Abstract
The Mediterranean fruit fly (medfly), Ceratitis capitata, is a key pest for fruit production in the Mediterranean and other areas of the world. With the aim of contributing to knowledge on the management of this pest, the present study analyses the spatial distribution of medfly sterile males released during a pilot application of Sterile Insect Technique (SIT) in a multi-varietal fruit farm in Central Italy. The spatio-temporal distribution was analysed with the Spatial Analyses by Distance Indices (SADIE) methodology. The wild male population and the released sterile males showed an aggregate distribution in the field, even though the latter were released uniformly in the experimental farm. Furthermore, the spatial dispersion of sterile flies mirrored that of the wild flies throughout the entire release period of the sterile males, which lasted about 4 months. This aspect is critical for the correct application of the SIT. These results provide useful insights on the application of sterile males to control medfly populations.
Similar content being viewed by others
Introduction
Due to its high capacity to spread and colonize new areas, its high adaptability to different climatic conditions, and the number of fruits on which it can develop, the Mediterranean fruit fly (medfly), Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), is considered one of the most damaging insects in the world1,2,3.
Medfly infestations are traditionally controlled with the use of synthetic insecticides applied as cover spray, which are generally cheaper than other types of control tools3,4. The use of these products has a severe impacts on beneficial arthropod fauna and human health. Therefore, new EU policy targets major reduction of pesticide use3. The contact insecticide can be mixed with a bait (attractive substance) to reduce the amount of product application. Other control approaches include biological control (e.g., parasitoids, entomopathogenic fungi, or nematodes), mass trapping, and attract-and-kill3,5,6,7,8. In an Integrated Pest Management (IPM) program, these approaches can be applied alone or in combination, according to the population density of the pest. In addition to these control methods, the Sterile Insect Technique (SIT) is used for Area Wide-Integrated Pest Management (AW-IPM). AW-IPM considers the total population of the target pest rather than a single plot or small number of plots of the area (as in IPM)3.
The SIT consists of insect mass rearing of the target pest. After being sterilized, usually by irradiation, sterile insects are released in the field with the wild population of the same species. Released sterile males, in competition with wild ones, mate with wild females. The eggs laid are not viable and result in no offspring. Over generations, this results in a decline of wild populations, below economic damage thresholds. As no chemicals are released into the environment, this technique is considered environmentally sustainable. SIT can be used for prevention (to exclude entry into the pest-free area), containment (to avoid the spread of invading alien pests), suppression (to maintain a low pest population level), and eradication (to eliminate the pest from a given area)9. The first successful application of SIT took place in the 1950s against the Cochliomyia hominivorax (Coquerel), the New World screwworm10. After this first application, the SIT was improved. It is currently is used for AW-IPM and eradication programs against moths [Cydia pomonela (L.), Pectinophora gossypiella (Saunders), and Thaumatotibia leucotreta (Meyrick)], tsetse flies (Glossinia spp.), and several fruit fly species [Anastrepha fraterculus (Wiedemann), A. ludens (Loew), A. obliqua (Marquart), A. suspensa (Loew), Zeugodacus cucurbitae (Coquillett), Bactrocera dorsalis (Hendel), B. tryoni (Froggatt) and C. capitata]11,12.
The SIT has been used against medflies in AW-IPM programs in several countries: in Australia, Chile, the US, and Mexico for prevention; in Guatemala for containment; in Brasil, Croatia, Israel, Jordan, Peru, Portugal, South Africa, Spain, and Tunisia for suppression; in Argentina, Dominican Republic, Guatemala, Israel, Jordan, Mexico, and Peru for eradication3,9,13.
The distribution of the target pest in the area (dispersion) and the movement of individuals (dispersal) of wild and sterile populations can impact the success of SIT programmes. These two parameters are influenced by a variety of ecological and behavioural factors intrinsic to the target species14. In order to optimally distribute sterile insects in the field, it is important to know the distribution of the wild population and the behaviour of the sterile insects once released15,16. In the case of strong clustering of the target insect population, the effectiveness of sterile insect releases may decrease; this can be overcome if the sterile insects are distributed, or redistributed after release, to mirror the distribution of wild insects14.
Most existing studies focus on the dispersal of sterile medfly males involving release-recapture trials17,18,19,20,21,22,23,24,25 or evaluating the recapture rate in sterile insect release programs26,27,28. Few studies have analysed the distribution of wild and sterile medflies during a SIT release programme29.
The aim of this study was to assess whether the spatially uniformly released sterile insects matched with the distribution of the wild population, by defining their spatial patchiness and association.
We aimed at characterise the spatial distribution of wild and sterile males in a multi-varietal fruit farm in the Latium region in central Italy. Here, in 2018 and 2019, a SIT pilot programme for the management of C. capitata was carried out, simultaneously to other pilot sites in the Adige Valley (Trentino Alto Adige region, North Italy)30,31.
Results
Quality control tests
The Quality Control tests were performed following the protocol in the FAO/IAEA/USDA/Quality Control Manual32.
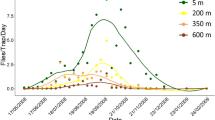
The average emergence rate (± SE) registered in the 32 batches (17 shipments) was 91.19 ± 1.21%. Two differently aged batches registered similar emergence rates, with an average of 91.13 ± 1.20% in the older batch and 91.25 ± 2.06% in the younger one. Figure 1 shows the estimated number of sterile males released in the field per release day according to the emergence rates recorded in the Quality Control (QC) test.
The QC test on lifespan showed that, on average, more than 90% of sterile flies remained alive after 5 days (Fig. 2). Percentage of live flies dropped to 65% after 10 days and 0% after 16 days.
Representative sample size
The number of catches observed in the 7 traps randomly selected to assess the representative sample size ranged from 312 to 959 flies, for a total of 3,928 flies. After counting was complete, all caught flies were observed in the dark room with UV light. The observation revealed 2,539 sterile and 1,389 wild flies. This represented the full set of data that we compared with the two sub-samples, whose size is reported on Table 1. Comparing the two sub-samples with the full set of data, accuracies of 93.65% and 98.63% were found using “sub-sample A” and “sub-sample B”, respectively (Table 1).
The χ2 test, using “sample size A”, showed a statistically significant result (χ2 = 26.107; df = 1; p < 0.001), instead, using “sample size B”, the χ2 test showed a not statistically differences (χ2 = 0.0939; df = 1; p = 0.759).
Based on the result of this test, the size reported in sub-sample B, not statistically different from the full set of data, was used for estimating the number of trapped fluorescent dye marked flies during the weekly monitoring trap control.
Adult monitoring
During the monitoring period, 220,918 medfly adults were collected in the experimental farm. Of these, 211,219 males were caught in the traps baited with trimedlure (59,306 wild and 151,913 sterile) and 9,699 females were caught in traps baited with BioLure.
The first wild male medfly adult was recorded on 2 July with a peak registered in mid-September. The last capture was recorded on 18 December (Fig. 3). The first adult female was recorded on 9 July. The flight registered 2 peaks in late August and late September, and the last capture was recorded on 4 December (Fig. 4).
The capture of sterile males took place from 2 July (the day after the first release) to 5 November (12 days after the last release) (Fig. 3). The captured sterile samples represented 2.97% of the total released sterile medflies which amounted to 5,106,500 (estimated considering the emergence rate for each release).
In total, more sterile males were trapped than wild males, with a ratio of 2.6:1. The only two dates when the number of wild males caught exceeded the number of sterile ones were 20 August and 5 November. In the first case, it was because there was a failure to release sterile males on 12 and 15 August; in the second case, it was because the release of sterile medflies had ended on 24 October.
Spatial analysis
Analysis of the cluster indices, performed using the Spatial Analysis by Distance Indices methodology (SADIE), showed that wild and sterile male medflies were patchly aggregated in the field (p = 0.018 for the wild male population; p < 0.001 for the sterile males); on the contrary, the female medflies did not report a statistically aggregated distribution (p = 0.061) (Table 2). Analysis of the single dates showed that aggregations of sterile males were statistically significant more often than in the wild male population. The maps, produced using geostatistical methods showed the hot spot distribution of wild and sterile male medflies and the local association with the magnitude of positive and negative association from 95th, 90th, and 75th percentiles during some exemplificative weeks for each month of monitoring (Fig. 5). The full set of maps is shown in Supplementary Figures S1-S5.
Example of contour maps (wild males and sterile males) obtained from four sampling dates, showing the medfly hot spot distribution and local association maps. The local association maps, obtained by SADIE procedure, indicate the magnitude of positive and negative association from 95th, 90th and 75th percentiles, coloured according to the following legend (blue square, positive 95th percentile; sky blue, positive 90th percentile; turquoise, positive 75th percentile; red, negative 90th percentile; pink, negative 75th percentile). Trap locations in the area are represented by white dots; x- and y-axes are expressed in UTM coordinates. The contour maps were generated using the software Surfer version 12 (Golden Software, Golden, CO, USA, https://www.goldensoftware.com/products/surfer/), the base map comes from Google Earth Pro (https://www.google.it/earth/).
In early July, both wild and sterile males were mainly aggregated in the apricot orchard in the northern part of the experimental farm. In the remainder of July, the two populations formed hotspots in the central-northern part of the experimental farm, which mainly features peach orchards. In August-September, the wild males formed hotspots in the central-northern part of the farm. The sterile males were localised in the same section of wild population but occupied a larger area that also included the apricot and plum orchards. In October, when the only fruits in the field were apples, the wild male population did not show any significant aggregation and was distributed randomly over the entire experimental farm. The sterile males were localised mainly in the central-northern part of the farm. The total spatial association among wild and sterile male medflies was positive and significant (p < 0.001). Statistical significance was found on all trap inspection dates except of the first three dates in October (Table 2).
The association maps highlight that the sterile males occupied the areas with the highest presence of the wild male population, forming positive associations. In July-August, the overlapping of wild and sterile male patches was in the central part of the farm (apricot and peach orchards), while the areas of positive association present in the northern and southern parts of the farm represented gaps (absence of both wild and sterile males). In September, the association observed in July-August continued, with smaller aggregation areas than in previous months. In October, the aggregation of patches was observed in the central part of the farm. The association maps show a negative local association in the north (in the plum orchard on 1 October and in cherry and fig orchards on 8 October); sterile males were present in these areas, but wild males were not. On the other hand, the negative local association observed in mid-October in apple orchards is due to the presence of wild males but not sterile ones.
In early July, females mainly aggregated in the apricot orchard in the northern part of the experimental farm (Fig. 6 and Supplementary Figures S6-S7). In the remainder of July and in August, they formed hotspots in the central and southern part of the experimental farm, which mainly feature peach orchards. In September and early October, females mainly aggregated in the pear orchards in the center of the experimental farm.
Example of contour maps (females) obtained from four sampling dates, showing the medfly hot spot distribution. Trap locations in the area are represented by white dots; x- and y-axes are expressed in UTM coordinates. The contour maps were generated using the software Surfer version 12 (Golden Software, Golden, CO, USA, https://www.goldensoftware.com/products/surfer/), the base map comes from Google Earth Pro (https://www.google.it/earth/).
Discussion
Given the isolation of the experimental farm, the initial purpose of the SIT pilot test in central Italy was to suppress the medfly population. Despite the intense release of sterile flies in 2019, with over 5 million sterile male medflies released in 4 months (from 1 July to 24 October), the ratio of sterile to wild catches was 2.6:1, far from those suggested by Enkerlin (2007)9 for suppression (25–100:1), eradication (100–150:1), containment (50–150:1), or preventive release (25–50:1) programmes.
The ratio of sterile to wild catches recorded in 2019 could indicate a partial effect of the SIT application on the medfly population. Looking at the male catches recorded in 2019 compared to previous years (Supplementary Figure S8), there was a 34% decrease in the male population compared to 2017 and a 54% decrease compared to 2018. As the experiment lasted only a few years and the climatic conditions of the area remained stable (Supplementary Figure S9), this decrease in density cannot be attributed with certainty to the application of SIT. It may result from natural variations in medfly population density.
The high population density in the experimental farm may also have occurred because the fruit was not harvested, and no management intervention was carried out on the wild medfly population before the release of the sterile insects (e.g., chemical control, mass-trapping, sanitation). The high population density of wild medflies, however, allowed the study of the spatio-temporal dynamics of wild males and comparison with that of released sterile medflies in 2019. After two years, the sterile insect releases were suspended, due to lack of funds.
The results of pre-shipping and post-shipping quality control tests of the sterile medfly males were in line with the parameters set out in the FAO/IAEA/USDA/Quality Control Manual32.
The temporal dynamics of wild medfly males observed in the experimental farm is typical of areas in Central Italy as reported in other studies4,33,34 with the flight period of males beginning in late June-early July and ending in late November. During this period, the highest population density usually occurs in September.
Although the releases were continuous over time, the number of sterile males recaptured weekly after the first launches was not uniform. This could be due to weather conditions that affected the lifespan, the fly activity of sterile samples, and/or the attractiveness of the trap (e.g., lure volatility). The latter may be the cause of the drastic reduction in the number of catches on the sampling dates of 15 July and 17 September, which was recorded in both wild and sterile medfly catches. The influence of weather on the recapture of sterile males is also reported by Barry et al. (2004)26 that analysed the recapture rate of sterile medflies in California’s release program between 1998 and 2001.
The last sterile medfly was sampled on 5 November. Since the last release of sterile insects occurred on 24 October, and the sampling date of 5 November was within the monitoring period of 29 October − 5 November, this study confirms that sterile medflies in the field can survive for at least 5 days (with a population density detectable with traps). Other studies emphasize the short lifespan of sterile medfly following release. Andress et al. (2013)28 reported a decline in the number of sterile medflies to approximately 66% of all captures within 3 days after release. Paranhos et al. (2010)23 reported that approximately 90% of sterile medflies were recaptured up to 5 days after release and only 10% from day 7 to 11.
The recapture rate registered in our case (2.97%) aligns with the results of other studies, e.g., 2.5% and 1.9% in Duarte et al. (2020)25 and 5% and 2.3% in Gavriel et al. (2012)24. However, these data are difficult to compare given the differences in the experimental areas and the monitoring protocols (type and density of traps used). In our case, the low percentage of adults responding to the trap’s lure was not related to the trap’s saturation, as the trap that recorded the highest number of captures (1,388 samples) still had space available to trap other samples. This shows that in pest monitoring programmes, traps only capture a small percentage of the population in the area at that time8.
Several factors determine the spatial distribution of insects in the field. For medflies, key factors are the lekking behaviour, the fruit availability, and the landscape composition33,34,35,36,37. As mentioned, for a sterile medfly release programme to work well, the released sterile male flies should be distributed in the field to mirror the wild male population, to enter competition for mating. As reported in other works34,36, male and female samples aggregate in areas to mate, and the females then move to other areas favourable for egg deposition. These aggregation areas are “leks”: non-resource-based mating aggregations38 in which males produce olfactory (pheromones), visual, and auditory signals to attract females and other males39.
In our study, as shown by the cluster indices, the wild male medfly population aggregated in hotspots. Despite their release from uniformly distributed Plastic Aerial Release Container (PARC) boxes in the experimental area, the male sterile samples also showed an aggregated distribution. As for the wild population, the total cluster index was statistically significant, and this statistical significance was also found on most sampling dates. The contour maps showed that the aggregation areas of the sterile males mirrored those of the wild males. This is confirmed by the positive and statistically significant association indices for both the total and most of the sampling dates.
While the female population was more evenly distributed, some female hotspots, overlapping with male aggregation areas, were observed. Sciarretta et al. (2018)34 showed that mated and virgin females occupy different areas in the field; the former search trees with ripening fruit for oviposition while the latter station in areas with males for mating. However, as female were monitored without differentiating reproductive status, it is not possible to determine whether the females formed hotspots for oviposition or mating.
The formation of significant positive associations of sterile and wild males does not guarantee that the released ones are successful at mating. Shelly and Whittier (1996)40, in a study on the evaluation of the mating competitiveness of sterile male of C. capitata, using the strain Maui-93, found that wild males achieves 91% of mating in leks formed by sterile and wild males even if they they wereonly 19% of the total males in these leks. Thus, sterile males forming their own leks not in the same place as wild males could be more likely to mate successfully with wild females. Further studies could examine the success rate of oviposition in captured females to assess whether they mated with sterile or wild males.
As shown on the contour maps, the sterile insects released in the southern part of the experimental field shifted northwards and aggregated in areas where more wild males were present. The orchards in the southern part of the experimental farm probably did not fulfil the requirements for the formation of leks, and sterile males, responding to the same stimuli as the wild ones, abandoned the area to find more favourable locations. Sterile males’ ability to move has been studied with experiments placing monitoring traps at various distances, generally with the furthest traps placed a few hundred metres from the sterile medfly release point. However, Andress et al. (2013)28 reported longer movement distances, showing that some sterile males move up to 1 km from the point of release.
Some distribution surveys report spatial dispersions of sterile flies mirroring those of wild flies, but these investigations focus on individual releases and cover small areas22,24,25. Our research shows that, in an operational condition during a SIT programme using ground release with PARC boxes, the sterile males showed a similar distribution to wild males throughout the entire release period. Furthermore, while our work did not specifically address the dispersal of individuals it clearly showed that sterile males make short-range movements that completely redefine their distribution in the field, moving from the release points to mating areas occupied by the wild population.
This research contributes to the knowledge of sterile medfly behaviour in a multi-varietal fruit-growing area and provides useful information for determining the protocols for released sterile flies in AW-IPM.
Methods
Study area
The investigation was conducted in an experimental farm (about 65 ha) managed by the Research Centre for Olive, Fruit and Citrus Crops (Council for Agricultural Research and Economics, CREA), in an agricultural area near Rome (Latium region, Central Italy), at an altitude ranging from 80 to 130 m a.s.l. The farm includes various fruit orchards occupying approximately 30 ha, composed mainly of peaches (9.6 ha), apricots (4 ha), cherries (3.6 ha), plums (3.1 ha), apples (2.8 ha), pears (1.9 ha), kiwifruit (1.2 ha), and pomegranates (0.8 ha) (Fig. 7A). There were also small plots of figs, walnuts, almonds, pistachios, blueberries, raspberries, hazelnut, feijoas, as well as hedgerows of bitter orange (Poncirus trifoliata) and isolated trees of persimmon and prickly pear.
Maps of the experimental area. Main orchards highlighted [plum (1), peach (2), cherry (3), apricot (4), apple (5), pomegranate (6), pear (7), feijoa (8)] (areas not highlighted are uncultivated fields or areas with sporadic or non-host trees) (A); and traps baited with Trimedlure (yellow circle) and release points of sterile medflies (white square) highlighted (B). The maps were generated using the software Google Earth Pro (https://www.google.it/earth/).
The fruits that medflies eat are present in the field from May to December due to the rich varietal collection (more than 4,000 modern and traditional cultivars). Since the farm is an experimental farm for variety selection, only a few fruits are used for tests, while most remain on the plants until they fall to the ground and decay. No insecticide treatments were carried out in the farm during our trial period.
The experimental farm is surrounded by fodder crops and uncultivated areas, so it can be considered an ecologically isolated territory, an indispensable feature to prevent fly immigration that would compromise the SIT trial. The climatic data were recorded by a meteorological station placed in the experimental farm.
Insects
Sterile medflies of the strain “Vienna 8” were obtained from the mass-rearing facility Empresa de Transformación Agraria S.A., TRAGSA (Valencia, Spain). Pupae were marked with red fluorescent dye with a dose of 1 g/l (to help identify the adult later), packed in plastic bags under hypoxia 24–72 h before the expected adult emergence, and irradiated with 100 Gy. Following irradiation, bags containing pupae were packed in an isothermal shipping box (kept at 10–16 °C) and transported via commercial airline to the Rome Fiumicino airport. The time spent under hypoxia during shipping was about 18–20 h.
Irradiated male pupae from two batches of different ages (2–3 days apart) were received weekly, from late June to mid-October 2019, except one missed shipment in early August.
Quality control tests
The supplier carried out the routine Quality Control (QC) tests and certified that the quality of the sterile flies was in line with the FAO/IAEA/USDA/Quality Control Manual32.
To verify the quality of the pupae after shipping, emergence rate and adult lifespan tests were performed following the protocols in the aforementioned manual32. The emergence rate was quantified for each shipment by taking 100 pupae from each batch and counting the individuals that emerged. In total, 32 tests were performed. This test was performed in external thermal conditions.
To assess the lifespan of sterile flies, 5 repetitions (from different shipments) were carried out with samples of 100 barely emerged adults kept in laboratory cages. The adult flies were fed an aqueous-sugar solution. The number of dead samples was recorded daily to evaluate the lifespan. This test was performed in ambient room conditions (24–28 °C).
Holding and release of sterile flies
Upon arrival at the airport, the material (175,000 pupae in each batch, for a total of 350,000) was transported to the experimental farm, removed from the plastic bags, and placed in ventilated Plastic Aerial Release Container (PARC) boxes (50 × 25 × 20 cm) containing water and food, following Enkerlin (2007)9. For each batch, 37 PARC boxes, containing approx. 4,700 pupae each (0.96 adults per cm2), were set up. The PARC boxes were kept outside until release, which occurred 5–6 days after the adults emerged.
Every 3–4 days, sterile male medflies were released in the field. The release was conducted homogeneously on the experimental farm by placing the 37 PARC boxes on the ground and opening them (Fig. 7B). About 2 h later, the empty PARC boxes were removed. The first release was on 1 July, and the last was on 24 October. Due to the interruption of sterile pupae shipment in early August, the releases scheduled for 12 and 15 August were not carried out. A total of 32 adult sterile male medfly releases were performed.
Adult monitoring
The activity of medfly adults was monitored using 57 Vaso Trap® devices (Carrello Roberto, Pianezza, Torino, Italy). Thirty-eight traps were baited with a male-targeted trimedlure dispenser (Isagro, Milano, Italy) (Fig. 7B), and 19 traps were baited with a female-targeted dispenser BioLure® Unipak (Suterra, Valencia, Spain). Traps were placed in the tree canopy at a height of 1.5–2.0 m. Trimedlure and BioLure dispensers were replaced every 6 weeks and every 4 months, respectively. The monitoring period started in April and ended in December 2019. Traps baited with trimedlure were installed with a density of approximately 1 per hectare of fruit orchard, to cover all fruit species present in the farm. Traps baited with BioLure were placed with a density of approximately 2 per hectare of fruit orchard. Traps were inspected weekly. For traps baited with the BioLure, male and female adults were identified, and only the females were counted; for those baited with trimedlure, all the males were counted, and a sample was subsequently taken to evaluate the percentages of sterile and wild flies. This was performed via the observation of fluorescent dye markings.
Representative sample size
Given the large number of medflies caught by the monitoring traps, the counting of sterile and wild medflies was carried out from a subset of the total catches per trap.
To assess whether the sample was representative of the total population, the results of the observation of fluorescent dye markings derived from two different sub-sample sizes were compared with data from the total population (see Table 1). This trial was done in 7 repetitions, i.e., counting catches from 7 traps. The results were compared by means of a contingency table and χ2 analysis.
Determination of the sterile or wild condition of male adult flies
Following Guillen-Aguilar et al. (2016)41, sterile males were identified in two steps. In the first step (step 1), the medflies were observed in a dark room with ultraviolet (UV) light (TL-D Blacklight Blue 36 W, Philips), to detect the fluorescent dye mark in the head frontal zone and the ptilinal suture. To facilitate observation, each medfly was pinned and fixed on a black card with its head facing the observer before placement in the dark room. Magnifying glasses were also used to get a clear view. Medflies with observable fluorescent markings were counted as sterile. The remaining flies were observed through fluorescence microscopy (step 2), separating the head from the body and placing the head on a slide. For this step, a Leica DLMB microscope lit with HBO mercury lamp (50 W/AC 34 V) was used. If the fluorescent mark was observable the fly was counted as sterile; otherwise, it was counted as wild.
After 3 inspection days (114 different trap samples), step 2 was eliminated, as only small percentage of flies were not correctly identified as sterile during step 1 (average error rate of 1%).
Due to the large number of flies caught in the monitoring traps, the counting took several months, which did not allow quick observation of fluorescent dye markings. The samples (labelled with trap number and sampling date) were frozen for later analysis.
Spatial analysis
Characterisation of the spatial distribution was carried out with SADIE (Spatial Analysis by Distance Indices) software (SADIEShell Version 2.0). This software is designed specifically for analysing counts of individuals at known locations42,43. It seeks to identify areas of clustering of two forms: patch and gap clusters. SADIE assigns each location an index of clustering (v) using the mean count (m); either a positive vi index for patch units with ci > m, or a negative vj index for gap units with cj < m. These cluster indices are then used to calculate overall cluster indices Vi and Vj for patches and gaps, respectively, with associated significance values43,44. Clustering analyses were carried out for each sampling date and for the whole sampling period, for the catches of C. capitata wild and sterile males and females.
SADIE (using the software SADIE N_AShell Version 1.0) was also used to perform an association analysis, measuring the local spatial association (χk) between two populations by overlaying the cluster maps of the two distributions45. Positive values of χk arise from coincidences of patches or gaps in both populations, while negative values arise from opposite cluster types. An overall index of spatial association (X) is calculated as the mean of local values. The significance of X may be tested by randomisations, after allowance for small-scale spatial autocorrelation from either population46. The association index was obtained for each sampling date and the whole sampling period, to compare the distributions of wild and sterile C. capitata males.
Geostatistical methods were used to map the spatial distribution of C. capitata wild and sterile males and females. Spatial distribution data and spatial association indices were contoured and mapped with Surfer version 12 (Golden Software, Golden, CO, USA), using the software’s default settings. On the maps, the positive local association (shown in blue) highlights areas occupied by both wild and sterile males (coincidences of patches) and areas not occupied by either (coincidences of gaps). The negative local association (shown in pink) highlights areas with opposite cluster type.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Morales, P., Cermeli, M., Godoy, F. & Salas, B. A list of mediterranean fruit fly Ceratitis capitata Widermann (Diptera: Tephritidae) host plants based on records on INIA-CENIAP museum of insects of agricultural interest. Entomotropica 19, 51–54 (2004).
Giunti, G. et al. Biology, ecology and invasiveness of the mediterranean fruit fly, Ceratitis capitata: a review. Entomol. Gen. 43 (6), 1211–1239. https://doi.org/10.1127/entomologia/2023/2135 (2023).
Giunti, G. et al. Management of the mediterranean fruit fly, Ceratitis capitata: past, present, and future. Entomol. Gen. 43 (6), 1241–1263. https://doi.org/10.1127/entomologia/2023/2136 (2023).
Colacci, M., Forleo, M. B., Lux, S. A. & Sciarretta, A. The status of Medfly and IPM practices based on case studies in Italy. Acta Hortic. 1352, 97–105. https://doi.org/10.17660/ActaHortic.2022.1352.13 (2022).
Navarro-Llopis, V. & Vacas, S. Mass trapping for fruit fly control. In Trapping and the Detection, Control and Regulation of Tephritid Fruit Flies. Lures, Area-Wide Programs, and Trade Implications (eds. Shelly, T., Epsky, N. B., Jang, E. B., Reyes-Flores, J. & Vargas, R.). 513–555 (Springer, 2014).
Piñero, J. C., Enkerlin, W. & Epsky, N. D. Recent developments and applications of bait stations integrated pest management of tephritid fruit flies. In Trapping and the Detection, Control and Regulation of Tephritid Fruit Flies. Lures, Area-Wide Programs, and Trade Implications (eds. Shelly, T., Epsky, N. B., Jang, E. B., Reyes-Flores, J. & Vargas, R.). 457–492 (Springer, 2014).
Tlemsani, M. & Boulahia-Khedr, S. Assessment of IPM using Conetrap® MagnetTM MED and Moskisan® attract-and-kill and mass-trapping devices to control Ceratitis capitata (Diptera: Tephritidae) in citrus orchards. J. Entomol. Zool. Stud. 5, 1511–1517 (2017).
Colacci, M., Trematerra, P. & Sciarretta, A. Evaluation of trap devices for mass trapping of Ceratitis capitata (Diptera: Tephritidae) populations. Insects 13, 941 (2022). https://doi.org/10.3390/insects13100941
Enkerlin, W. R. Guidance for Packing, Shipping, Holding and Release of Sterile Flies in Area-Wide Fruit Fly Control Programmes. FAO Plant Production and Protection Paper 190. 1–134 (Rome, 2007).
Knipling, E. F. Sterile insect technique as a screwworm control measure: the concept and its development. In Symposium on Eradication of the Screwworm from the United States and Mexico (ed. Graham O. H.). 1–62. (Misc. Publ. Entomol. Soc. America, 1985).
Enkerlin, W. R. Impact of fruit fly control programmes using the sterile insect technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management. 2 Ed. (eds. Dyck, A., Hendrichs, J. & Robinson A. S.). 979–1006 (CRC Press, 2021).
Hendrichs, J. & Robinson, A. Sterile insect technique in Encyclopedia of Insects. Second Edition (eds Resh, V. H. & Cardé, R. T.) 935–957 (Academic, Elsevier, (2009).
Plá, I. et al. Sterile insect technique programme against mediterranean fruit fly in the Valencian community (Spain). Insects 12, 415. https://doi.org/10.3390/insects12050415 (2021).
Lance, D. R. & McInnis, D. O. CRC Press,. Biological basis of the sterile insect technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management. 2nd Ed. (eds. Dyck, A., Hendrichs, J. & Robinson A. S.). 113–142 (2021).
Lindquist, D. A., Butt, B. A. & Moore, I. Ecological requirements of the sterile male technique. In Proceedings: FAO Conference on Ecology in Relation to Plant Pest Control, 11–15 December 1972, Rome, Italy. 249–262 (FAO, 1974).
Itô, Y., Yamamura, K. & Manoukis, C. Role of population and behavioural ecology in the sterile insect technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management. 2nd Ed. (eds. Dyck, A., Hendrichs, J. & Robinson A. S.). 245–282 (CRC Press, 2021).
Wakid, A. M. (ed Shoukry, A.) Dispersal and flight range of mediterranean fruit fly, Ceratitis capitata Wied. In Egypt. Z. Angew Entomol. 81 214–218 https://doi.org/10.1111/j.1439-0418.1976.tb04228.x (1976).
Wong, T. T. Y. et al. Mediterranean fruit fly: dispersal of wild and irradiated and untreated Laboratory-Reared males. Environ. Entomol. 11, 339–343 (1982).
Baker, P. S., Chan, A. S. T. & Zavala, M. A. Dispersal and orientation of sterile Ceratitis capitata and Anastrepha ludens (Tephritidae) in Chiapas, Mexico. J. Appl. Ecol. 23 (1), 27–38 (1986).
Plant, R. C. & Cunningham, R. T. Analyses of the dispersal of sterile mediterranean fruit flies (Diptera: Tephritidae) released from point source. Environ. Entomol. 20 (6), 1493–1503 (1991).
Meats, A. & Smallridge, C. J. Short- and long-range dispersal of Medfly, Ceratitis capitata (Dipt., Tephritidae), and its invasive potential. J. Appl. Entomol. 131, 518–523 (2007).
Horta-Lopes, D. J. et al. The importance of dispersal surveys on the behavioural knowledge of mediterranean fruit fly sterile males (Ceratitis capitata Wiedemann) (Diptera: Tephritidae) released at an orchard at biscoitos and in the Angra urban area, Terceira Island, Azores. IOBC/WPRS Bull. 38, 161–169 (2008).
Paranhos, B. J. et al. Field dispersal and survival of sterile Medfly males aromatically treated with ginger root oil. Environ. Entomol. 39 (2), 570–575. https://doi.org/10.1603/EN08309 (2010).
Gavriel, S., Gazit, Y., Leach, A., Mumford, J. & Yuval, B. Spatial patterns of sterile mediterranean fruit fly dispersal. Entomol. Exp. Appl. 142, 17–26. https://doi.org/10.1111/j.1570-7458.2011.01197.x (2012).
Duarte, F. et al. Release-Recapture test of dispersal and survival of sterile males on Ceratitis capitata (Diptera: Tephritidae). Neotrop. Entomol. 49, 893–900. https://doi.org/10.1007/s13744-020-00801-x (2020).
Barry, J. D., Blessinger, T. & Morse, J. G. Recapture of sterile mediterranean fruit flies (Diptera: Tephritidae) in California’s preventative release program. J. Econ. Entomol. 97 (5), 1554–1562 (2004).
Meats, A., Smallridge, C. J. & Dominiak, B. C. Dispersion theory and sterile insect technique: application to two species of fruit fly. Entomol. Exp. Appl. 119, 247–254. https://doi.org/10.1111/j.1570-7458.2006.00415.x (2006).
Andress, E., Walters, I., del Toro, M. & Shelly, T. Release-Recapture of sterile male mediterranean fruit flies (Diptera: Tephritidae) in Southern California. Proc. Hawaii. Entomol. Soc. 45, 11–29 (2013).
Puche, H., Midgarden, D. G., Ovalle, O. & Kendra, P. E. Effect of elevation and host availability on distribution of sterile and wild mediterranean fruit flies (Diptera: Tephritidae). Fla. Entomol. 88 (1), 83–90. https://doi.org/10.1653/0015-4040(2005)088 (2005). [0083:EOEAHA]2.0.CO;2.
Chiesa, S. G., Angeli, G., Cristofaro, M., Arnone, S. & Ioriatti, C. The sterile insect technique for mediterranean fruit fly control: a North-Italy pilot project. Pheromones and other semiochemicals in integrates production and integrated protection of fruit crops. IOBC-WPRS Bull. 146, 85–87 (2019).
Chiesa, S. G. et al. Validazione Della tecnica Dell’insetto sterile per La gestione Della Mosca Della frutta Ceratitis capitata in Meleto. Atti Gior Fitopatolog. 1, 141–146 (2020).
FAO/IAEA/USDA. Product Quality Control for Sterile Mass-Reared and Released Tephritid Fruit Flies, Version 6.0. 1–164 (International Atomic Energy Agency, 2014).
Sciarretta, A. & Trematerra, P. Spatio-temporal distribution of Ceratitis capitata population in a heterogeneous landscape in central Italy. J. Appl. Entomol. 135, 241–251. https://doi.org/10.1111/j.1439-0418.2010.01515.x (2011).
Sciarretta, A. et al. Analysis of the mediterranean fruit fly [Ceratitis capitata (Wiedemann)] spatio-temporal distribution in relation to sex and female mating status for precision IPM. PLos ONE. 13 (4), e0195097. https://doi.org/10.1371/journal.pone.0195097 (2018).
Kaspi, R. & Yuval, B. Mediterranean fruit fly Leks: factors affecting male location. Funct. Ecol. 13, 539–545. https://doi.org/10.1046/j.1365-2435.1999.00344.x (1999).
Papadopoulos, N. T., Katsoyannos, B. I. & Nestel, D. Spatial autocorrelation analysis of Ceratitis capitata (Diptera: Tephritidae) adult population in a mixed deciduous fruit orchard in Northern Greece. Environ. Entomol. 32 (2), 319–326. https://doi.org/10.1603/0046-225X-32.2.319 (2003).
Midgarden, D., Lira, E. & Silver, M. Spatial analysis of Tephritid fruit fly traps. In Trapping and the Detection, Control and Regulation of Tephritid Fruit Flies. Lures, Area-Wide Programs, and Trade Implications (eds. Shelly, T., Epsky, N. B., Jang, E. B., Reyes-Flores, J. & Vargas, R.). 277–320 (Springer, 2014).
Höglund, J. & Alatalo, R. V. Leks. 1–248 (Princeton University Press, 1995).
Shelly, T. E. & Whittier, T. S. Lek behavior of insects. In Mating Systems in Insects and Arachnids (eds. Choe, J. C. & Crespi, J.). 273–293 (Cambridge University Press, 1997).
Shelly, T. E. & Whittier, T. S. Mating competitiveness of sterile male mediterranean fruit flies (Diptera: Tephritidae) in male-only release. Ann. Entomol. Soc. Am. 89 (5), 754–758 (1996).
Guillen-Aguilar, J. C. et al. Manual to Differentiate Wild Mediterranean Fruit Flies Ceratitis capitata (Wied.) from Non-Irradiated (Fertile) and Irradiated (Sterile) VIENNA Temperature Sensitive Lethal Strain Flies. 1–51 (Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture, 2016).
Perry, J. N., Winder, L., Holland, J. M. & Alston, R. D. Red-blue plots for detecting clusters in count data. Ecol. Lett. 2, 106–113. https://doi.org/10.1046/j.1461-0248.1999.22057.x (1999).
Winder, L. et al. Twenty years and counting with SADIE: Spatial analysis by distance indices software and review of its adoption and use. Rethinking Ecol. 4, 1–16. https://doi.org/10.3897/rethinkingecology.4.30890 (2019).
Perry, J. N. Measures of Spatial pattern for counts. Ecology 79, 1008–1017. https://doi.org/10.1890/0012-9658 (1998). (1998)079[1008:MOSPFC]2.0.CO;2.
Perry, J. N. & Dixon, P. A new method to measure Spatial association for ecological count data. Ecoscience 9, 133–141. https://doi.org/10.1080/11956860.2002.11682699 (2002).
Dutilleul, P. Modifying the t test for assessing the correlation between two Spatial processes. Biometrics 49, 305–314. https://doi.org/10.2307/2532625 (1993).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.C., M.R.T., A.S.; Data curation: M.C., C.C.; G.B.; Formal Analysis: M.C., D.L., P.F., A.S.; Funding acquisition: M.R.T.; Investigation: M.C., M.R.T., D.L., P.F., C.C.; G.B.; Methodology: M.C., M.R.T., A.S.; Resources: M.C., M.R.T., A.S.; Supervision: M.R.T.; Writing – original draft: M.C, A.S.; Writing – review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Colacci, M., Tabilio, M.R., Lolletti, D. et al. Spatio temporal association between sterile and wild males in a Ceratitis capitata SIT programme. Sci Rep 15, 19584 (2025). https://doi.org/10.1038/s41598-025-04369-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04369-7