for \({V}_{b}\) and \({V}_{a}\), respectively.
Statistical analysis
All statistical analysis and graphical representation were obtained using JMP® Pro software (version 15.0.0, SAS Institute Inc., Cary, NC, USA). All measurements were carried out in triplicates. Two-way analysis of variance was used to evaluate the effect of factors, while Tukey-HSD post-hoc analysis was carried out for mean comparison at a 5% significance level.
Results and discussion
Effect of primary emulsion (PE) parameters
Homogenization speed (rpm)
The impact of varying homogenization speeds (6000, 10,000, and 15,000 rpm) during primary emulsification on the properties of PE, DE, and antibubble is presented in Fig. 2. At 6000 rpm, \({D}_{PE}\) was maximum, which decreased with increased homogenization speed. The higher shear generated by elevated homogenization speed creates intense turbulence and high-velocity gradients, which stretch and break the droplets into more uniform and smaller sizes36. All PEs were polydisperse with a mean \({DI}_{PE}\) above 0.5. Generally, higher homogenization intensity provides more energy to overcome the interfacial tension that holds larger droplets, thus breaking them to smaller ones with narrow distribution37,38,39. In contrast, at a low degree of homogenization, insufficient droplet break-up yields big-sized droplets with a wider distribution as observed for 6000 rpm. Despite a wide distribution (high \(\sigma\)), the \({DI}_{PE}\) prepared at 6000 rpm was lower than that of 10,000 rpm because of a higher \({D}_{PE}\). From Eq. (1), it is evident that DI is directly proportional to the square of \(\sigma\) and inversely proportional to the square of \(\mu\) size.
The effect of homogenization speed on (a) primary emulsion (PE), (b) double emulsion (DE), (c) antibubble, and (d) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axis, respectively. The values were obtained from triplicate samples, each with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (b) and (c) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
\({D}_{DE}\) and \({DI}_{DE}\) decreased with increasing homogenization speed used for PE preparation. This decrease was due to the reduced droplet size of PE with increased homogenization speed. Smaller PE droplets create a larger interfacial area, which increases the resistance to flow i.e. the viscosity36,40 that typically results in smaller and more uniform DE droplets41,42. However, at a low dispersed phase fraction, the change in the effective viscosity of PEs as a function of droplet size is minimal43. Herein, 10% \({W}_{1}\) was used to prepare PE, hence, the difference in the effective viscosity difference in PEs due to the difference in droplet size and subsequent effect in \({D}_{DE}\) can become nominal as compared to the effect of the PE droplet size (\({D}_{PE})\). \({D}_{PE}\) sets a baseline for \({D}_{DE}\)44,45,46. It is plausible that smaller internal droplets can be more tightly packed, potentially resulting in smaller more predictable, and controlled droplet formation in microfluidic channels. Conversely, larger PE droplets impose flow instability promoting irregular droplet breakup and subsequent collision and coalescence41,42,46. The common flow instability observed during the study was slug flow (due to adhering of the large droplets on tubing and microchannel), dripping-to-jetting transition, irregular interfacial deformation and pinch-off, and back-pressure fluctuations. Yet, all \({DI}_{DE}\) was < 0.01, indicating they were monodispersed.
Upon freeze-drying, a slight shrinkage in the structure was visually evident. This resulted in smaller \({D}_{AB}\) than corresponding \({D}_{DE}\), thus RC < 1. Such shrinkage during freeze-drying has been previously reported and typically depends on the solid content47. While the \({D}_{AB}\) from PE prepared at 10,000 and 15,000 rpm were comparable, RC was closer to 1 for higher homogenization speeds. Interestingly, 6000 rpm resulted in smaller \({D}_{AB}\) with the lowest RC. This reduction was due to the selective formation of antibubbles from small-sized DE droplets, while the large-sized DE droplets (formed by coalescence of DE droplets) underwent structural collapse, releasing \({W}_{1}\) droplets to \({W}_{2}\). The outer interface of large DE droplets is more susceptible to such disturbances during freeze-drying due to the formation of larger crystals during freezing, which can protrude through the interface48. Although the added cryoprotectant (maltodextrin) is expected to reduce ice formation and maintain the integrity of the emulsion during freezing18,19, the protective effect may be limited with increasing droplet size. \({DI}_{AB}\) of all antibubbles were above 0.01, which is beyond the monodispersity criteria. Overall, a high homogenization speed (rpm) resulted in small-sized DEs and yielded antibubbles with higher RC.
Internal aqueous phase content (\({{\varvec{W}}}_{1}\), %)
To evaluate the effect of W1 (%), PEs were prepared with 5, 10, or 15% of \({W}_{1}\) in the oil phase. The resulting changes in the properties of PE, DE, and antibubble are presented in Fig. 3. Higher \({W}_{1}\) content (15%) resulted in larger \({D}_{PE}\) and smaller \({DI}_{PE}\). Large \({D}_{PE}\) at 15% \({W}_{1}\) was due to the reduction in the particle-to-dispersed phase ratio37,49, resulting in a condition comparable to low particle concentration, as discussed in "Particle concentration in the oil phase (, %)". Additionally, increased \({W}_{1}\) content can lead to more collisions, resulting in coalescence and thus larger \({D}_{PE}\), especially at limited particle concentrations50,51. The low DI for the PE prepared with a high \({W}_{1}\) (%) was due to the inverse relationship with μ (μ was high for high \({W}_{1}\) content), as shown in Eq. (1).
The effect of the internal aqueous phase content (\({W}_{1}\), %) on (a) primary emulsion (PE), (b) double emulsion (DE), (c) antibubble, and (d) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axes, respectively. The values were obtained from triplicate samples, each with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (b) and (c) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
\({D}_{DE}\) and \({DI}_{DE}\) were minimum at 5% \({W}_{1}\) and increased at a higher \({W}_{1}\) content. At low \({W}_{1}\) content, PE comprised very fine droplets and hence acted as a single dispersed phase with minimal influence of \({W}_{1}\). However, higher \({W}_{1}\) content resulted in bigger \({D}_{PE}\), which impose greater resistance to interfacial deformation, especially during the necking and pinch-off stages, resulting in big-sized \({D}_{DE}\). In addition, higher \({W}_{1}\) content can increase the viscosity of the PE52, which provides additional resistance against the pinch-off of PE, thereby increasing the DE droplet size53,54. The effect of \({W}_{1}\) (%) on \({D}_{DE}\) was also reflected on \({D}_{AB}\), with higher \({W}_{1}\) fraction resulting in larger \({D}_{AB}\). All \({DI}_{AB}\) was above 0.01, indicating they were polydisperse. All RC was < 1, indicating some degree of shrinkage during freeze-drying. Interestingly, RC was higher for higher \({W}_{1}\) (%), suggesting that an increase in \({W}_{1}\) can provide resistance to shrinkage of the system. This is due to the increase in the total solid content in the system. The liquid phase with very low solid content is likely to undergo shrinkage during freeze-drying as compared to when high solid content is present47. Previous studies also reported that the cryoprotectants added in \({W}_{1}\), herein maltodextrin plays a crucial role in resisting shrinkage and the overall collapse of the structure15,18,19. Hence, low \({W}_{1}\) (%) resulted in small-sized DE, but maximum RC was achieved at higher \({W}_{1}\) (%).
Particle concentration in the oil phase (\({{{P}}{{C}}}_{{{O}}}\), %)
The effects of different concentrations of R972 particles in the oil phase, i.e., \({PC}_{O}\), were investigated in the range of 0.5% to 1.5% during PE preparation. Their impact on the properties of PE, DE, and antibubble is presented in Fig. 4. With higher \({PC}_{O}\) (%), \({D}_{PE}\) and \({DI}_{PE}\) decreased. All concentrations successfully stabilized PEs, indicating that these concentrations were above the minimum particle concentration (MPC). MPC refers to the minimum concentration of particles below which the droplets are not stable11. At low particle concentrations, interfacial coverage is incomplete or uneven, leaving newly formed droplet interfaces temporarily unprotected. This results in surface tension gradients that drive Marangoni flows across the interface. These flows counteract shear-induced deformation, making droplet breakup less efficient and leading to larger, irregular droplets with higher polydispersity55,56. As particle concentration increases, interfacial coverage improves, reducing Marangoni stresses and enhancing droplet breakup. This produces smaller, more uniform droplets. Additionally, higher coverage decreases collision frequency and efficiency, minimizing coalescence and effectively stabilizing the droplets up to a critical particle concentration (CPC). Above CPC, the interfaces are saturated, and the additional particles contribute to the emulsion viscosity and stability but do not affect droplet size and dispersity57,58.
The effect of particle concentration (%) in the oil phase on (a) primary emulsion (PE), (b) double emulsion (DE), (c) antibubble, and (d) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axis, respectively. The values were obtained from triplicate samples, each with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (b) and (c) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
With increased \({PC}_{O}\) (%), \({D}_{DE}\) and \({DI}_{DE}\) also decreased. This could be attributed to the larger \({D}_{PE}\) at low \({PC}_{O}\) (%) as \({D}_{PE}\) sets a baseline for \({D}_{DE}\). In addition, larger PE droplets impose greater resistance to interfacial deformation, especially during the necking and pinch-off stages, thereby promoting collision and coalescence41,42,46 as discussed in "Homogenization speed (rpm)". Although DE prepared with low \({PC}_{O}\) (0.5%) was in the borderline, all \({DI}_{DE}\), was < 0.01, indicating they were monodispersed.
The RC for all samples was < 1, indicating \({D}_{AB}\) < \({D}_{DE}\), i.e., shrinkage. However, low \({PC}_{O}\) (0.5%) resulted in smaller \({D}_{AB}\) and RC, as compared to higher \({PC}_{O}\) (%). A comparably bigger \({D}_{DE}\) resulting in comparably smaller \({D}_{AB}\) at low particle concentration was due to the bursting of larger DE droplets, releasing \({W}_{1}\) to \({W}_{2}\)as discussed in "Homogenization speed (rpm)". In addition, it could also be partly attributed to the shrinkage due to lower solid content in the system. Ideally, freeze-drying should not result in shrinkage and/or collapse. However, shrinkages are often attributed to products with high moisture and low surface-area-to-volume ratio59. All \({DI}_{AB}\) was > 0.01, indicating further optimization is required for preparing monodisperse antibubbles. Overall, higher \({PC}_{O}\) (%) resulted in small-sized DE and antibubbles with maximum RC.
Effect of second emulsification parameters
Flow rate ratio (FRR)
FRR was defined as the ratio of the volumetric flow rate of \({W}_{2}\) to that of PE. The effects of different FRR (5, 20, and 35) on the properties of DE, and antibubble, were evaluated and presented in Fig. 5. The \({D}_{DE}\) and \({DI}_{DE}\) decreased with increased FRR. In shear-driven systems like T-junction microfluidics, dispersed phase droplets (herein, PE) are formed due to shear exerted by the flow of continuous phase (herein, \({W}_{2}\)), but the size and dispersity may be affected by properties of the liquids (e.g. density, viscosity, ion strength, and pH), and system conditions (e.g. temperature, pressure, or any externally applied forces)27,28. But when the same liquids and systems are used and operated at a constant dispersed phase flow rate, the increased FRR results in an increased drag force and faster pinch-off reducing the volume and the size of each droplet53,54, herein the DE droplet. All \({DI}_{DE}\) were < 0.01, and thus were monodispersed.
The effect of flow rate ratio (FRR) during second emulsification on (a) double emulsion (DE), (b) antibubble, and (c) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axis, respectively. The values were obtained from triplicate samples with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (a) and (b) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The trends in \({D}_{AB}\) and \({DI}_{AB}\) were similar to those observed for DE droplets. The RC was also < 1 for all samples. However, RC was higher for small-sized antibubbles that corresponded to higher FRR. This high RC for small-sized antibubble could be attributed to higher Laplace pressure, which stabilizes the droplet and resists disruption or shrinkage as compared to larger DE droplets. Laplace pressure is inversely proportional to droplet size60, and higher Laplace pressure indicates a robust internal force that maintains the droplet’s integrity, providing high resistance against deformation61. All \({DI}_{AB}\) was > 0.01, indicating further optimization is required for preparing monodisperse antibubbles. Hence, a higher FRR resulted in small-sized DE droplets and antibubble with maximum RC.
Particle concentration in continuous phase (\({{\varvec{P}}{\varvec{C}}}_{{\varvec{W}}2}\))
The effects of different concentrations of R816 particles in \({W}_{2}\) (1, 2, and 3%), i.e., \({PC}_{W2}\) (%) on the properties of DE, and antibubble are presented in Fig. 6. In general, with increased \({PC}_{W2}\) (%), the viscosity of the continuous phase increases thereby reducing the shear force at the interface and making it more difficult to make the small-sized droplets62,63. In contrast, the \({D}_{DE}\) and \({DI}_{DE}\) were the highest for low \({PC}_{W2}\) (1%) and reduced at higher \({PC}_{W2}\) (3%). This was attributed to higher DE droplet coalescence at low (1%) \({PC}_{W2}\), which was depicted by frequent big sized drops twice the normal drops, resulting in high σ and \({DI}_{DE}\) > 0.01 and hence polydisperse. Whilst, more uniform small-sized \({D}_{DE}\) at high \({PC}_{W2}\) (3%) could be attributed to the robust interface and viscous continuous phase capable of resisting the DE droplet coalescence. \({DI}_{DE}\) for higher \({PC}_{W2}\) (2–3%) were < 0.01, indicating monodispersity. In addition, the results could also be partly influenced by the spatial availability of particles to quickly accommodate at the newly created shear-induced interface to minimize the interfacial tension gradient and subsequent Marangoni stress. At low \({PC}_{W2}\) (1%), less particles are spatially available to quickly accommodate the newly created shear-induced interface, resulting in higher interfacial garadient and Marangoni stress. The Marangoni stress counteracts shear-induced deformation, making droplet breakup less efficient55,56, herein delay the droplet pinch-off64 resulting in bigger droplets.
The effect of particle concentration (%) in the continuous phase (\({W}_{2}\)) during second emulsification on (a) double emulsion (DE), (b) antibubble, and (c) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axis, respectively. The values were obtained from triplicate samples, each with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (a) and (b) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In contrast to \({D}_{DE}\), \({D}_{AB}\) was larger for higher \({PC}_{W2}\) (%). This could be attributed to the reduced ability of the outer interface at low particle concentration to resist the disruption of the structure releasing \({W}_{1}\) to \({W}_{2}\), especially for the cases of large DE droplets formed by coalescence as discussed in "Homogenization speed (rpm)". All \({DI}_{AB}\) values were > 0.01, thus the antibubbles were polydisperse. The RC of all samples were < 1, indicating some degree of shrinkage during the process. The highest \({PC}_{W2}\) (i.e., 3%) resulted in antibubbles with maximum RC, due to the formation of densely packed, rigid layers of particles that resist deformation or disruption. Hence, a higher \({PC}_{W2}\) (%) resulted in smaller DE droplets and antibubbles with maximum RC.
Microchannel size
T-junction microchips with different microchannel diameters (100, 140, and 190 µm) were used to prepare DE. However, due to frequent clogging with the 100 µm microchannels, samples could not be produced smoothly, so this size was excluded from further evaluation. The effects of the 140 µm and 190 µm microchannel sizes on the properties of DE, and antibubble are presented in Fig. 7. The larger microchannel resulted in a larger \({D}_{DE}\) and smaller \({DI}_{DE}\). In the larger microchannel, the shear stress from the continuous phase (which helps drag and pinch the dispersed phase herein PE) is lower compared to smaller microchannels. This allows the dispersed phase to occupy a larger volume before the droplet pinches off, producing larger droplets53,65,66. The smaller \({DI}_{DE}\) at larger microchannel was due to inverse relation of DI and μ. Both \({DI}_{DE}\) were < 0.01 and thus monodispersed.
The effect of microchannel size (μm) during second emulsification on (a) double emulsion (DE), (b) antibubble, and (c) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axis, respectively. The values were obtained from triplicate samples, each with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (a) and (b) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The trend for \({D}_{AB}\) and \({DI}_{AB}\), was similar to those for DEs. However, \({DI}_{AB}\) was > 0.01 for both samples indicating polydispersity. Additionally, \({D}_{AB}\) was smaller compared to \({D}_{DE}\) for both samples, resulting in RC < 1. The small-sized microchannel yielded antibubbles with higher RC due to their higher Laplace pressure, which stabilizes the droplet, resists shrinkage, and enhances resistance to deformation compared to larger DE droplets60,61, as discussed above in "Flow rate ratio (FRR)". Overall, the smaller microchannel (i.e., 140 μm) resulted in smaller DE droplets, and antibubble with maximum RC.
Additional cryoprotectants in the continuous phase (\({{\varvec{W}}}_{2}\))
In addition to maltodextrin added in the internal aqueous phase (\({W}_{1}\)) and continuous phase (\({W}_{2}\)), an additional amount of maltodextrin (5 and 10%) or mannitol (5 and 10%) was added to \({W}_{2}\), and used to prepare DEs. The effects of these additional cryoprotectants and their concentrations in \({W}_{2}\) on the properties of DE, and antibubble are presented in Fig. 8. In general, increased cryoprotectant concentration increases the density and viscosity of \({W}_{2}\), which reduces the drag force for pinch-off resulting in larger droplets53,65,66. In contrast, a higher cryoprotectant concentration resulted in smaller \({D}_{DE}\). The reduced \({D}_{DE}\) was attributed to the increases in the density and viscosity of \({W}_{2}\) which reduced DE droplet collision and coalescence that would form larger droplets28. Yet all \({DI}_{DE}\) were < 0.01 and thus monodisperse.
The effect of additional cryoprotectants and their concentrations (%) in the continuous phase (\({W}_{2}\)) during second emulsification on (a) double emulsion (DE), (b) antibubble, and (c) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axis, respectively. The values were obtained from triplicate samples, each with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (a) and (b) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). NA: No additional cryoprotect. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Despite \({D}_{AB}\) was < \({D}_{DE}\), resulting RC < 1. \({D}_{AB}\) and RC were larger for higher cryoprotectant concentrations as compared to lower concentrations. This was in contrast to \({D}_{DE}\) and could be attributed to a dense \({W}_{2}\) phase with increased solid content providing resistance against deformation during freeze-drying47,67. Moreover, increased cryoprotectant concentration can reduce ice formation and maintain the integrity of the emulsion better18,19. Additionally, mannitol provided better results for antibubble than maltodextrin. While maltodextrin in the system can act as a bulking and viscosifying agent, to preserve the physical properties and chemical constituents of freeze-dried products68, mannitol offers additional preservation to the interface against the potential deterioration induced by ice crystals67,69. The higher cryoprotectant concentrations also resulted in the least \({DI}_{AB}\), yet all were above 0.01 and hence polydisperse. Overall, the additional 10% cryoprotectant in \({W}_{2}\) resulted in small-sized DE and antibubble with higher RC. Mannitol addition resulted in a higher RC (0.81 ± 0.01) compared to maltodextrin (0.78 ± 0.01).
Effect of freezing temperature (℃)
The prepared DEs were frozen at − 20 and – 80 °C to evaluate the effect of post-emulsification freezing temperature on the antibubble size and the RC, as presented in Fig. 9. Freezing the DE at − 80 °C resulted in larger \({D}_{AB}\) and higher RC, indicating that lower freezing temperatures provide better protection against deformation of the antibubble structure. Ideally, the freeze-drying process should not result in shrinkage or collapse and retain the initial morphology and volume, yet in practice, it’s inevitable, especially when the solid content is low47,67. Freezing at lower temperatures (− 80 °C) enhances the rate of freezing. This results in more small-sized ice crystals and a lower fraction of unfrozen water. Consequently, it provides better resistance against shrinkage or collapse compared to freezing temperatures near the freezing point70,71. Although, \({D}_{AB}\) was substantially reduced upon freezing at − 80 °C, it is still > 0.01, hence polydisperse.
The effect of freezing temperature on (a) double emulsion (DE), (b) antibubble, and (c) the reconstitution coefficient (RC). The bars (shaded) and bullets (red) represent the left and right y-axis, respectively. The values were obtained from triplicate samples, each with at least 100 evaluations. The error bars represent the standard deviation. The dotted horizontal lines in (a) and (b) represent the monodispersity criteria of 0.01. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Monodisperse antibubble
Final process parameters
The final process parameters were selected based on their ability to produce smaller DE droplet sizes and antibubbles with higher RC, as shown in Table 1. When small DE size and maximum RC were not achieved with the same parameter value, the value that resulted in maximum RC was selected. Despite all DE droplets being monodispersed (\({D}_{DE}\) < 0.01), all antibubbles had \({D}_{AB}\) > 0.01, thus not meeting the stringent monodispersity criteria. Therefore, the final DE and antibubbles were prepared under the selected conditions to obtain more uniform antibubbles.
Emulsions (PE and DE) and antibubble characteristics
The characteristics of PE, DE, and antibubble prepared using the selected process parameters (Table 1) are presented in Fig. 10. The resulting PE (Fig. 10a) exhibited substantially smaller \({D}_{PE}\) (3.36 ± 2.04 μm) and \({DI}_{PE}\) (0.37 ± 0.07) compared to earlier preparations, where \({D}_{PE}\) and \({DI}_{PE}\) reached up to 12.95 ± 9.61 μm and 0.62 ± 0.12, respectively. When this PE was used to produce DE (Fig. 10b) under the selected conditions (Table 1), the resulting DE was more uniform (\({DI}_{DE}\) ~ 0.001) compared to all previous samples. Upon freeze-drying and reconstitution (Fig. 10c), \({D}_{AB}\) remained smaller than \({D}_{DE}\), but the RC reached 0.873 ± 0.01, which is the highest among all antibubbles studied. This indicates that these antibubbles exhibited the greatest physical stability against the preparation process. Importantly, \({DI}_{AB}\) was less than 0.01, indicating that the prepared antibubbles were monodispersed, in contrast to previous antibubbles, which exhibited \({DI}_{AB}\) values exceeding 0.01 and reaching up to 0.06. The results obtained were the cumulative outcome of all the above-mentioned preparation parameters.
The size, dispersity index (DI) and optical images of primary emulsion (PE) (a), double emulsion (b), and antibubbles (c), and (d) the concentration of calcein in DE, freeze dried double emulsion (FDDE), and antibubbles during storage. \({W}_{1}\): internal aqueous phase, O: oil phase (intermediate phase in DE), \({W}_{2}\): continuous aqueous phase, and A: air phase (intermediate phase in antibubble). The bars and error bars represent the mean and standard deviation, respectively obtained from triplicate samples, each with at least 100 evaluations. Different small letter alphabets above the error bars indicate significant differences among the sample means (p < 0.05).
Encapsulation efficiency (EE) of calcein
The prepared DE showed a very high calcein loading efficiency (98.82 ± 0.28%), which significantly decreased during the storage up to 336 h (2 weeks) at 4 ± 2 °C (Fig. 10d). The release of hydrophilic components from \({W}_{1}\) to \({W}_{2}\) of DE occurs via diffusion52,72. Upon freeze-drying, the encapsulated calcein content in FDDE was reduced to 89.81 ± 0.17%. This reduction could be attributed to the collapse of some of the DE droplets, releasing \({W}_{1}\) to \({W}_{2}\) of DE. Previous studies in the preparation of antibubbles have also reported that the encapsulation efficiency of antibubbles were comparatively lower than that of their parent DEs18,19, although a direct comparison of their encapsulation stability was not conducted. However, the encapsulated calcein content in FDDE was maintained during storage, which resulted in considerably higher encapsulated calcein content in FDDE than in DE within a week (168 h) of storage at 4 ± 2 °C. The reduction in encapsulated calcein content in FDDE during the storage (4 ± 2 °C) was negligible. However, after rehydration, some additional release of calcein was observed until the first few hours, after which the release seemed to level off. Calcein encapsulation was 84.31 ± 0.26% even after 48 h of rehydration. This is in line with the work of Zia et al.17, who, albeit for a different release marker (methylene blue instead of calcein), found that after some initial release, potentially due to rupturing of some unstable antibubbles, there was no further release of encapsulated marker for at least 14 days.
Conclusions
DE-templated antibubbles were successfully prepared using a combination of HSH and T-junction microfluidics. All studied emulsification parameters significantly influenced (p < 0.05) on the properties of emulsions (PE and DE) and antibubbles. While most DEs exhibited monodispersity, achieving monodisperse antibubbles required the optimal combination of multiple parameters. The optimal conditions for antibubble formation included a higher homogenization speed (15,000 rpm), higher \({W}_{1}\) (15%), higher \({PC}_{O}\) (2.4%), higher FRR (35), higher \({PC}_{W2}\) (3%), smaller microchannel size (140 μm), additional 10% mannitol in \({W}_{1}\), and a freezing temperature of − 80 °C. Antibubbles were consistently smaller than their corresponding DEs, resulting in RC < 1, indicating some shrinkage during formation.
Although DEs demonstrated high loading of active components, their EE significantly declined during storage. Freeze-drying of DE slightly reduced EE, but the encapsulated calcein was stable in \({W}_{1}\) throughout the study. Reconstituting FDDE to form antibubbles restores their stable three-phase structure with an intermediate gaseous phase, presenting a viable strategy for bioactive component loading. Long term storage of FDDE followed by rehydration into antibubbles proved to be more effective than direct DE delivery. Future studies should focus on scaling up production, improving EE during the DE-to-antibubble transition, and evaluating stability and release dynamics under in-vitro gastrointestinal simulations using human digestive fluids and tissues for more accurate in vivo predictions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Zia, R., Nazir, A., Poortinga, A. T. & van Nostrum, C. F. Advances in antibubble formation and potential applications. Adv. Colloid Interface Sci. 305, 102688 (2022).
Niroula, A. & Nazir, A. New insights into antibubble formation by single drop impact on a same-liquid pool. J. Colloid Interface Sci. 662, 19–30 (2024).
Hughes, W. & Hughes, A. R. Liquid drops on the same liquid surface. Nature 129, 59 (1932).
Baird, M. H. I. The stability of inverse bubbles. Trans. Faraday Soc. 56, 213–219 (1960).
Skogen, N. Inverted soap bubbles-A surface phenomenon. Am. J. Phys. 24, 239 (1956).
Stong, C. L. The amateur scientist. Sci. Am. 230, 116–121 (1974).
Wang, W. et al. Antibubble formation through single drop impact: Effect of density difference. Phys. Fluids 36, (2024).
Vitry, Y., Dorbolo, S., Vermant, J. & Scheid, B. Controlling the lifetime of antibubbles. Adv. Colloid Interface Sci. 270, 73–86 (2019).
Poortinga, A. T. Long-lived antibubbles: stable antibubbles through Pickering stabilization. Langmuir 27, 2138–2141 (2011).
Schroën, K., Shen, X., Hasyyati, F. I., Deshpande, S. & van der Gucht, J. From theoretical aspects to practical food Pickering emulsions: Formation, stabilization, and complexities linked to the use of colloidal food particles. Adv. Colloid Interface Sci. 334, 103321 (2024).
Niroula, A., Gamot, T. D., Ooi, C. W. & Dhital, S. Biomolecule-based pickering food emulsions: Intrinsic components of food matrix, recent trends and prospects. Food Hydrocoll. 112, 106303 (2021).
Anuj, Niroula Akmal, Nazir Karin, Schroën (2025) Particle-dominated double emulsions: concept of Pickering stabilization interfacial challenges and emerging opportunities in food systems. Future Foods 100668. https://doi.org/10.1016/j.fufo.2025.100668.
Albert T., Poortinga (2011) Long-Lived Antibubbles: Stable Antibubbles through Pickering Stabilization. Langmuir 27(6), 2138-2141. https://doi.org/10.1021/la1048419.
Silpe, J. E. & McGrail, D. W. Magnetic antibubbles: Formation and control of magnetic macroemulsions for fluid transport applications. J. Appl. Phys. 113, 17B304 (2013).
Poortinga, A. T. Micron-sized antibubbles with tunable stability. Colloids Surf. A Physicochem. Eng. Asp. 419, 15–20 (2013).
Kotopoulis, S. et al. Formulation and characterisation of drug-loaded antibubbles for image-guided and ultrasound-triggered drug delivery. Ultrason. Sonochem. 85, 105986 (2022).
Araya-Hermosilla, R. et al. Pickering emulsions and antibubbles stabilized by PLA/PLGA nanoparticles. Langmuir 38, 182–190 (2022).
Zia, R., Poortinga, A. T., Nazir, A., Aburuz, S. & van Nostrum, C. F. Triple-emulsion-based antibubbles: A step forward in fabricating novel multi-drug delivery systems. Pharmaceutics 15, 2757 (2023).
Zia, R., Poortinga, A. T., Nazir, A., Ayyash, M. & van Nostrum, C. F. Preparation of acid-responsive antibubbles from CaCO3-based Pickering emulsions. J. Colloid Interface Sci. 652, 2054–2065 (2023).
Kim, J. W. et al. Recent advances in the microfluidic production of functional microcapsules by multiple-emulsion templating. Lab Chip 22, 2259–2291 (2022).
Wei, X. et al. Recent advances on the generation, stabilization, and potential applications of double emulsions based on the microfluidic strategy. Food Eng. Rev. 16, 129–145 (2024).
Nekouei, M. & Vanapalli, S. A. Volume-of-fluid simulations in microfluidic T-junction devices: Influence of viscosity ratio on droplet size. Phys. Fluids 29, 32007 (2017).
Abate, A. R. et al. Impact of inlet channel geometry on microfluidic drop formation. Phys. Rev. E Stat. Nonlinear. Soft Matter Phys. 80, 026310 (2009).
Loizou, K., Wong, V. L. & Hewakandamby, B. Examining the effect of flow rate ratio on droplet generation and regime transition in a microfluidic t-junction at constant capillary numbers. Inventions 3, 54 (2018).
Liu, H. & Zhang, Y. Droplet formation in a T-shaped microfluidic junction. J. Appl. Phys. 106, 034906 (2009).
Li, X. B. et al. Study on the mechanism of droplet formation in T-junction microchannel. Chem. Eng. Sci. 69, 340–351 (2012).
Maan, A. A., Nazir, A., Khan, M. K. I., Boom, R. & Schroën, K. Microfluidic emulsification in food processing. J. Food Eng. 147, 1–7 (2015).
Ho, T. M., Razzaghi, A., Ramachandran, A. & Mikkonen, K. S. Emulsion characterization via microfluidic devices: A review on interfacial tension and stability to coalescence. Adv. Colloid Interface Sci. 299, 102541 (2022).
Oveysi, M., Bazargan, V., Nejat, A. & Marengo, M. Exploring the stability of single emulsion created by microfluidics and its use in the production of core–shell microparticles. Microfluid. Nanofluidics 28, 1–17 (2024).
Silpe, J. E., Nunes, J. K., Poortinga, A. T. & Stone, H. A. Generation of antibubbles from core-shell double emulsion templates produced by microfluidics. Langmuir 29, 8782–8787 (2013).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schmitt, V., Leal-Calderon, F. & Bibette, J. Preparation of Monodisperse Particles and Emulsions by Controlled Shear. In Colloid Chemistry II 195–215 (Springer, 2003). https://doi.org/10.1007/3-540-36412-9_8.
Maan, A. A., Schroën, K. & Boom, R. Spontaneous droplet formation techniques for monodisperse emulsions preparation—Perspectives for food applications. J. Food Eng. 107, 334–346 (2011).
ten Klooster, S., Sahin, S. & Schroën, K. Monodisperse droplet formation by spontaneous and interaction based mechanisms in partitioned EDGE microfluidic device. Sci. Rep. 9, 7820 (2019).
Jiao, Y. et al. Enhancing the formation and stability of oil-in-water emulsions prepared by microchannels using mixed protein emulsifiers. Front. Nutr. 9, 822053 (2022).
Yi, L. et al. Physical mechanisms for droplet size and effective viscosity asymmetries in turbulent emulsions. J. Fluid Mech. 951, A39 (2022).
Niroula, A., Alshamsi, R., Sobti, B. & Nazir, A. Optimization of pea protein isolate-stabilized oil-in-water ultra-nanoemulsions by response surface methodology and the effect of electrolytes on optimized nanoemulsions. Colloids Interfaces 6, 47 (2022).
L’Estimé, M., Schindler, M., Shahidzadeh, N. & Bonn, D. Droplet size distribution in emulsions. Langmuir 40, 275–281 (2024).
Yulianingsih, R. & Gohtani, S. The influence of stirring speed and type of oil on the performance of pregelatinized waxy rice starch emulsifier in stabilizing oil-in-water emulsions. J. Food Eng. 280, 109920 (2020).
Chideme, N. & De Vaal, P. Effect of liquid viscosity and surface tension on the spray droplet size and the measurement thereof. J. Appl. Fluid Mech. 17, 2652–2672 (2024).
Xie, B. et al. Numerical investigation of the effect of microfluidic flow parameters and physical properties on double emulsion droplet forming. Meccanica 59, 157–168 (2024).
Opalski, A. S., Makuch, K., Derzsi, L. & Garstecki, P. Split or slip—passive generation of monodisperse double emulsions with cores of varying viscosity in microfluidic tandem step emulsification system. RSC Adv. 10, 23058–23065 (2020).
Farah, M. A., Oliveira, R. C., Caldas, J. N. & Rajagopal, K. Viscosity of water-in-oil emulsions: Variation with temperature and water volume fraction. J. Pet. Sci. Eng. 48, 169–184 (2005).
Kamnerdsook, A. et al. Formation of double emulsion micro-droplets in a microfluidic device using a partially hydrophilic–hydrophobic surface. RSC Adv. 11, 35653–35662 (2021).
Wang, X., Li, D., Pang, Y. & Liu, Z. Pinch-off dynamics of double-emulsion droplets with/without the influence of interfacial coupling effect. Phys. Fluids 34, (2022).
Liu, Z. et al. Dynamic mechanism of double emulsion droplets flowing through a microfluidic T-junction. Phys. Fluids 35, (2023).
Wanning, S., Süverkrüp, R. & Lamprecht, A. Impact of excipient choice on the aerodynamic performance of inhalable spray-freeze-dried powders. Int. J. Pharm. 586, 119564 (2020).
Marefati, A., Rayner, M., Timgren, A., Dejmek, P. & Sjöö, M. Freezing and freeze-drying of pickering emulsions stabilized by starch granules. Colloids Surfaces A Physicochem. Eng. Asp. 436, 512–520 (2013).
Niroula, A. et al. Natural stabilizers for functional foods: The role of optimized date seed extracts in nanoemulsion applications. LWT 208, 116732 (2024).
Håkansson, A. Experimental methods for measuring coalescence during emulsification—A critical review. J. Food Eng. 178, 47–59 (2016).
Williams, Y. O. N., Wensveen, M., Corstens, M. & Schroën, K. Coalescence kinetics of high internal phase emulsions observed by a microfluidic technique. J. Food Eng. 362, 111739 (2024).
Heidari, F., Jafari, S. M., Ziaiifar, A. M. & Malekjani, N. Stability and release mechanisms of double emulsions loaded with bioactive compounds; a critical review. Adv. Colloid Interface Sci. 299, 102567 (2022).
Liu, Z., Chai, M., Chen, X., Hejazi, S. H. & Li, Y. Emulsification in a microfluidic flow-focusing device: Effect of the dispersed phase viscosity. Fuel 283, 119229 (2021).
Guerrero, J. et al. Capillary-based microfluidics—coflow, flow-focusing, electro-coflow, drops, jets, and instabilities. Small 16, 1904344 (2020).
Van Puyvelde, P., Velankar, S., Mewis, J., Moldenaers, P. & Leuven, K. U. Effect of marangoni stresses on the deformation and coalescence in compatibilized immiscible polymer blends. Polym. Eng. Sci. 42, 1956–1964 (2002).
Schmitt, M. & Stark, H. Marangoni flow at droplet interfaces: Three-dimensional solution and applications. Phys. Fluids 28, (2016).
Hinderink, E. B. A., Münch, K., Sagis, L., Schroën, K. & Berton-Carabin, C. C. Synergistic stabilisation of emulsions by blends of dairy and soluble pea proteins: Contribution of the interfacial composition. Food Hydrocoll. 97, 105206 (2019).
Sun, Z. et al. Pickering emulsions stabilized by colloidal surfactants: Role of solid particles. Particuology 64, 153–163 (2022).
Rey, L. & May, J. C. Freeze-Drying/Lyophilization Of Pharmaceutical & Biological Products, Revised and Expanded. Freeze-Drying/Lyophilization Of Pharmaceutical & Biological Products, Revised and Expanded (Taylor & Francis Group, 2004). https://doi.org/10.1201/9780203021323.
Jiao, J. & Burgess, D. J. Multiple Emulsion Stability: Pressure Balance and Interfacial Film Strength. In Multiple Emulsions: Technology and Applications (ed. Aserin, A.) 1–27 (Wiley, Ltd, 2007). https://doi.org/10.1002/9780470209264.CH1.
Kumar, H. & Kumar, V. Preparation of water-in-diesel oil nano-emulsion using nonionic surfactants with enhanced stability and flow properties. J. Dispers. Sci. Technol. 39, 560–570 (2018).
Garstecki, P., Fuerstman, M. J., Stone, H. A. & Whitesides, G. M. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab Chip 6, 437–446 (2006).
Moon, S. K., Cheong, I. W. & Choi, S. W. Effect of flow rates of the continuous phase on droplet size in dripping and jetting regimes in a simple fluidic device for coaxial flow. Colloids Surf. A Physicochem. Eng. Asp. 454, 84–88 (2014).
Ponce-Torres, A., Rubio, M., Herrada, M. A., Eggers, J. & Montanero, J. M. Influence of the surface viscous stress on the pinch-off of free surfaces loaded with nearly-inviscid surfactants. Sci. Rep. 10, 1–12 (2020).
Vladisavljević, G. T. et al. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv. Drug Deliv. Rev. 65, 1626–1663 (2013).
Gulati, S. et al. Microdroplet formation in rounded flow-focusing junctions. Microfluid. Nanofluidics 20, 1–9 (2016).
Morais, A. R. D. V. et al. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 503, 102–114 (2016).
Chuacharoen, T., Moolwong & Chysirichote, T. Effects of maltodextrin on physicochemical properties of freeze-dried avocado powder. J. Homepage 5, 178–186 (2021).
Wu, H. Y., Sun, C. B. & Liu, N. Effects of different cryoprotectants on microemulsion freeze-drying. Innov. Food Sci. Emerg. Technol. 54, 28–33 (2019).
Nowak, D. & Jakubczyk, E. The freeze-drying of foods⇔the characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 9, 1488 (2020).
Tchessalov, S. et al. Practical advice on scientific design of freeze-drying process: 2023 update. Pharm. Res. 40, 2433–2455 (2023).
Gharehbeglou, P., Jafari, S. M., Homayouni, A., Hamishekar, H. & Mirzaei, H. Fabrication of double W1/O/W2 nano-emulsions loaded with oleuropein in the internal phase (W1) and evaluation of their release rate. Food Hydrocoll. 89, 44–55 (2019).
Funding
This study was funded by United Arab Emirates University (Grant no. G00003356).
Author information
Authors and Affiliations
Contributions
A.N.: Conceptualization; data curation; formal analysis; investigation; methodology validation; visualization; writing—original draft. R.Z.: Investigation; methodology. A.P.: Methodology; writing—review and editing. A.N.: Conceptualization; methodology; supervision; writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
Albert Poortinga holds a patent related to microbubble encapsulation technology, which may be considered a potential competing financial interest. All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Niroula, A., Zia, R., Poortinga, A. et al. Double emulsion templated monodisperse antibubbles via combined high-shear homogenization and T-junction microfluidics. Sci Rep 15, 23124 (2025). https://doi.org/10.1038/s41598-025-04009-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04009-0