Abstract
Saliva, known for better patient compliance and simpler collection, is ideal for monitoring antiseizure medication (ASM) levels. This study aimed to validate saliva for measuring lacosamide, develop a pharmacokinetic (PK) model, and determine the optimal saliva concentration for seizure control in epilepsy patients. In our prospective study at Seoul National University Hospital from August 2021 to November 2022, we enrolled lacosamide-prescribed epilepsy patients, collecting their saliva and blood samples. We developed a population PK model with nonlinear mixed-effects modeling, incorporating a saliva compartment and plasma-to-saliva distribution scaling factor. The model, factoring in CYP2C19 genotypes, demographics, and concurrent ASM use, estimated optimal saliva lacosamide concentration cutoffs for well-controlled seizures in high seizure burden patients. These values were validated through a two-year longitudinal analysis. In our study, 123 epilepsy patients prescribed lacosamide were finally analyzed. We identified 74 matched pairs of blood and saliva samples, finding a linear relationship between their lacosamide concentrations (R = 0.62, P < 0.001). Using our PK model, we estimated individual peak (Cmax) and trough concentrations in saliva and blood based on dosage, determining optimal saliva cutoffs for well-controlled seizure status in lacosamide: 15.94 mg/L for Cmax and 9.056 mg/L for trough, with 72.7% sensitivity and 88.2% specificity. Longitudinal analysis showed well-controlled seizure status achievement aligning with times when estimated Cmax and trough surpassed these cutoffs. Our research presents the potential and validity of using saliva concentration as an alternative to blood concentration for lacosamide TDM, advancing personalized pharmacotherapy in epilepsy treatment.
Similar content being viewed by others
Introduction
Lacosamide, a recently developed voltage-gated ion channel inhibitor approved by the FDA in 2009, is considered one of the new-generation antiseizure medications (ASMs)1,2. It offers advantages such as effectiveness, tolerability, and minimal drug-drug interactions compared to previous ASMs1,3,4. However, current management guidelines only provide a therapeutic dose range, leaving the determination of an appropriate dose for individual patients largely dependent on the clinician’s discretion1,5. To achieve personalized management with objective indicators, therapeutic drug monitoring (TDM) is crucial, especially in high seizure burden patients6,7. However, even with TDM in place, measuring a patient’s blood concentration regularly is challenging due to the invasive nature of blood sampling, making it less feasible in outpatient settings.
As an alternative to blood sampling, saliva has emerged as a promising method for measuring drug concentrations8,9. The correlation between saliva concentration and blood concentration has been demonstrated for some new-generation ASMs in small-scale pilot studies9,10,11,12. However, such evidence is not well-established in a larger patient population, and population pharmacokinetic (PK) models have not been developed for this purpose. Furthermore, there is a lack of research confirming whether saliva can be used for TDM and its association with the anti-seizure effect.
Therefore, in this study, we aimed to first confirm whether there is a correlation between lacosamide’s saliva and blood concentrations in a larger number of patients. Additionally, we developed a population PK model capable of estimating both blood and saliva concentrations, incorporating individual genetic polymorphism information of Cytochrome P450 enzymes. Through this research, we sought to identify the optimal cutoff value for estimated saliva concentration that demonstrates an anti-seizure effect and investigate whether saliva samples can be used for TDM based on longitudinal data.
Materials and methods
Patient enrollment
In our prospective study, we recruited patients with epilepsy who had been prescribed lacosamide between August 2021 and November 2022 at Seoul National University Hospital (SNUH). ASM regimens including total daily dose of lacosamide was maintained at least for one month before the enrollment in order to assume a steady state13. The time points at which the last lacosamide dose was taken and the sample collection of blood and saliva were measured. Additionally, we considered potential confounding factors by investigating whether patients had recently consumed food, smoked, or brushed their teeth within an hour of sample collection9,10. We also assessed for any oral health issues, including dental cavities, to account for their potential influence on the study variables. This study was approved by SNUH Institutional Review Board (IRB number: 2104-146-1213) and all informed consent was received from enrolled patients and/or legal guardians. All methods were performed in accordance with the relevant guidelines and regulations.
Sample collection
Saliva and blood samples were simultaneously collected during outpatient sessions, ensuring that there was no more than a 30-min gap between the collection times. For blood samples, a minimum of 5 mL was collected into sodium-heparin tubes, serum-separate tubes, and/or ethylene diamine acetic acid tubes (BD Biosciences). To maintain consistency and eliminate potential confounding conditions, the collection of saliva samples followed a standardized procedure10. Initially, patients rinsed their mouths with water and expectorated a minimum of 1–3 mL of unstimulated saliva into untreated polypropylene tubes (BD Biosciences). All samples were promptly stored at − 70 °C within 3 h of collection.
Determination of lacosamide concentration
The plasma, serum, and saliva concentrations of lacosamide was analyzed by validated liquid chromatography‒mass spectrometry (LC‒MS) using Waters Acquity system with Hypersil Gold column (100 × 2.1 mm, 3 µm, Thermo Scientific, USA) and Xevo TQ MS (Waters Corporation, USA). Lacosamide-d3 was used as an internal standard. The mobile phase was consisted with 0.1% formic acid in distilled water and acetonitrile in a 60:40 ratio under gradient conditions with a flow rate of 0.25 mL/min. The lacosamide concentration was calculated using the peak area ratio of lacosamide to lacosamide-d3. The calibration curve was linear in the concentration range of 0.01–10 mg/L in saliva and 0.1–10 mg/L in blood (plasma and serum). The within- and between-batch precision results of lacosamide were within the range of 1.40–3.81%, and accuracies were within the range of 99.6–104.3% in blood sample. The corresponding values were 0.43 to 2.81% and 97.8 to 104.7%, respectively, in lacosamide saliva sample.
Analysis of clinical profiles
In our study, we assessed the baseline seizure burden by querying participants about their seizure events over a minimum six-month period. The number of days with seizure events was calculated, and participants were classified into groups: seizure-free, less than one seizure event day per month, more than one seizure event day per month, more than one seizure event day every three weeks, and more than one seizure event day every two weeks. The high seizure burden group included those experiencing more than one seizure event day per month. Within this group, the severe seizure burden group consisted of patients with more than one seizure event day every two weeks, while others were categorized as the less-severe seizure burden group. In addition to the seizure burden, to assess the effectiveness of seizure management, we defined well-controlled seizure status as the point at which no further adjustments were made for seizure control for at least two months, with patients expressing satisfaction with their current condition. Furthermore, comprehensive clinical data were collected, including height, body weight, seizure type (focal or generalized), ASM numbers prescribed, adverse events due to any ASM, laboratory findings, electroencephalogram (EEG) data, and brain magnetic resonance imaging (MRI) scans.
For longitudinal analysis, we collected seizure frequency, lacosamide total daily dose, ASM numbers prescribed, and well-controlled seizure status at each of the following time points: at the time of study enrollment (baseline) and every two months (± 2 weeks) for two years. Time points without available data were recorded as blank14.
Genetic analysis
DNA extraction from peripheral whole blood samples was carried out to analyze the genotype of CYP2C9 and CYP2C19, using the Maxwell CSC Blood DNA Kit and Maxwell CSC Instrument (Promega). Subsequently, we employed TaqMan allelic discrimination assays on a real-time polymerase chain reaction (RT-PCR) system (Applied Biosystems). The results of allelic discrimination were determined through the utilization of 7500 Real-Time PCR System software, version 2.0.6 (Applied Biosystems).
In terms of CYP2C9 genotypes, individuals were classified as normal metabolizers (*1/*1) and intermediate metabolizers (*1/*3). For CYP2C19, patients were categorized into three distinct phenotypes: normal metabolizers (*1/*1), intermediate metabolizers (*1/*2 or *3), and poor metabolizers (*2/*2, *2/*3, or *3/*3)13.
Pharmacokinetic analysis and modeling
The population PK model of lacosamide was developed using nonlinear mixed-effects modeling using NONMEM (version 7.4.0, ICON Development Solutions, Ellicott City, USA) with Pirana (version 2.9.9, Certara, Princeton, USA) interface15,16. The parameters were estimated using the first-order conditional estimation method with the interaction option. The previously published population PK model of lacosamide in healthy subjects was the starting point of the base model17. It was a one compartment model with first-order absorption and elimination, where creatinine clearance (CLcr) affected the apparent clearance (CL/F) and body surface area (BSA) impacted on apparent volume of distribution (Vd/F). Because our patients’ data were obtained by sparse sampling, we fixed the absorption rate (Ka), Vd/F, and the power coefficient of BSA to Vd/F based on values from the published model. Saliva compartment model and scaling factor for plasma to saliva distribution model was assessed to explain the saliva PK of lacosamide. Proportional error model was explored for the residual error of blood and saliva concentration. The PK model was evaluated based on numerical and graphical diagnostics, including goodness-of-fit plots, prediction-corrected visual predictive check, and precision of final estimates by bootstrap. Covariate effects were assessed including CYP2C19 genotypes, demographic characteristics, and concomitant use of other ASMs, such as topiramate, perampanel, phenytoin, valproate, carbamazepine, phenobarbital, lamotrigine, oxcarbazepine, pregabalin, zonisamide, levetiracetam, gabapentin, clobazam, vigabatrin, lorazepam, and rufinamide.
Statistics
The data were presented as mean ± standard deviation (range), median [interquartile range], or number (percentage) as appropriate. Continuous variables were analyzed using T-tests, while categorical variables were assessed using chi-square tests or Fisher’s exact tests. To explore relationships between variables, correlation analysis was performed using Pearson correlation coefficients. Additionally, the Jonckheere-Terpstra test was employed for trend analysis among multiple groups to evaluate the presence of ordered relationships. For analyzing the predictive power of estimated saliva concentration for well-controlled seizure status, variables with P-values < 0.10 in univariate analysis were included in multivariable analyses.
To visually interpret longitudinal clinical profiles and estimated saliva concentration over time, we utilized locally estimated scatterplot smoothing lines (LOESS) for flexible nonlinear regression18. To compare the estimated saliva concentration between time points before and after achieving well-controlled seizure status, we employed a mixed model implemented in GraphPad Prism 9.5.1 instead of repeated measures ANOVA, considering the presence of missing values. Multiple comparisons between time points were adjusted using Tukey’s method.
We employed linear mixed-effects models (LMM) to examine variations in the time-dependent changes in clinical profiles. These models included random slopes and intercepts at the participant level, considering pertinent variables like age, baseline lacosamide total daily dose, and baseline ASM numbers prescribed. Additionally, an interaction term between time and the clinical factor under investigation was incorporated.
Statistical analyses were carried out with a significance level set at P < 0.05 and were performed using R software version 4.3.0 (2023; R Team, Vienna, Austria) and GraphPad Prism software version 9.5.1 (2023; GraphPad software, MA, U.S.A.).
Results
Patient characteristics
Out of the 128 patients prescribed lacosamide, five individuals were excluded from the study. Four patients dropped the study, and one patient did not provide a sufficient saliva sample. As a result, the final study analysis included 123 patients (Supplementary Fig. 1).
For the patients included in the analysis, the average age was 41.3 ± 14.2 years (Table 1). Of these, 67 patients (54.5%) were male. The majority of the patients experienced focal-type seizures (113, 91.9%). All patients were prescribed lacosamide on a twice-a-day basis, and the median dose was 300 mg/day [interquartile range, 200–400]. Among them, 77 patients (63.1%) were receiving polytherapy with ASMs. The median number of ASMs prescribed was 3 [2,3,4,5]. Additionally, 37 patients (30.3%) reported experiencing adverse events related to the use of any ASMs. At the time of study enrollment, approximately half of the patients (63, 51.2.%) had achieved seizure freedom and 76.4% (94/123) of the patients were well controlled showing tolerable seizure events, which no additional ASM management was done at least for two months.
All patients except two had available EEG data. Among them, epileptiform discharges were documented in 83.5% (101/123) of the participants and temporal lobe epilepsy was the most common (79/121, 65.3%). At brain MRI, 51.4% (55/107) of the patients showed structural lesion.
Saliva effectively represented lacosamide concentration as blood
Among the 123 enrolled patients, fourteen saliva samples (11.4%) were excluded from the analysis due to quality control issues, and an additional 37 patients (30.1%) did not adhere to the blood collection process. As a result, the final analysis comprised 109 saliva samples from 109 unique patients and 102 blood samples from 86 patients. Among these, 74 matched pairs were identified, representing 74 patients (Supplementary Fig. 1). The median time for sample collection was 4.25 h [interquartile range, 2.57–7] for saliva and 4.67 h [3.03–6.86] for blood (Table 1).
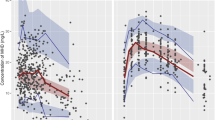
Our investigation revealed a linear correlation between lacosamide concentration and daily dose in both blood and saliva, irrespective of BSA normalization (Fig. 1A, B, and Supplementary Fig. 2). Additionally, we observed a linear relationship between saliva and blood lacosamide concentrations (R = 0.62, P < 0.001) (Linear regression; Csaliva = 4.932 + 0.652 × Cblood, adjusted R2 = 0.38) (Fig. 1C). This finding validates the reliability of saliva samples as representative of lacosamide concentration in clinical assessments.
Analysis of lacosamide Levels in blood and saliva in relation to dosage and genotype. (A, B) The linear correlation was shown between BSA-normalized daily dose of lacosamide (mg/m2/day) and its concentration in (A) blood (n = 86) and (B) saliva (n = 109), respectively. (C) A linear regression model described the relationship between blood and saliva lacosamide levels (n = 74) (Csaliva = 4.932 + 0.652 × Cblood, adjusted R2 = 0.38). (D, E) Boxplots represent the dose-normalized (D) blood (n = 146) and (E) saliva (n = 37) levels of lacosamide across three CYP2C19 genotype group, respectively, with significance determined using the Jonckheere-Terpstra test (blood level: P < 0.001, saliva level: P = 0.029). Dose normalization was calculated as concentration × 300 mg/day/daily dose (mg/day). Data in blood sample were merged with the previous study (Supplementary Table 1). BSA body surface area.
Next, we performed genetic analysis of CYP2C9 and CYP2C19 in 37 patients. For CYP2C9, 97.3% (36/37) were normal metabolizers, so further analysis was limited. On the other hand, CYP2C19 analysis revealed a diverse distribution: 35.1% (13/37) normal metabolizers, 48.6% (18/37) intermediate, and 16.2% (6/37) poor metabolizers. Merging data from blood samples with a larger previous study (Supplementary Table 1)13, we observed a distinct trend in lacosamide concentrations across CYP2C19 phenotypes (Fig. 1D and E). Both saliva and blood samples showed significantly gradient concentrations, with the highest in poor metabolizers, followed by intermediate and normal metabolizers (Blood: P < 0.001, Saliva: P = 0.029). This result also underscores the effective use of saliva samples in representing lacosamide concentration compared to blood samples.
Population pharmacokinetic model of lacosamide
In the development of the lacosamide PK model, we incorporated not only 109 spot saliva and 102 spot blood samples but also 59 follow-up saliva samples that were self-collected by patients during their subsequent outpatient visits (Supplementary Fig. 1). The lacosamide PK model in epilepsy patients was appropriately described with a one-compartment model, first-order absorption, and linear elimination with scaling factor for plasma to saliva distribution (Fig. 2A). The scaling factor of 1.44 well explained the saliva lacosamide concentration (Supplementary Table 2). The lacosamide CL/F was explained by following equation, having inter-individual variability of 23.8%: CL/F (L/h) = θ1 × (1 + 0.771 × carbamazepine + 0.467 × phenobarbital) × (CLcr/107)0.579 L/h, where θ1 was 1.78 L/h in CYP2C19 normal and intermediate metabolizer, and 1.43 in CYP2C19 poor metabolizer. Concomitant use of carbamazepine and phenobarbital increased the typical value of lacosamide CL/F with 77.1% and 46.7%, respectively (Supplementary Table 2). The goodness of fit plots showed adequate model structure to predict the lacosamide concentration in saliva and blood sample (Fig. 2B). The prediction-corrected visual predictive check plots, stratified by saliva and blood sample, also showed that the observed lacosamide levels were within the 95% prediction intervals (Fig. 2C). The final parameter estimates were similar to the median bootstrap estimates, showing a reasonably narrow range within the 95% confidence interval of the bootstrap analysis (Supplementary Table 2). Overall, the model was robust and adequate with good precision to characterize the blood and saliva PK properties of lacosamide in epilepsy patients.
Development of the population PK model for lacosamide. (A) Structure of lacosamide PK model, including a scale factor for plasma-to-saliva distribution of lacosamide. Ka, first order rate constant; CL/F, apparent clearance. (B) Goodness-of-fit-plots for the PK model of lacosamide, comparing observations with population and individual predictions, and displaying CWRES versus population predictions and time after last dose. (C) A prediction corrected visual predictive check of lacosamide model, stratified by blood and saliva groups. This part includes 500 simulated datasets. The observed concentrations are indicated by closed circles, with solid lines representing the 5th (blue), median (red), and 95th (blue) percentiles of the observed concentrations. The shaded blue and red areas highlight the 95% confidence intervals of the simulated concentrations for each percentile. CWRES conditional weighted residuals; PK pharmacokinetics.
Based on our pharmacokinetic model, we estimated the individual patients’ peak (Cmax) and trough concentrations of lacosamide in both saliva and blood with their dose regimen (Saliva: Cmax, 12.8 ± 4.65 mg/L, Trough, 7.56 ± 2.90 mg/L; Blood: Cmax, 8.89 ± 3.23 mg/L, Trough, 5.25 ± 2.02 mg/L) (Table 1 and Supplementary Table 3).
The prediction of the anti-seizure effect with saliva level of lacosamide in high seizure burden group
Subsequently, to explore the predictive capability of estimated saliva concentration for the anti-seizure effect of lacosamide, we classified patients based on their seizure burden over a minimum six-month period, as outlined in the method section. The low seizure burden group consisted of 95 patients experiencing fewer than one seizure event day per month, while the high seizure burden group comprised 28 patients with more than one seizure event day per month (Table 1). Notably, the high seizure burden group exhibited a younger age (P = 0.029), a higher daily dose of lacosamide (P = 0.018), a greater number of ASM prescribed (P < 0.001), more structural brain lesions in MRI (P = 0.045), and demonstrated poorer compliance to blood sample collection (P = 0.032) compared to the low seizure burden group. Detailed characteristics of the two groups were described in Table 1.
Given that the anti-seizure response within the low seizure burden group and lacosamide monotherapy group was already saturated with low concentrations of lacosamide (Supplementary Fig. 3), we focused on the high seizure burden group, which exhibited a higher demand for ASMs in both dose and number. Then, we analyzed the predictive factors for well-controlled seizure status in the high seizure burden group. In the multivariate analysis, the estimated saliva concentrations of lacosamide in Cmax, trough, and area under curve (AUC) remained significantly associated with well-controlled seizure status after accounting for age, sex, and the number of ASMs prescribed (Cmax: Odds ratio [OR] = 1.440, 95% confidence interval [CI] = 0.242–2.568, P = 0.024, Trough: OR = 1.398, 95% CI = 0.038–0.720, P = 0.046; AUC: OR = 1.029, 95% CI = 0.007–0.061, P = 0.027) (Supplementary Table 4). Based on the results, we identified optimal cutoff saliva concentrations for well-controlled seizure status in lacosamide, determining values of 15.94 mg/L for Cmax and 9.056 mg/L for trough, with a sensitivity of 72.7% and specificity of 88.2% (Fig. 3A and D).
Determination and validation of optimal cutoff values for saliva lacosamide concentration in the high seizure burden group. (A) In this group (n = 28), the optimal cutoff for peak saliva lacosamide concentration (Cmax) for achieving well-controlled seizure status was 15.94 mg/L, with a sensitivity of 72% and specificity of 88.2%. (B) The longitudinal analysis revealed a temporal correlation between achieving well-controlled seizure status and instances where Cmax exceeded 15.9 mg/L. The solid lines represent corresponding LOESS regression lines, with shaded areas indicating 95% CIs. The various colored spaghetti plots represent the longitudinal data for individual patients, tracking the changes in their lacosamide levels over time. (C) Comparison of estimated peak saliva concentrations before and after achieving seizure control showed significant differences (pre-4 months: 13.72 mg/L, at achievement: 16.51 mg/L, post-4 months: 15.87 mg/L, multiple comparison correction by Tukey’s method). (D) The optimal trough level cutoff was 9.056 mg/L, with the same sensitivity and specificity. (E) Well-controlled seizure status achievement times corresponded with trough levels above 9.06 mg/L. The solid lines represent corresponding LOESS regression lines, with shaded areas indicating 95% CIs. The various colored spaghetti plots represent the longitudinal data for individual patients, tracking the changes in their lacosamide levels over time. (F) Notable differences were observed in trough concentrations before and after seizure control (pre-4 months: 8.65 mg/L, at achievement: 10.32 mg/L, post-4 months: 9.92 mg/L, multiple comparison correction by Tukey’s method). CI confidence interval; LOESS locally estimated scatterplot smoothing.
Longitudinal analysis of PK estimates and well-controlled seizure status in the high seizure burden group
To validate these saliva lacosamide cutoff values in real-world scenarios, we analyzed longitudinal data over two years from the 28 participants in the high seizure burden group (Fig. 3 and Supplementary Table 5). Almost all patients, except two (92.9%), had a follow-up of at least one year, with 16 (57.1%) followed for one year and a half, and 4 (14.3%) for the full two years. A total of 1104 out of 1456 (75.8%) clinical data points were successfully collected at 13 different time points. Based on the clinical data, we estimated Cmax and trough saliva concentration at each time points by our developed PK model.
At the time of study enrollment, only 39.3% (11/28) of the patients were in a well-controlled seizure status, but within a mean duration of 4.75 ± 5.74 months (range 0–18), 85.75% (24/27) of the high seizure burden group eventually achieved a well-controlled seizure status (Supplementary Table 5). The median daily dose of lacosamide was 400 mg [IQR 337.5–500], and the median number of ASMs prescribed was 3 [IQR 2–4]. The estimated Cmax and trough saliva concentrations of lacosamide were 16.51 ± 4.24 mg/L (range 7.44–24.3) and 10.32 ± 3.18 mg/L (3–16.3), respectively, surpassing the predicted optimal cutoff values (15.94 mg/L for Cmax and 9.056 mg/L for trough).
According to visual inspection of the LOESS curves, the actual achievement of a well-controlled seizure status closely aligned with the time points when the estimated Cmax (Fig. 3B) and trough (Fig. 3E) surpassed their respective predicted cutoff values. Notably, the estimated saliva concentration within 4 months before achieving a well-controlled seizure status showed a significant difference compared to the post-achievement periods (Cmax: 13.72 mg/L vs 16.51 mg/L, P < 0.001; Trough: 8.65 mg/L vs 10.32 mg/L, P = 0.001, corrected for multiple comparisons using Tukey’s method) (Fig. 3C and F). These findings illustrate that the optimal cutoff values for lacosamide saliva concentration, obtained through the PK model, can reliably predict the anti-seizure effect in real-world scenarios.
Subgroup analysis showed greater influence of lacosamide dose than ASM polytherapy for seizure control in the high seizure burden group
In the results of the multivariate analysis (Supplementary Table 4), we confirmed that well-controlled seizure status had a significant association not only with the estimated saliva concentration of lacosamide but also with the number of ASMs prescribed. This prompted the need to ascertain whether seizure control was more influenced by lacosamide concentration in the body or by the impact of ASM polytherapy. To explore this, we conducted a subgroup analysis within the high seizure burden group (Fig. 4, Supplementary Table 3, 5, and 6). Within the high seizure burden group, 9 patients (32.1%) were categorized as the severe seizure burden group, experiencing more than one event day every two weeks, while the other 19 patients were in the less-severe seizure burden group.
Subgroup analysis in the high seizure burden group assessing the impact of lacosamide dosage increments on seizure control. (A) Saliva concentrations of lacosamide (spot, Cmax, and trough) in the high seizure burden group exhibited significant trends in the Jonckheere-Terpstra test, with the highest levels in patients having more than one seizure event per day and the lowest in those with one event every two weeks (spot level: P = 0.033; Cmax: P = 0.041; Trough: P = 0.033). (B) A longitudinal analysis comparing the severe (n = 9) and less-severe (n = 19) seizure burden groups showed a significantly faster decline in seizure frequency in the severe group (β = − 0.234, 95% CI, − 0.376 to − 0.090, P = 0.002). (C) In the same comparison, the lacosamide dose increased more rapidly in the severe group (β = 2.043, 95% CI, 0.315 to 3.816, P = 0.023). (D) There was no significant time-dependent difference in the number of ASM prescribed between the two groups (β = − 0.009, 95% CI, − 0.020 to 0.002, P = 0.126). The solid lines of (B), (C), and (D) represent corresponding LOESS regression lines, with shaded areas indicating 95% CIs. (E) The heatmaps illustrate the time-dependent changes in lacosamide dose and ASM number, highlighting a more intense augmentation of lacosamide dose in the severe seizure burden group compared to the less-severe group, without significant differences in ASM number. ASM antiseizure medication; CI confidence interval; LOESS locally estimated scatterplot smoothing.
At the time point of the study enrollment, lacosamide saliva concentrations in spot, Cmax, and trough exhibited significant trends, with the highest levels observed in patients experiencing more than one seizure event day per month and the lowest in those with one seizure event day every two weeks (spot level: P = 0.033; Cmax: P = 0.041; Trough: P = 0.033) (Fig. 4A and Supplementary Table 3). These findings suggest a positive correlation between higher lacosamide saliva concentration and improved seizure control.
Subsequently, we analyzed the longitudinal data of seizure frequency, lacosamide dose, and ASM prescription number for the severe and less-severe seizure burden groups. It was observed that the seizure frequency in the severe group significantly declined at a faster rate over time compared to the less-severe group (β = − 0.234, 95% CI, − 0.376 to − 0.090, P = 0.002) (Fig. 4B and Supplementary Table 6). Additionally, the lacosamide dose increased significantly faster in the severe group (β = 2.043, 95% CI, 0.315 to 3.816, P = 0.023) (Fig. 4C and Supplementary Table 6). However, the ASM number prescribed did not show a significant difference in time-dependent change between both groups (β = − 0.009, 95% CI, − 0.020 to 0.002, P = 0.126) (Fig. 4D and Supplementary Table 6). These trends were visually confirmed through a heatmap illustrating the changes in individual lacosamide dose and ASM number over time (Fig. 4E). Consequently, we obtained a clue that within the high seizure burden group, seizure control was influenced more towards supplementing insufficient concentrations through increasing lacosamide dose rather than via ASM polytherapy.
Discussion
This prospective study involved 123 participants to investigate the correlation between lacosamide’s saliva concentration and blood concentration. Additionally, we developed a population PK model that incorporates individual Cytochrome P450 genetic polymorphism information to estimate both saliva and blood concentrations. Through this approach, we determined optimal lacosamide cutoff values for saliva concentration that demonstrate an anti-seizure effect in the high seizure burden group: 15.94 mg/L for Cmax and 9.056 mg/L for trough. These values were further confirmed with longitudinal data to establish their validity in real-world scenarios.
Saliva sampling presents several advantages over blood in measuring ASM concentrations19. Notably, saliva sampling is associated with higher patient adherence compared to blood9,10. Indeed, in our study, while only 86 out of 123 enrolled participants provided blood samples, all 123 participated in saliva sampling. This is significant considering the 30.1% (37/123) non-adherence rate for blood sampling, much higher than the 11.4% (14/123) failure rate due to saliva quality control issues. Furthermore, in the high seizure burden group, there was a significantly higher non-adherence rate compared to the low seizure burden group (46.4% vs. 25.3%, P = 0.032), highlighting the usefulness of saliva samples, especially for TDM in patients with high seizure burden.
Building on the advantages of saliva sampling, saliva-based TDM presents a non-invasive, patient-friendly alternative, especially beneficial for those requiring optimized care amidst ASM polytherapy19. The optimal lacosamide cutoff values in saliva we propose can serve as a pivotal reference for deciding on dose augmentation or adjustments in add-on therapy for patients with poorly controlled seizures. Moreover, saliva-based monitoring enables the frequent tracking of ASM concentrations without the constraints of location and time8,19. This flexibility is particularly valuable for patients experiencing breakthrough seizures or those undergoing continuous dose adjustments, as it allows for TDM through home self-sampling. In fact, our developed PK model incorporated 59 self-collected follow-up samples from patients’ homes, demonstrating its practical application in real-world settings.
To address variability in sampling times, we applied a population PK approach to estimate individual trough, peak concentrations, and systemic exposure, considering CYP genotypes and concomitant medications as covariates. The model well described lacosamide distribution, with a saliva-to-plasma concentration ratio of 1.44, indicating preferential excretion into saliva. According to the salivary excretion classification system, lacosamide can be classified as a Class I compound, characterized by high intestinal permeability and low plasma protein binding (< 15%)20,21. This classification suggests that lacosamide is likely to undergo salivary excretion, which is consistent with our findings and supports the feasibility of saliva-based TDM. Although an empirical PK model was used, a physiologically based pharmacokinetic model approach could be considered in future research to mechanistically simulate salivary distribution and further validate our findings.
Our study faced the challenge of assessing the high seizure burden group, who were receiving ASM polytherapy alongside lacosamide, which made it difficult to isolate the sole effects of lacosamide monotherapy. Nevertheless, the lacosamide concentrations we identified—15.94 mg/L for Cmax and 9.056 mg/L for trough—are consistent with the therapeutic ranges reported in other studies6,13. Subgroup analysis within the high seizure burden group indicated that dosage increases of lacosamide were more influential in controlling seizure frequency than the augmentation of ASM polytherapy, underscoring the substantial role of lacosamide concentration in its anti-seizure activity. Yet, this analysis provides indirect evidence, and further well-designed studies are needed to confirm these findings through actual saliva-based TDM.
Our invesigation has several limitations; Our longitudinal data collection focused only on lacosamide doses, leaving open the possibility that the observed anti-seizure effects might have been influenced by dose adjustments in other ASMs. Adverse events were also intertwined with ASM polytherapy, complicating the assessment of an upper lacosamide threshold without other drug interactions. Furthermore, the classification of patients’ seizure burden was based on self-reporting, which could introduce recall bias. Future research should involve larger and more diverse patient populations and include a range of ASMs to be monitored via saliva sampling. Saliva-based TDM holds particular promise for pediatric research, where blood collection poses a greater challenge. The findings from this study lay the groundwork for such progressive research, moving towards more patient-centric and less invasive monitoring methods in epilepsy pharmacotherapy.
Conclusion
The primary objective of our study was to explore personalized pharmacotherapy with lacosamide. We validated the utility of saliva as a convenient and adherent sampling method for patients. By developing a population PK model that incorporates individual patient characteristics, our study not only established a therapeutic range for lacosamide levels in saliva among patients with a high demand for TDM but also demonstrated the practical feasibility of implementing saliva-based TDM in clinical practice.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request upon reasonable request of any qualified investigator for purposes of replicating procedures and results. If such data are used for a publication, its methods should be communicated, and internationally recognized authorship rules should be applied.
References
Kanner, A. M. et al. Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs II: Treatment-resistant epilepsy. Neurology 91, 82–90. https://doi.org/10.1212/WNL.0000000000005756 (2018).
Ben-Menachem, E. et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia 48, 1308–1317. https://doi.org/10.1111/j.1528-1167.2007.01188.x (2007).
Chung, S. et al. Lacosamide as adjunctive therapy for partial-onset seizures: A randomized controlled trial. Epilepsia 51, 958–967. https://doi.org/10.1111/j.1528-1167.2009.02496.x (2010).
Zaccara, G., Perucca, P., Loiacono, G., Giovannelli, F. & Verrotti, A. The adverse event profile of lacosamide: A systematic review and meta-analysis of randomized controlled trials. Epilepsia 54, 66–74. https://doi.org/10.1111/j.1528-1167.2012.03589.x (2013).
Babar, R. K., Bresnahan, R., Gillespie, C. S. & Michael, B. D. Lacosamide add-on therapy for focal epilepsy. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD008841.pub3 (2021).
Patsalos, P. N., Spencer, E. P. & Berry, D. J. Therapeutic drug monitoring of antiepileptic drugs in epilepsy: A 2018 update. Ther. Drug Monit. 40, 526–548. https://doi.org/10.1097/ftd.0000000000000546 (2018).
Patsalos, P. N. et al. Antiepileptic drugs–best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE commission on therapeutic strategies. Epilepsia 49, 1239–1276. https://doi.org/10.1111/j.1528-1167.2008.01561.x (2008).
Liu, H. & Delgado, M. R. Therapeutic drug concentration monitoring using saliva samples. Focus on anticonvulsants. Clin. Pharmacokinet. 36, 453–470. https://doi.org/10.2165/00003088-199936060-00006 (1999).
Greenaway, C., Ratnaraj, N., Sander, J. W. & Patsalos, P. N. Saliva and serum lacosamide concentrations in patients with epilepsy. Epilepsia 52, 258–263. https://doi.org/10.1111/j.1528-1167.2010.02751.x (2011).
Kim, D.-Y. et al. Usefulness of saliva for perampanel therapeutic drug monitoring. Epilepsia 61, 1120–1128. https://doi.org/10.1111/epi.16513 (2020).
Rolan, P., Sargentini-Maier, M. L., Pigeolet, E. & Stockis, A. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy men. Br. J. Clin. Pharmacol. 66, 71–75. https://doi.org/10.1111/j.1365-2125.2008.03158.x (2008).
Brandt, C., Bien, C. G., Helmer, R. & May, T. W. Assessment of the correlations of lacosamide concentrations in saliva and serum in patients with epilepsy. Epilepsia 59, e34–e39. https://doi.org/10.1111/epi.14023 (2018).
Ahn, S.-J. et al. Effects of CYP2C19 genetic polymorphisms on the pharmacokinetics of lacosamide in Korean patients with epilepsy. Epilepsia 63, 2958–2969. https://doi.org/10.1111/epi.17399 (2022).
Lee, W. J. et al. Seronegative autoimmune encephalitis: clinical characteristics and factors associated with outcomes. Brain 145, 3509–3521. https://doi.org/10.1093/brain/awac166 (2022).
Jang, Y. et al. Population pharmacokinetic model development and its relationship with adverse events of oxcarbazepine in adult patients with epilepsy. Sci. Rep. 11, 6370. https://doi.org/10.1038/s41598-021-85920-0 (2021).
Lee, S. et al. Topiramate dosage optimization for effective antiseizure management via population pharmacokinetic modeling. Ann. Clin. Transl. Neurol. https://doi.org/10.1002/acn3.51962 (2023).
Kim, S. E. et al. Pharmacokinetics of lacosamide in healthy korean male volunteers. Pharmacology 89, 172–178. https://doi.org/10.1159/000336774 (2012).
Erickson, P. et al. Prevalence and clinical implications of a β-amyloid–negative, tau-positive cerebrospinal fluid biomarker profile in alzheimer disease. Jama Neurol. 80, 969–979. https://doi.org/10.1001/jamaneurol.2023.2338 (2023).
Patsalos, P. N. & Berry, D. J. Therapeutic drug monitoring of antiepileptic drugs by use of saliva. Ther. Drug. Monit. 35, 4–29. https://doi.org/10.1097/FTD.0b013e31827c11e7 (2013).
Idkaidek, N. & Arafat, T. Saliva versus plasma pharmacokinetics: Theory and application of a salivary excretion classification system. Mol. Pharm. 9, 2358–2363. https://doi.org/10.1021/mp300250r (2012).
(FDA), U.S. Food and Drug Adminstration VIMPAT® (lacosamide) Tablets and Injection Label, <https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022253lbl.pdf> (2008).
Funding
This research was supported by a grant from SK Chemicals Pharmaceuticals (Grant No. 0620213640).
Author information
Authors and Affiliations
Contributions
Y.J. and S.L. drafted the manuscript. K-Y.J., K-S.Y., K.C., I-J.J., and S.K.L. revised the manuscript. Y.J., S-J.A., and S.L. prepared tables. Y.J. and S.L. prepared figures. Y.J., S-J.A., and Y.K. reviewed patients’ medical records. Y.J. analyzed the clinical data. J-Y. C. analyzed the concentration of lacosamide. S.L. developed the PK model. Y.J., Y.K., and S.K.L. interpreted EEG. Y.J., H.S.L., Y-K.L., Y.W.S., S-T.L., K-H.J, K-I.P., K-Y.J., K.C., and S.K.L. collected clinical data. S-J.A., K.C., I-J.J. and S.K.L. provided study concepts. K-Y.J, SH.L., K-S. Y., K.C., I-J.J, and S.K.L. supervised the study. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Seoul National University Hospital Institutional Review Board (IRB No. 2104-146-1213) and written informed consent was obtained from all patients and/or legal guardians. All co-authors have reviewed and approved the contents of the manuscript, and the Scientific Reports requirements for authorship have been met. We confirm that we have read the journal’s position on issues related to ethical publication and affirm that this report is consistent with those guidelines. We certify that the submission (aside from an abstract) is not under review by any other publication. No previous report overlaps with the current work. We have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jang, Y., Ahn, SJ., Lee, S. et al. Saliva-based lacosamide monitoring paves the way toward personalized epilepsy pharmacotherapy. Sci Rep 15, 19181 (2025). https://doi.org/10.1038/s41598-025-04044-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04044-x