Abstract
Accurate identification and preservation of parathyroid glands (PTG) during thyroidectomy are crucial for minimizing postoperative complications. In this study, we evaluated the effectiveness of diffuse reflectance spectroscopy (DRS) and near infrared autofluorescence (NIRAF) as complementary, non-invasive techniques for real-time PTG identification and characterization during surgery. Our study revealed a correlation between intraoperative DRS-derived oxygenation indexes and parathyroid hormone (PTH) levels, suggesting that DRS may predict PTG perfusion status and subsequent PTH fluctuations, allowing assessment of tissue viability during surgery. As the control, a decrease in oxygenation was assessed during parathyroidectomies when the blood supply to the PTG was stopped by the vessels’ ligation. It has been also shown that DRS, reliably distinguishes PTG from surrounding tissues based on their unique reflectance properties. DRS-based classification achieved an F1-score of 0.88, demonstrating its potential as a robust standalone tool for PTG identification. Furthermore, when DRS data were integrated with NIRAF measurements, the classification accuracy improved significantly, achieving an F1-score of 0.98. These findings underscore the potential of combining DRS and NIRAF for superior real-time PTG identification and viability assessment, that could improve surgical outcomes by reducing the postoperative complications related to PTG injury.
Similar content being viewed by others
Introduction
Identification and preservation of the parathyroid glands (PTG) during thyroidectomy can be a challenging task. Possible visual similarities of parathyroid and thyroid tissues in combination with their morphological variabilities lead to unintentional removal of PTG in 5–22% of thyroid surgeries, followed by temporary hypocalcemia in approximately 13% of patients1,2,3 and postoperative hypoparathyroidism (low level of parathyroid hormone) in approximately 8% of patients4,5. The quality of life of patients with this complication is significantly lower than that of patients without hypoparathyroidism, and some studies suggest that this condition may be associated with increased mortality6.
In recent decade, near-infrared autofluorescence (NIRAF) has emerged as a promising method for the intraoperative identification of PTG preventing its damage in thyroidectomy. This technique is non-invasive, label-free, and offers a rapid means of tissue differentiation7,8. The sensitivity of NIRAF ranges from 81 to 100%, with specificity between 80 and 100%9. For instance, the FDA-approved PTeye system offers an accuracy rate of 94.3% in identification of PTG against other tissues without requiring the overhead lights to be turned off10. Sehnem et al. demonstrated that NIRAF prevented inadvertent parathyroidectomy in 21% of cases, highlighting the importance of reliable PTG detection during surgery. Additionally, 50% of surgeons reported that NIRAF improved their ability to identify and assess PTG during parathyroidectomy11. Namely, 70% of senior surgeons used NIRAF to confirm parathyroid tissue, while 100% of junior surgeons used it for both identification and confirmation8. It has been also shown that NIRAF detection enhances patient outcomes following total thyroidectomy. In a randomized study, Dip et al. observed a significant reduction in severe transient hypocalcemia in the NIRAF group, with rates decreasing from 18 to 4%12. DiMarco et al. reported that NIRAF-assisted surgeries successfully identified accidentally excised PTG in 85% of cases, allowing for timely autotransplantation, though it did not significantly reduce the rates of missed PTG excisions or postoperative hypocalcemia13.
In addition to the well-established and FDA-certified NIRAF visualization technique, several other label-free optical methods have been proposed for the PTG identification. Some promising approaches are based on the analysis of diffuse reflectance spectra from tissues in the visible and NIR ranges, utilizing optical fiber probes14,15 or multi- or hyperspectral cameras16,17. The properties of diffusely reflected light arise from the scattering and absorption of light by tissues, providing potential insights into both their morphological characteristics and molecular composition18. Indeed, diffuse reflectance spectroscopy (DRS) has demonstrated high sensitivity and specificity values in the task of PTG identification (on the level of 80–90%). Yet, compared to numerous works devoted to NIRAF, the potential of DRS in PTG identification is significantly underexplored.
Besides PTG identification, assessment of its viability and perfusion during surgery is also a critical issue since when removing pathological thyroid tissue, the blood supply to the parathyroid gland may be disrupted19. The existing approaches mainly rely on the surgeon’s experience with visual inspection and palpation20. For this purpose, the method for evaluating PTG perfusion using Indocyanine Green (ICG) has been proposed21,22,23. ICG, a safe and cost-effective dye approved by the FDA, is widely utilized across various medical applications. Following intravenous administration, ICG binds to plasma proteins, circulates within the intravascular space, and is eventually excreted via the hepatobiliary system. By ICG fluorescence intensity assessed via intra-operative imaging, one can estimate perfusion of the PTG and evaluate its viability. It is anticipated that the use of ICG will eliminate the need for the gold-standard of PTG viability assessment, PTH level measurement, after thyroidectomy23. However, significant variations exist among published studies regarding research design, inclusion criteria, and patient management protocols24,25,26. Moreover, there is no standardized system for assessing ICG fluorescence intensity, making image interpretation subjective. Additionally, ICG accumulation in the thyroid gland can cause diagnostic confusion27.
PTG perfusion and viability could be also monitored in a label-free manner using various optical techniques. Mannoh et al. demonstrated PTG perfusion assessment without labels by utilizing laser speckle contrast imaging, achieving an accuracy of 91.5%28. The analysis of PTG viability is also possible by means of DRS. Since hemoglobin is the dominant chromophore in tissues, the DRS spectra in the visible and NIR ranges provide information on the content of both oxygenated and deoxygenated hemoglobin, thereby indicating the oxygenation status of tissues. This approach is widely used for the assessment of oxygenation status of various tissues both ex vivo and in vivo, including PTG29,30; however, studies specifically focused on PTG are still in the early stage. Hence, label-free optical methods are powerful tools in PTG identification and characterization, with different optical techniques studied to different extents. At the same time there is a lack of investigations that combine multiple optical modalities for the simultaneous identification and viability assessment of PTG31.
To address this issue, in the present study we explored the use of the multimodal approach combining DRS and NIRAF for simultaneous intraoperative localization of PTG and assessment of its viability. Namely, the aims of the study were (1) to verify whether DRS can be used to track changes in PTG oxygenation during surgery as a marker of its viability, (2) to test the capabilities of DRS in discrimination between PTG and other tissues based on their reflectance in the Vis-NIR spectral range, (3) to explore the performance of the multimodal DRS-NIRAF probe in PTG detection. The obtained results pave the way for advanced techniques of PTG assessment during thyroidectomy aimed at improving patient’s outcome.
Materials and methods
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Endocrinology Research Centre (protocol code 10, 26.05.2020). The methods were carried out in accordance with relevant guidelines and regulations. The patients/participants provided their written informed consent to participate in this study.
In vivo experiments during thyroidectomy
Optical measurements were taken during 26 thyroidectomy surgeries. The muscles and all identified parathyroid glands were measured before and after thyroid removal, as well as the thyroid itself. During the first 11 surgeries, only diffuse reflectance spectra were measured; and during the latter 15 surgeries, the NIRAF measurements were added.
In addition to measuring optical parameters, vein blood analysis was conducted to determine PTH levels before surgery and immediately after thyroidectomy to further assess changes occurring in the parathyroid glands according to standard procedures.
Experimental setup
The multimodal experimental setup was built for the simultaneous detection of the NIRAF and DRS data (Fig. 1A, B). The signal collection scheme utilized a motorized filter holder with an LP 800 nm filter (OFE1LP-800, JCOPTIX, China), which allowed the filter to be removed during DRS recording and inserted into the beam path during fluorescence measurement to cut the excitation. A halogen lamp (BIM-6214, Brolight) providing illumination in 400–2500 nm range was used as the broadband light source for DRS measurement. The reference spectrum was measured using a reflectance standard with a 99% reflection coefficient (LabSphere, North Sutton, NH, USA). The light from the lamp passed through a collimator containing an optical filter OF1 (СЗС 26) to attenuate signal intensity in the NIR region. Then the light was focused on a surgical optical fiber with a core diameter of 550 μm and a numerical aperture of 0.22 and transmitted through the optical fiber to the tissue. The signal reflected from the tissue was recorded by the second identical optical fiber placed adjacent to the first one. The reflected signal then passed through a two-lens system and was detected by a spectrometer (YIXIST, spectral resolution 12 nm) in the range of 300–1100 nm with an integration time of 20 ms. A 650 nm laser (0.2 W, OXlaser) was used to excite fluorescence. Prior to initiating clinical measurements, we performed ex vivo fluorescence measurements on several types of human tissues using both 650 nm and 785 nm excitation wavelengths. These preliminary studies revealed that autofluorescence signals detected at around 800 nm were strongly correlated under both excitation regimes. The laser beam passed through a narrowband cleanup laser line filter OF3. Then the laser beam was directed through two mirrors into the optical fiber that was also used to collect the fluorescence signal. The intensity of the exciting radiation delivered on the tissue surface was 270 mW/cm2, below the permissible exposure level32. Fluorescence collection was performed through the same channel used for DRS registration, with the LP 800 filter inserted into the detection system. The fluorescence signal was recorded by the same spectrometer with an integration time of 500 ms. The optical fibers were positioned side-by-side in a custom-designed, sterilizable pen-shaped probe (Fig. 1E).
(A) Multimodal setup combining DRS and NIRAF modes, (B) image of the multimodal setup and the fiber probe, (C) representative optical density spectrum of PTG, (D) representative NIRAF spectrum of PTG, cut below 800 nm with a long pass filter, (E) image of the surgical field with the fiber probe (dashed rectangle) targeting the thyroid gland.
At each point of interest, measurements of reflectance spectra, NIR fluorescence spectra, and background illumination were performed by sequentially switching between the laser and the lamp. The probe was applied in direct contact with the tissue surface by the surgeon during all procedures. While the angle was frequently close to perpendicular (approximately 90°), it was not strictly standardized. The surgeon adjusted the orientation as needed, based on convenience and accessibility within the surgical field. Notably, the measurement results were found to be robust with respect to moderate angular variation. The probe’s contact-based design allowed for quick and intuitive use, without requiring precise alignment. According to the surgical team, the device was easy to operate, integrated smoothly into the existing workflow, and enabled rapid data collection without disrupting the procedure. The surgeon specifically noted that the probe was efficient, user-friendly, and did not cause any delays or complications. To ensure consistency, all measurements were performed by a small, trained surgical team familiar with the protocol. No technical malfunctions or ergonomic issues were encountered throughout the study.
Data processing
Diffuse reflectance spectroscopy
The effective optical density of tissues was calculated as follows:
where \(\:\text{O}\text{D}\left(\lambda\:\right)\) is the effective optical density spectrum, \(\:I\left(\lambda\:\right)\) is the recorded diffuse reflectance spectrum of the tissue, \(\:{I}_{ref}\left(\lambda\:\right)\) is the reference spectrum, and \(\:{I}_{bg}\left(\lambda\:\right)\) is the background spectrum measured during surgery, associated with the presence of overhead lighting in the surgery room. The representative optical density spectrum of PTG is depicted in (Fig. 1C). The reference spectrum was measured from a standard with a 99% reflection coefficient at the distance of 15 mm, and the background spectrum was measured in the absence of illumination. Both spectra were taken once before the start of in vivo measurements.
To assess the total hemoglobin content, oxygenation, and tissue scattering, the optical density spectra of tissues in the range of 510–590 nm were fitted by the linear combination of the absorption spectra of oxyhemoglobin and deoxyhemoglobin taken from the literature and scattering33:
where \(\:{\mu\:}_{HbO2}\) and \(\:{\mu\:}_{Hb}\) are the absorption coefficients of oxyhemoglobin and deoxyhemoglobin, respectively, \(\:\text{H}\text{b}{\text{O}}_{2},\:\text{H}\text{b}\) are the indices (decomposition amplitudes) of oxyhemoglobin and deoxyhemoglobin, and \(\:{a}_{s},\:\:{b}_{s}\) are the scattering indices. In the considered spectral range, the contribution of other chromophores is negligible, and scattering was modeled as a linear function of wavelength.
For a visual assessment of changes in the ratio of oxyhemoglobin to deoxyhemoglobin concentrations in tissue, \(\:\text{O}{\text{D}}_{sub}\) spectra were calculated. Specifically, a linear approximation —derived from OD values at 500 nm and 600 nm—was subtracted from the original OD spectrum. This procedure effectively highlights spectral features associated with hemoglobin oxygenation dynamics by removing the baseline trend.
The main absorbers in the range of 800–1050 nm are water and oxyhemoglobin, with scattering also making a significant contribution. The spectra in this range were fitted by the expression34:
where \(\:{\mu\:}_{Water}\) is the absorption coefficient of water, \(\:\text{H}\text{b}{\text{O}}_{2}^{{\prime\:}},\:\text{W}\text{a}\text{t}\text{e}\text{r}\) are the indices (decomposition amplitudes) of oxyhemoglobin and water in the NIR range, and \(\:{c}_{s},\:{d}_{s}\) are the scattering indices.
The oxygenation, water indices and total hemoglobin were calculated using the following formulas:
NIR autofluorescence
To calculate the NIRAF intensity, the spectrum of the background illumination was subtracted from the measured spectrum with the laser on (Fig. 1D). The amplitude of fluorescence signal was determined by calculating the maximum of the resulting spectrum.
Data analysis and classification models
To evaluate the predictive ability of the optical features extracted from the diffuse reflectance data and NIRAF, classification models based on logistic regression with L2-regularization were developed to predict whether the measured tissue site was PTG or not. Two datasets were used: the first contained only the DRS data and included 26 surgeries, and the second combined DRS and fluorescence data and included 15 surgeries. For each surgery from 5 to 10 DRS and NIRAF spectra were collected from various sites of PTG and from 5 to 10 spectra of surrounding tissues available at the operational field: tissues of thyroid gland and muscles, forming a balanced dataset. In total, for DRS 244 spectra were collected for PTG tissues, 74 spectra were collected from thyroid glands and 118 spectra from muscles. For NIRAF, 124, 50 and 70 spectra were recorded from PTG, thyroid glands and muscles, respectively.
Using the obtained dataset, four models were built: (1) the model, which utilizes water index, estimated from the DRS, (2) the model that uses amplitudes extracted via principle component analysis (PCA) to discriminate PTG, (3) the model, that uses NIRAF intensity solely, and (4) the model that uses both water index, extracted from the DRS data, and NIRAF intensity.
To build the predictive model, which used PCA amplitudes as input features, the OD spectra were preliminary standardized, i.e., the mean value was subtracted and then the spectra were divided by the standard deviation of OD for each wavelength, resulting in a distribution with a zero mean and one standard deviation. For the fluorescence data, the logarithm of the fluorescence intensity values was used as an input feature. The quality and the optimal hyperparameters, such as the regularization parameter and the number of principal components, were estimated using grouped cross-validation with data from a single surgery used as a hold-out set. The F1-score was chosen as the optimization metric for evaluating the model quality, with its mean value and standard deviation calculated across the folds:
where precision is the ratio of instances predicted as PTG, that are indeed PTG, and recall is the ratio of all PTG instances that the model correctly predicted.
Additionally, for accurate comparison of results, aforementioned models were subjected to the same cross-validation procedure on the consistent dataset. The analysis was restricted to data from surgeries where fluorescence and DRS measurements of tissues were concurrently recorded (15 surgeries).
All data preprocessing, visualization of results, training and validation of models were carried out with custom Python 3 scripts using the Pandas, NumPy, Matplotlib, Scikit-learn, Scikit-image, Seaborn libraries.
Results
Diffuse reflectance spectroscopy of PTG – oxygenation and viability
To assess PTG oxygenation, DRS in the 500–1100 nm spectral range was used. An experiment was conducted to analyze tissue oxygenation dynamics during surgical procedures by measuring diffuse reflectance spectra before and after the blood vessels supplying the PTG adenoma were ligated before excision. The experiment was performed during two parathyroidectomies, where temporary vessel occlusion allowed for the assessment of oxygenation changes. The results demonstrated a significant decrease in tissue oxygenation index calculated according to the Eq. (4) after vessel clamping compared to baseline levels (Fig. 2C, D). The most pronounced decrease in oxygenation was observed during the surgery, where complete vessel occlusion led to a total drop in adenoma oxygenation to zero (Fig. 2D). We note that in this case a pronounced drop of oxygenation occurred in a 1 min, while in other cases it was slower and could be observed just in 3–5 min (Fig. 2C). Spectral analysis revealed the transformation of two distinct oxyhemoglobin absorption peaks into a single deoxyhemoglobin absorption peak, indicating a complete cessation of oxygenation (Fig. 2B). In the other case, full occlusion was not confirmed, however, a visual change in tissue color to a bluish hue was noted by the surgeon, indirectly confirming a decrease in oxygenation. For visual assessment, \(\:\text{O}{\text{D}}_{sub}\:\)spectra are shown in (Fig. 2A, B), illustrating a gradual reduction in hemoglobin absorption peaks over time. A corresponding decline in the total hemoglobin index, calculated according to Eq. (5), was observed in both surgeries, closely correlating with the time elapsed since vessel ligation (Fig. 2E, F). This may indicate that the total hemoglobin index can also be used to predict PTG damage. To ensure measurement accuracy, spectral data were collected at the same tissue location with maximum possible precision. However, anatomical variability in vessel positioning and tissue heterogeneity made it difficult to achieve perfect alignment of measurement points, contributing to some variability in oxygenation and total hemoglobin indices.
The effective optical density spectra of PTG corresponding to different blood oxygenation levels are shown in (Fig. 3A). The dip between oxyhemoglobin twin peaks (located at 540 nm and 575 nm) in the spectrum colored with blue is more pronounced, indicating a higher oxygenation index (SpO2 = 0.9) compared to the spectrum colored with orange (SpO2 = 0.5). Representative decomposition of the effective optical density spectrum into the sum of the absorption spectra of oxyhemoglobin and deoxyhemoglobin and the scattering according to Eq. (4) is shown in (Fig. 3B). We note the blue and orange spectra in Fig. 3A correspond to the PTG before and after surgery, and the corresponding blood PTH levels were 69 and 24 pg/ml. Hence, given the evident decrease in the oxygenation level (from 0.9 to 0.5) and the decrease in PTH the day after surgery, it can be suggested that the PTG perfusion could have been compromised, and the DRS features can predict this fact.
(A) Mean effective optical density spectra of PTG obtained during the surgery, measured before and after thyroid removal. The translucent areas indicate the standard deviation. The legend indicates the calculated oxygenation indices (SpO2) for the mean spectra. (B) Representative decomposition of the experimental OD spectrum (orange dots) into the sum of the absorption spectra of deoxyhemoglobin (green dashed line) and oxyhemoglobin (red dashed line) and a straight-line representing the light scattering (black dashed line). (C) Box plots of PTG oxygenation indices before (blue) and after (orange) thyroid removal for different surgeries, with PTH values measured before and after surgery shown above.
The distributions of the oxygenation indices of PTG for three representative surgeries before and after thyroid gland removal are shown in (Fig. 3C). The corresponding PTH levels are indicated at the top of the panel 3 C. It is evident that a decrease in PTH levels is accompanied by a decrease in the oxygenation indices of PTG. Hence, the results demonstrate that the intraoperative assessment of PTG oxygenation may reveal correlation with the PTH level prognosis after thyroidectomy.
Diffuse reflectance spectroscopy for PTG identification
As the next step, we questioned whether the spectral features from DRS could be indicative of the tissue type, namely, whether it is possible to discriminate PTG from the surrounding tissues based on its reflectance spectrum.
The average OD spectra of PTG and other tissues (thyroid gland and muscles) are presented in (Fig. 4A), where a notable difference both in the visible and NIR spectral ranges can be observed. These differences are particularly pronounced near the wavelengths of 470 nm and 970 nm. To illustrate these differences, the dependence of OD values at 970 versus 470 nm was plotted (Fig. 4B), where the data separation into two clusters (PTG and other tissues) can be seen, indicating the potential applicability of DRS for tissue type determination.
(A) Averaged effective optical density spectra of the PTG and other tissue types. The translucent areas indicate the standard deviation. The vertical dashed lines correspond to 470 and 970 nm. (B) The dependence of the effective optical density values at the wavelengths of 970 nm versus 470 nm for the parathyroid gland (orange) and other tissue types (blue).
In the NIR spectral range oxyhemoglobin and water significantly contribute to the light absorption by tissues. The mean effective optical density spectra of the PTG and other tissues highlight that the amplitude of the water absorption peak at 970 nm is significantly lower for PTG (Fig. 5A). The water content was assessed as the water index according to the Eqs. (3–6), and the effective optical density spectra were decomposed into the sum of water and deoxyhemoglobin absorption spectra and scattering (Fig. 5B). Distributions (box plots) of the water index values were constructed, and the Mann-Whitney test was performed, indicating that this feature was statistically different for PTG and other tissues (p-value < 10− 4, Fig. 5C).
(A) Averaged effective optical density spectra of the PTG and other tissues in the 800–1050 nm range with the value subtracted at 800 nm. (B) Decomposition of the representative experimental OD spectrum into the sum of deoxyhemoglobin, water, and scattering spectra according to Eq. (3). (C) Distributions (boxplots) of the water index values for the parathyroid gland and other tissue types, (****) indicate p-value < 10− 4.
Therefore, the water index was chosen as a classification feature for PTG identification. The average F1-score was 0.80 ± 0.27, demonstrating the model’s ability to separate the data and indicating potential for accuracy improvement with more advanced methods.
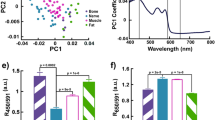
To build a more accurate classifier that accounts for the variability of DRS spectra across the entire wavelength range (450–1050 nm), the effective optical density spectra were processed using principal component analysis (PCA) with 5 components, which explained 99.99% of the variance. The major PCA components with the highest amplitudes reveal contributions of oxy- and deoxyhemoglobin and water (Fig. 6A): the first component up to 600 nm resembles deoxyhemoglobin absorption spectrum, and the second – that of oxyhemoglobin. When plotting the decomposition coefficients (amplitudes) of the first component against the second one, two almost non-overlapping clusters are formed (Fig. 6B), indicating significant differences between tissue types. The contribution of the second component, which resembles the oxyhemoglobin absorption spectrum, to the PTG OD spectra was substantially lower compared to its contribution to the OD spectra of other tissues.
Considering the potential impact of the probe pressure on the inspected tissue site caused by occlusion and changes in water and hemoglobin concentration, we preformed investigation of the probe pressure effect on contributions of two major PCA components to the DRS spectrum of thyroid. The results are plotted in (Fig. 6B), where the red points correspond to low probe pressure on a tissue site, and the black ones – to high pressure. The probe pressure is colored with gradient as shown in the right panel in (Fig. 6C). The effective optical density spectra of the thyroid gland under varying probe pressures on the inspected tissue are presented in (Fig. 6C). When pressure is applied to the tissue, water is expelled, leading to a decrease in the amplitude of the water absorption peak at 970 nm in the effective optical density spectra, as observed in the inset (green curve). Concurrently, the oxyhemoglobin index \(\:\text{H}\text{b}{\text{O}}_{2}^{{\prime\:}}\) within the NIR range according to Eq. (3), shows an increase (orange curve).
(A) The first two normalized components of the OD spectra obtained using PCA (n = 5 components). (B) The graph of the first PCA component plotted versus the second PCA component for the parathyroid gland (orange) and other tissue types (blue). (C) Effective optical density spectra of the thyroid under different probe pressures on the inspected tissue site. For panels (B,C) the black-to-red gradient indicates the data obtained by varying the probe pressure on the inspected tissue site. The graph of the water index (green) and \(\:\text{H}\text{b}{\text{O}}_{2}^{{\prime\:}}\) (orange) plotted versus relative probe pressure is shown in the inset.
Despite the changes in component contributions to the OD spectra due to pressure variation, the points remain within their class cluster, i.e., by varying probe pressure it is not possible to achieve such spectral variations that would lead to thyroid spectrum classification as PTG. Hence, the probe pressure does not significantly affect tissue type classification.
Classification of tissue type (PTG/other) was performed using the amplitudes of PCA components of the DRS spectra. During cross-validation, the use of five PCA components was found to be optimal, and the achieved F1-score of the model of 0.88 ± 0.18.
NIR autofluorescence/drs of PTG: multimodal measurements in vivo
The NIR autofluorescence at 650 nm excitation was measured during surgeries for PTG and surrounding tissues with the developed multimodal setup, i.e., for each tissue site both NIRAF and DRS spectra were obtained. Given the knowledge of elevated NIRAF in PTG, we first examined the performance of PTG identification using the only feature – the logarithm of the NIR fluorescence intensity. The resulting F1-score was 0.94 with a standard deviation of 0.10.
Next, the data from two optical methods (NIRAF and DRS) were combined. The dependence of the water index on the natural logarithm of NIR fluorescence intensity for PTG and other tissues is shown in (Fig. 7). The data clearly group into two clusters, suggesting high classification accuracy. It should also be noted that classification of PTG based solely on fluorescence (corresponding to the vertical line in Fig. 7) yields worse results compared to the classification using both features, where the boundary is represented by a sloped line.
The diagram where the water index (obtained from DRS) is plotted against the natural logarithm of the NIRAF intensity for the parathyroid gland and other tissue types. The position of the sloped dashed line corresponds to the decision boundary when classifying the data combined from DRS and NIRAF, and the position of the vertical dashed line was determined by a classifier using only NIRAF.
Thus, a classification model was developed with the water peak and the logarithm of NIRAF intensity as the input features. The F1-score was 0.98 with a standard deviation of 0.04. The scatter of the F1-score values significantly decreased, and the average value substantially exceeded those of the other models discussed (Table 1).
Discussion
During thyroidectomy, there is a significant risk of inadvertent damage to the PTG caused by vascular rupture. Accurate assessment of PTG viability during surgery would enable surgeons to make timely adjustments to the procedure. Intraoperative and postoperative measurements of parathyroid hormone levels in blood can help to assess PTG function, but this method is labor-intensive, and its results are available too late to adjust the course of the surgery21. As previously mentioned, the commonly used method for intraoperative perfusion assessment is the visual evaluation of PTG after the administration of ICG combined with fluorescence detection. Another method is based on Laser Speckle Contrast Imaging (LSCI), which does not require the use of exogenous markers and can be used without turning off the overhead lights. However, this method is sensitive to inadvertent movements of the surgical field caused by patient respiration or the motion of hands holding retractors. The proposed DRS method also allows for the assessment of PTG oxygenation levels without the use of additional markers. The experimental findings demonstrate that vessel ligation, resulting in a decrease in deoxyhemoglobin concentration due to tissue oxygen consumption, significantly reduces the oxygenation index, as determined by spectral analysis. Moreover, during three surgeries, it was shown that changes in the oxygenation index correlated with the decrease in blood PTH levels. Thus, the use of DRS potentially allows the monitoring of PTG perfusion during surgery and predicts hypoparathyroidism-related complications.
The majority of measurements were performed during hemithyroidectomy procedures. In these cases, our assessment focused on the parathyroid glands located on the side of the thyroid lobe scheduled for resection—typically one or two glands. However, it is important to note that not all parathyroid glands were visually identified by the surgeon in every case due to anatomical variability and intraoperative constraints. Furthermore, in our analysis, we presented only those parathyroid glands that demonstrated a measurable decrease in tissue oxygenation, as detected by DRS. This selection was made to emphasize cases where impaired perfusion was most evident. It is possible that in the remaining glands, perfusion remained stable and no significant changes occurred. In addition to the DRS-derived oxygenation data, we also relied on the surgeon’s intraoperative visual assessment. The surgeon provided real-time evaluations of potential gland damage based on standard visual cues such as color, turgor, and bleeding characteristics, which were considered alongside the quantitative measurements. This combined approach helped us to correlate spectral findings with clinical judgment and enhance the reliability of our interpretation.
Our study demonstrated that the PTG can be distinguished from other tissue types based solely on the water index with an F1-score of 80% (the water content in PTG is lower). Furthermore, employing PCA for DRS data analysis increases the F1-score to 88%, making it comparable to methods based on NIRAF. The DRS analysis also reveals that the impact of oxyhemoglobin in PTG DRS is lower compared to that in other tissues (Fig. 4). These findings suggest promising avenues for the development of new devices capable of simultaneously detecting the PTG and assessing its viability.
Moreover, we observed that as the probe pressure on thyroid tissue increased, the water index decreased. This trend likely results from the displacement of water from the tissue cavities under increased pressure, a phenomenon theoretically described for skin by Zhang et al.35. Additionally, increased probe pressure may lead to a higher density of scatterers per unit volume36. It is also important to emphasize that conducting such experiments on the PTG is particularly challenging due to its small size. However, the DRS experiments performed on the thyroid with different pressure showed that this factor does not compromise the classification performance (Fig. 6).
Currently, the most popular method for PTG detection is the NIRAF spectroscopy37. In a meta-analysis conducted by Wang et al., which included 24 studies involving 2,062 patients and 6,680 samples, the combined sensitivity and specificity of the NIRAF method were found to be 96%, a result that aligns closely with the outcomes of our study9. Furthermore, by integrating NIRAF data with DRS, the accuracy of PTG detection reaches 98%, with the standard deviation reduced to 4%. This combined approach not only significantly enhances diagnostic precision but also provides a more comprehensive assessment of tissue status. The obtained results underscore the benefits of the suggested multimodal approach.
Conclusion
In this study, we have demonstrated the efficacy of DRS and NIRAF as complementary tools for the identification and characterization of PTG during thyroid surgeries. The results underscore a correlation between PTG oxygenation levels and PTH levels, indicating the potential of intraoperative DRS in predicting PTG perfusion status and subsequent PTH fluctuations. Moreover, the study highlights the distinct optical properties of PTG compared to surrounding tissues, with significant differences observed in the optical density spectra, particularly in the visible and NIR ranges.
Notably, even without the use of NIRAF, DRS alone showed substantial promise in differentiating PTG from other tissues. The classification based solely on the water index, derived from DRS spectra, achieved an F1-score of 0.80. This result, while lower than that obtained with combined modalities, still underscores the standalone capability of DRS in PTG identification. Further enhancement in classification performance was observed when PCA was applied to the DRS data, yielding an F1-score of 0.88. These findings highlight the robustness of DRS as a reliable method for tissue differentiation, even in the absence of fluorescence-based features. The combination of water index and NIRAF intensity as classification features yielded an impressive F1-score of 0.98, demonstrating increased accuracy compared to models utilizing individual features.
In conclusion, our results validate the use of DRS and NIRAF as powerful, non-invasive techniques for real-time PTG identification during surgery. The integration of these optical modalities into surgical practice could enhance the precision of PTG preservation, thereby reducing the risk of postoperative hypoparathyroidism and improving patient outcomes.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
Ritter, K., Elfenbein, D., Schneider, D. F., Chen, H. & Sippel, R. S. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J. Surg. Res. 197, 348–353 (2015).
Sitges-Serra, A. Etiology and diagnosis of permanent hypoparathyroidism after total thyroidectomy. J. Clin. Med. 10, 543 (2021).
Applewhite, M. K. et al. Quality of life in thyroid cancer is similar to that of other cancers with worse survival. World J. Surg. 40, 551–561 (2016).
Büttner, M., Musholt, T. J. & Singer, S. Quality of life in patients with hypoparathyroidism receiving standard treatment: a systematic review. Endocrine 58, 14–20 (2017).
P Bilezikian, J. et al. Evaluation and management of primary hyperparathyroidism: summary statement and guidelines from the fifth international workshop. J. Bone Min. Res. 37, 2293–2314 (2020).
Almquist, M., Ivarsson, K., Nordenström, E. & Bergenfelz, A. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. J. Br. Surg. 105, 1313–1318 (2018).
Tjahjono, R., Nguyen, K., Phung, D., Riffat, F. & Palme, C. E. Methods of identification of parathyroid glands in thyroid surgery: a literature review. ANZ J. Surg. 91, 1711–1716 (2021).
Sehnem, L. Jr et al. A multicenter evaluation of near-infrared autofluorescence imaging of parathyroid glands in thyroid and parathyroid surgery. Surgery 173, 132–137 (2023).
Wang, B., Zhu, C. R., Liu, H., Yao, X. M. & Wu, J. The accuracy of near infrared autofluorescence in identifying parathyroid gland during thyroid and parathyroid surgery: a meta-analysis. Front. Endocrinol. (Lausanne). 12, 701253 (2021).
Kiernan, C. M., Thomas, G., Baregamian, N. & Solόrzano, C. C. Initial clinical experiences using the intraoperative probe-based parathyroid autofluorescence identification system—PTeye™ during thyroid and parathyroid procedures. J. Surg. Oncol. 124, 271–281 (2021).
Kose, E., Kahramangil, B., Aydin, H., Donmez, M. & Berber, E. Heterogeneous and low-intensity parathyroid autofluorescence: patterns suggesting hyperfunction at parathyroid exploration. Surgery 165, 431–437 (2019).
Dip, F. et al. Randomized controlled trial comparing white light with near-infrared autofluorescence for parathyroid gland identification during total thyroidectomy. J. Am. Coll. Surg. 228, 744–751 (2019).
DiMarco, A. et al. Does fluoroscopy prevent inadvertent parathyroidectomy in thyroid surgery? Ann. R Coll. Surg. Engl. 101, 508–513 (2019).
Schols, R. M., Alic, L., Wieringa, F. P., Bouvy, N. D. & Stassen, L. P. Towards automated spectroscopic tissue classification in thyroid and parathyroid surgery. Int. J. Med. Robot Comput. Assist. Surg. 13, e1748 (2017).
Serra, C., Serra, J. & Ferreira Machado, I. L. Vieira ferreira, L. F. Spectroscopic analysis of parathyroid and thyroid tissues by ground-state diffuse reflectance and laser induced luminescence: a preliminary report. J. Fluoresc. 31, 1235–1239 (2021).
Maktabi, M. et al. Classification of hyperspectral endocrine tissue images using support vector machines. Int. J. Med. Robot Comput. Assist. Surg. 16, 1–10 (2020).
Clancy, N. T., Jones, G., Maier-Hein, L., Elson, D. S. & Stoyanov, D. Surgical spectral imaging. Med. Image Anal. 63, 101699 (2020).
Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol. 58, R37 (2013).
Dionigi, G. et al. Prospectives and surgical usefulness of perioperative parathyroid hormone assay in thyroid surgery. Expert Rev. Med. Devices. 5, 699–704 (2008).
Madill, E. M., Cooray, S. D. & Bach, L. A. Palpation thyroiditis following subtotal parathyroidectomy for hyperparathyroidism. Endocrinol. Diabetes Metab. Case Reports (2016).
Spartalis, E. et al. Intraoperative indocyanine green (ICG) angiography for the identification of the parathyroid glands: current evidence and future perspectives. Vivo (Brooklyn). 34, 23–32 (2020).
Kim, D. H., Kim, S., Jung, J., Kim, S. W. & Hwang, S. H. Indocyanine green fluorescence for parathyroid gland identification and function prediction: systematic review and meta-analysis. Head Neck. 44, 783–791 (2022).
Vidal Fortuny, J. et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. J. Br. Surg. 105, 350–357 (2018).
Lavazza, M. et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg. 5, 512 (2016).
Chang, Y. K. & Lang, B. H. H. To identify or not to identify parathyroid glands during total thyroidectomy. Gland Surg. 6, S20 (2017).
Sadowski, S. M., Fortuny, J. V. & Triponez, F. A reappraisal of vascular anatomy of the parathyroid gland based on fluorescence techniques. Gland Surg. 6, S30 (2017).
Wong, A., Wong, J. C. Y., Pandey, P. U. & Wiseman, S. M. Novel techniques for intraoperative parathyroid gland identification: a comprehensive review. Expert Rev. Endocrinol. Metab. 15, 439–457 (2020).
Mannoh, E. A., Thomas, G., Solórzano, C. C. & Mahadevan-Jansen, A. Intraoperative assessment of parathyroid viability using laser speckle contrast imaging. Sci. Rep. 7, 14798 (2017).
Sircan-Kucuksayan, A., Uyuklu, M. & Canpolat, M. Diffuse reflectance spectroscopy for the measurement of tissue oxygen saturation. Physiol. Meas. 36, 2461 (2015).
Romine, M., Moazzen, A., Luong, L., Cho, K. & Lee, S. Y. Portable diffuse reflectance spectroscopy for non-invasive and quantitative assessment of the parathyroid glands viability. In 2023 IEEE International Opportunity Research Scholars Symposium (ORSS) 63–66 (IEEE, 2023).
Kim, Y. et al. A co-axial excitation, dual-RGB/NIR paired imaging system toward computer-aided detection (CAD) of parathyroid glands in situ and ex vivo. J. Biophotonics. 15, e202200008 (2022).
Ziegelberger, G. ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation. Health Phys. 105, 74–96 (2013).
Stratonnikov, A. A. & Loschenov, V. B. Evaluation of blood oxygen saturation in vivo from diffuse reflectance spectra. J. Biomed. Opt. 6, 457–467 (2001).
Davydov, D. A. et al. Monitoring the skin structure during edema in vivo with spatially resolved diffuse reflectance spectroscopy. J. Biomed. Opt. 28, 57002 (2023).
Zhang, X. U., Faber, D. J., Van Leeuwen, T. G. & Sterenborg, H. J. C. M. Effect of probe pressure on skin tissue optical properties measurement using multi-diameter single fiber reflectance spectroscopy. J. Phys. Photonics. 2, 34008 (2020).
Reif, R. et al. Analysis of changes in reflectance measurements on biological tissues subjected to different probe pressures. J. Biomed. Opt. 13, 10502 (2008).
Kim, S. W., Lee, H. S. & Lee, K. D. Intraoperative real-time localization of parathyroid gland with near infrared fluorescence imaging. Gland Surg. 6, 516 (2017).
Acknowledgements
The research was carried out within the state assignment of Ministry of Health of the Russian Federation (theme No. 124020600011-5). P.T. was supported by the personal scholarship from the Theoretical Physics and Mathematics Advancement Foundation “BASIS” (24-2-1-31-1). V.T. was supported by the Russian Science Foundation grant 23-14-00287.
Author information
Authors and Affiliations
Contributions
Conceptualization, E.S., L.U., G.B.; methodology, E.S., G.B., L.U., V.T., A.M., I.K. and N.K.; software, G.B., P.T., N.K.; experimental setup, V.P., G.B., N.K.; resources, L.U., A.V., N.P., P.W.; validation, P.T., G.B., N.K., B.Y., P.W., A.M. and E.S.; formal analysis, P.T., N.K., B, Y.; investigation, P.T., N.K., I.K., N.P., S.S., V.P., A.V., A.S.; data curation, P.T., G.B., N.K. and B.Y.; writing—original draft preparation, P.T., G.B., B.Y. and E.S.; supervision, E.S.; project administration, L.U. and N.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tseregorodtseva, P., Budylin, G., Korneva, N. et al. Parathyroid glands viability assessment and identification during surgery with multimodal diffuse reflectance spectroscopy and NIR autofluorescence probe. Sci Rep 15, 22097 (2025). https://doi.org/10.1038/s41598-025-04059-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04059-4