Abstract
In plants, potassium (K+) serves multiple functions, despite being scarce due to strong soil adsorption. This study examined how the presence of arbuscular mycorrhiza fungi (AMF) like Rhizophagus irregularis and Funneliformis mosseae influenced the absorption and transport of K+ in bean roots through symbiotic interactions. In a symbiotic relationship, AMF had the potential to enhance potassium absorption and storage in various tissues of bean seedlings. Under symbiotic conditions, the concentration of potassium in stem tissues was observed to increase almost four times more than control conditions. The genome of beans was shown to contain a total of nineteen PvAKT genes and two PvHKT genes. Based on phylogeny analysis, PvAKT family members and their corresponding orthologs were categorized into four distinct groups. Subfamily 3 of the PvAKT phylogeny tree exhibited distinct variations from other subfamilies in terms of gene structure, conserved domains, and potential phosphorylation sites. The presence of cis-regulatory element related to ABA responsiveness in the upstream region led to the division of PvAKT and PvHKT genes into two specific groups. Gene expression analysis disclosed that PvAKT and PvHKT genes are induced by AMF and have tissue specific expression. PvAKT6 and PvAKT11 genes and both PvHKT genes showed differential expression in root and shoot tissues, while PvAKT3 gene increased expression in both root and shoot tissues. The results suggest that AMF had a significant impact on increasing the solubility of K+ and ultimately enhancing the function of K+ transporters.
Similar content being viewed by others
Introduction
The second highest mineral nutrient found in plants is Potassium (K +), making up 2–10% of their dry weight1. K+ is a crucial necessity for all cells to function properly, and it must come from the external environment. Within the plant cytoplasm, K+ plays a vital role as a major inorganic cation that is necessary for enzyme activity, particularly in primary metabolism2. K+ plays a crucial role in enabling plants to carry out important biochemical and physiological functions like stomatal movement, osmoregulation, photosynthesis, and protein synthesis3,4. The evolution of a multitude of K+ transporters and channels in plants allows for the regulation of K+ transport, which is essential for diverse physiological and developmental processes3,5.
Investigations into plant DNA sequences unveiled three groups of genes responsible for producing K+ transporters in the plasma membrane: the HKT, HAK/KUP/KT, and AKT family6,7. The HKT (High-Affinity Potassium Transporters) gene family consists of potassium and sodium transporters that are essential for absorbing and controlling these elements in different environmental situations8. Specific characteristics distinguish the various subgroups within the HKT family. There are two clearly defined subfamilies within the HKT family that can be distinguished by their ion selectivity8,9. Subfamily I transporters are specific to only Na+, whereas those in subfamily II have the ability to transport either Na + or K + or even both ions9,10,11. The AKT (Arabidopsis K + Transporter) is a main type of K+ transporter in plants. The role of potassium transporters from the AKT gene family is significant in controlling the absorption and regulation of K+ in plants12,13. Besides, it was stated that AKTs are involved in response to abiotic stresses14. In soil environments with higher K+ concentrations, AKT1 transport activity becomes crucial for mediating independent K+ transport15. Furthermore, Plants that overexpress AKT1 exhibit enhanced proton efflux capacity and are more resistant to ABA inhibition13.
In many agricultural soils, the concentration of K+ in the soil is optimal for the growth and development of plants, but the main problem is the binding of potassium to the mineral particles of the soil grains, which reduces its absorption by the roots16. Consequently, plants must come up with successful approaches to increase the absorption of K+ from the soil, which may involve using high-affinity transport systems or establishing symbiotic relationships with microorganisms17. Arbuscular mycorrhizal fungi (AMF) have become well-known for their role in exchanging nutrients and enhancing plant resilience during environmental challenges18. AMF establish a mutually beneficial bond with their host plants, bartering essential nutrients and water for carbon-rich sugars and lipids19,20,21. Furthermore, AMF play a crucial role in increasing a plants utilization of phosphorus and nitrogen when facing abiotic stress such as drought22. Our understanding of how AMF impacts plant uptake of K+ is extremely lacking.
The species Phaseolus vulgaris, commonly referred to as common bean, represents a leguminous plant that is extensively utilized in the dietary practices of humans due to its nutritional value, and it possesses the remarkable capability to establish mutualistic symbiotic relationships with both arbuscular mycorrhizal fungi, which play a crucial role in enhancing nutrient uptake, particularly phosphorus, and nitrogen-fixing rhizobacteria, which are essential for converting atmospheric nitrogen into a form that is accessible and beneficial for plant growth, thereby contributing significantly to soil fertility and ecosystem sustainability23.In the current study, the AKT and HKT family members were identified and characterized in genome of common bean. Moreover, the absorption of potassium and transcription levels of PvHKT and PvAKT genes were analyzed in bean roots undergoing symbiosis with AMFs like Funneliformis mosseae and Rhizophagus irregularis. Our hypothesis in this study was that symbiosis with mycorrhizal fungi increases potassium uptake and potassium transporter activity. Results indicated that AMFs have a positive effect on the solubilization and absorption of K+ ions, subsequently altering the expression levels of PvHKT and PvAKT genes.
Materials and methods
Plant materials and treatments
Common bean seeds, Kermanshah variety prepared by Karaj Seedling and Seed Research Center, were treated with 3% sodium hypochlorite solution for 5 min to sterilize and then washed twice with sterile water. The seeds were planted in pots with sterile soil and 50 g of mycorrhizal inoculum. The tested soil had 45 ppm sodium and a pH of 6.8, and the soil texture was silty. The soil that underwent rigorous testing procedures was subjected to sterilization through the application of an autoclave. The sterilized soil was carefully distributed into pots with height of 25 cm and a diameter of 20 cm. In this experiment, three treatments were investigated, which included treatment with inoculum of Funneliformis mosseae, Rhizophagus irregularis and not using fungus (using mock inoculum). The inoculum of F. mosseae and R. irregularis was prepared from Turan Biotechnology Company, Shahrood, Iran. The pots were watered at intervals of four days and kept at a temperature of 26 ± 3 °C and a light length of 14 h. In this experiment, fertilizers were not used to feed the seedlings. After six weeks, to confirm the cloning of AMF, the roots were gently removed from the soil and washed with water. Phillips and Heyman’s method24 was used to color the roots and it was examined using light microscopes for confirming symbiosis. Different organs of 7-week-old seedlings were harvested and stored in liquid nitrogen and then transferred to a -80 freezer. This experiment was performed in four independent repetitions and three seedlings were examined in each repetition.
Measurement of potassium concentration in plant organs
In order to measure potassium in the root, stem and leaf tissues, the tissues were dried for 24 h in the oven at a temperature of 42 °C. In the next step, 2 g of each tissue was completely powdered and placed at a temperature of 550 °C for 5 h. Then 20 cc of 6% hydrochloric acid solution was added to each sample and the samples were kept at 80 °C Bain-Marie for 20 min. The solution is poured into a balloon and diluted to 100 ml with distilled water. Next, we utilize the film photometer to measure both the standard series and the current samples at a wavelength of 766.5 nm.
Identification and characterization of AKT and HKT genes in bean genome
In the Ensembl Plants database25, the BLASTp program was used to identify orthologous sequences in bean plants by querying the known sequence of AKT and HKT proteins from Arabidopsis thaliana. The sequences that were identified underwent verification and validation by the CDD database based on their possession of a distinct domain. The identified sequences were named according to their location. The arrangement of PvAKT and PvHKT genes was visualized by TBtools v. 0.665 (https://github.com/CJ-Chen/TBtools-II)26 through the depiction of exon and intron distributions. The ProtParam tool27 was utilized to analyze the physicochemical properties of PvAKT and PvHKT proteins including isoelectric point (pI), molecular weight (MW), the grand average of hydropathy (GRAVY) value, and instability index.
In order to create the phylogenetic tree, it was necessary to align the sequences of PvAKT and PvHKT proteins with their homologs using Clustal Omega28. In the next step based on the multiple alignment results, IQTREE website29 created a phylogenetic tree using Maximum Likelihood method with 1000 Bootstrap. Through multiple alignment analysis using the ClustalW method, duplicated genes within the PvAKT and PvHKT gene family were pinpointed by identifying genes with greater than 70% similarity in their CDS sequences. Furthermore, the TBtools software computed the Ka and Ks indices.
In order to predict the phosphorylation modifications sites, the PvAKT and PvHKT protein sequences were fed into the NetPhos 3.1 server platform30. Areas rich in serine, threonine, and tyrosine amino acids that exhibited a confidence level of over 90% were detected and assembled.
An analysis was conducted on a segment of 1500 nucleotides leading up to the initiation site of the PvAKT and PvHKT genes, serving as the promoter area. The sequences were extracted from the Ensembl Plants database and the promoter sequence of PvAKT and PvHKT genes underwent analysis in the PlantCare database31. Finally, the cis-regulatory elements were grouped according to their function.
RNA extraction and real-time PCR analysis
Hot phenol method was used to extract RNA from root and shoot tissues. The quality and quantity of RNA samples were checked using Nanodrop (Implen N50, Munich, Germany). Suitable samples were selected and converted to cDNA using a reverse transcriptase kit (Yekta Tajhiz Azma Co., Tehran, Iran). In order to check the expression pattern of genes, ABI Step One device and Ampliqon 2X qPCR Master Mix green-High Rox (Ampliqon, Stenhuggervej, Denmark) kit were used. In this study, the expression of four PvAKT genes (one gene from each phylogenetic group) along with two PvHKT genes were investigated. Actin gene with access code XM_007139153.1 was used as a reference gene. The primers of all genes were designed using Primer Blast program and based on the coding sequence (Supplementary Table 1). The delta-delta ct method32 was utilized to compute the relative expression levels of each gene.
Data analysis
The average potassium concentration in different organs was compared using Tukey’s test (P-value < 0.05). The relative gene expression data was compared with the control sample using t-test.
Results
K+ concentration in different tissues
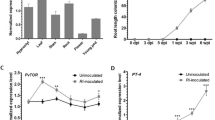
The trend of K+ concentration changes in different bean tissues showed that symbiosis with AMF could increase K+ absorption and accumulation (Fig. 1). In leaf tissues, K+ concentration increased 1.6 and 2.3 times compared to the control, respectively, in symbiosis with F. mosseae and R. irregularis. The highest increase in K+ concentration was observed in the stem tissues, which increased almost 4 times under the symbiotic conditions compared to the control. Potassium accumulation was more accumulated in root tissues than other tissues. Under the conditions of symbiosis with R. irregularis, the concentration of K+ in the roots increased almost 1.4 times compared to the control.
Physiochemical properties of PvAKTs and PvHKTs
In the current study, nineteen PvAKT and two PvHKT genes were identified in the genome of common bean (Table 1). The protein length of PvAKTs ranged from 309 aa (PvAKT17) to 875 aa (PvAKT6) and exon number varied from 1 (PvAKT3 and PvAKT19) to 12 (PvAKT11 and PvAKT14). Moreover, the GRAVY index ranged from − 0.247 to 0.271 and most PvAKTs showed negative GRAVY (hydrophilic proteins). Both PvHKT proteins showed positive GRAVY indicating hydrophobic proteins. In addition, pI value varied between 5.13 and 9.55 in PvAKTs. PvHKT proteins were very similar based on physicochemical properties, but differences were observed between PvAKTs.
Phylogenetic tree of PvAKT and PvHKTs
Based on phylogeny analysis, PvAKT family members and their orthologs were divided into four groups (Fig. 2a). Six members were in group 1, one member in group 2, five members in group 3 and seven members in group 4. It was also found that there is the most similarity between AKT homologues of green pea with PvAKT members. The results of the phylogeny tree for HKT members showed that both PvHKT proteins are in the same group and the closest ortholog to them is green pea HKT, Psat1g050520 (Fig. 2b). The structure of PvAKT genes revealed that these genes are structurally different from each other, only the members of phylogeny group 3 were slightly different from each other (Fig. 3a). All members of phylogeny group 3 have two pore domain potassium channel while other PvAKT members showed to have ERG, potassium channel/voltage-dependent domain (Fig. 3b). PvHKTs showed similar gene structure and having cation transporter domain.
Duplication events in PvAKTs and PvHKTs
Based on the position of genes, PvAKTs were located on 10 chromosomes of bean genome and both PvHKT genes were located on chromosome number 4 (Fig. 4). Duplication events between PvAKTs estimated that two pairs of genes including PvAKT7-PvAKT17 and PvAKT8-PvAKT18 were segmentally duplicated. It was also found that two PvHKT genes are duplicated in tandem. All duplicated genes showed the Ka/Ks less than 1, indicating that they were under purifying selection. These results indicate that PvAKT family members are less affected by duplication events.
Prediction of phosphorylation modifications
Phosphorylation modification sites in PvAKT and PvHKT proteins were predicted based on the position of three amino acids, serine, tyrosine, and threonine (Fig. 5). The results revealed that PvAKT13 protein has the highest potential phosphorylation site and PvAKT18 has the lowest site. Comparison between different groups of PvAKT revealed that members of group 3 have fewer phosphorylation sites than other groups. In group 3, there are proteins that all have two pore domain potassium channels. Among PvHKT proteins, PvHKT2 had more potential phosphorylation modification sites.
Upstream analysis of PvAKT and PvHKT genes
Analysis of the upstream region of PvAKT and PvHKT genes showed that there are differences between these genes based on the frequency of cis-regulatory elements (Fig. 6). For example, eight ABA response cis-regulatory elements (ABRE) were identified in the promoter of PvAKT7 gene, while this cis-element was not identified in the promoter region of PvAKT1, PvAKT3, PvAKT5, PvAKT6, PvAKT16 and PvAKT19 genes. Based on the grouping, the highest frequency belongs to cis-elements related to response to light and growth. Among the stress response cis-regulatory elements, MYB showed the highest frequency in the promoter of PvAKT and PvHKT genes. PvHKT1 was richer in terms of distribution of cis-regulatory elements than PvHKT2.
Expression analysis of PvAKTs and PvHKTs
The expression profile of PvAKT genes in shoot tissues showed that the transcription level of these genes is affected by the symbiosis with AMF (Fig. 7). PvAKT3 and PvAKT11 genes showed a similar expression pattern, both of them were increased under the conditions of symbiosis with AMF, although they were more induced under symbiosis with F. mosseae. The transcription level of PvAKT5 gene showed some increase under both conditions of symbiosis with AMF. A different expression pattern was observed in PvAKT6 gene and its expression was down-regulated in response to symbiosis with AMF. Both PvHKT genes showed almost similar expression patterns. Both showed a significant decrease in expression in response to symbiosis with R. irregularis.
Under the conditions of symbiosis with R. irregularis, the expression of PvAKT3, PvAKT5 and PvAKT6 genes significantly increased in root tissues, while these genes were less induced under the conditions of symbiosis with F. mosseae (Fig. 8). The transcription level of PvAKT11 gene was decreased in root tissues symbiotic with F. mosseae and was not induced under symbiosis with R. irregularis. The expression levels of both PvHKT genes were up-regulated in response to symbiosis with AMF, although their expression was more clearly induced by R. irregularis.
Discussion
K+ plays a critical role as the primary cation in regulating the proper ionic environment within plant cells. The balance of K+ within cells is vital for supporting essential functions and ultimately ensuring viability33. One of the problems in receiving K+ is its absorption by the soil and its low availability for plant roots. The symbiosis of plant roots with AMF can increase the absorption of ions, including K+. The symbiotic relationship between bean seedlings and AMF was shown to increase K+ absorption and accumulation in leaf, stem, and root tissues according to our findings. The increase in K+ concentration reached its peak in the stem tissues, where it grew nearly fourfold under symbiotic conditions as opposed to the control. There are few reports that have shown that symbiosis of plant roots with AMF increases K+ absorption. For example, it has been reported that the AM fungus Rhizophagus irregularis in tomato plants leads to improved growth and increased K+ acquisition, which prevents K+ deficiency for optimal root growth34. The presence of increased levels of K+ in plant tissues as a result of mycorrhizal associations with varying AMF species has been confirmed in studies on Pelargonium peltatum shoots35 and Lactuca sativa leaves36. The stem plays a role as a storage organ in plants. It seems that K+ accumulation in the stems of beans supports their growth and development processes.
K+ transporters play an important role in absorbing and moving and maintaining the balance of this ion. In this study, 19 PvAKT family members and 2 PvHKT genes were identified in the bean genome. PvAKT family members had differences in structure and physicochemical properties and were separated into four subfamilies based on the phylogenetic tree. These differences can be due to evolutionary pressures that have caused changes in their sequence. The members of subfamily 3 of the phylogeny tree showed more obvious differences with other subfamilies, they had less number of exons, less potential phosphorylation sites and had two pore domain potassium channel. It seems that the members of this subfamily have gone through a different evolutionary process. A low number of exons affects the expression of genes and can increase the speed of gene induction37,38. Gene duplication serves as the main pathway for enlarging gene families within an organism39,40. Whole-genome duplication, segmental, and tandem duplication are all forms of gene duplication that have been significant in the evolution of gene families. Between PvAKT family members, two segmental duplication events were predicted. This shows that most of the doubling was probably before the derivation of monocots and dicots. Both PvHKT members showed a tandem duplication. This duplication event may explain why these two genes exhibit such striking similarities in their physicochemical characteristics.
Analysis of promoter regions of PvAKT and PvHKT genes revealed that PvAKTs and PvHKT1 are highly related to light-induced factors involved in growth. These results establish that these transporters are related to upstream pathways involved in growth and development. Based on the presence of the ABA hormone response cis-regulatory element in the upstream region, PvAKT and PvHKT genes were divided into two groups. It seems that genes that lack ABA response elements are not in direct ABA-dependent signaling pathways. However, further studies are needed. The expression pattern of PvAKT and PvHKT genes showed that these genes are involved in the response to AMF. The transcription level of PvAKT3, PvAKT6 and both PvHKT genes increased in root tissues symbiosis with AMF. It has been reported that potassium transporters can be induced under symbiosis with AMF34,41. AKT transporters contribute to K+ uptake under different K+ concentration conditions and they are particularly active in roots and associated tissues42. It has been found that AKT1 is mainly expressed in Arabidopsis root epidermal cells43. PvAKT6 and PvAKT11 genes and both PvHKT genes showed differential expression in root and shoot tissues, which indicates that these genes have tissue specific expression. PvAKT3 gene increased expression in both root and shoot tissues, it seems that this transporter is more involved in K+ movement. K+ initially enters roots in plants, then moves to the aerial portions and disperses throughout cells into separate compartments7. Based on the results, it can be concluded that both AMF increased K+ solubility and thus increased the activity of K+ transporters.
Conclusion
In this study, the effect of symbiosis of arbuscular mycorrhiza fungi (AMF), Rhizophagus irregularis and Funneliformis mosseae with bean roots on the absorption of K+, and activity of K+ transporters was investigated. Symbiosis with AMF could increase K+ accumulation in bean tissues, especially stems, which shows the efficiency of AMF in increasing K+ solubility and absorption. The analysis of beans K+ transporters, PvAKT and PvHKT, demonstrated distinct structural variations among members of the PvAKT family likely caused by evolutionary pressures. PvAKT and PvHKT were divided into two groups based on the presence of cis-regulatory elements in response to ABA, indicating that some members of these transporters are not in the direct pathway of ABA signaling. The expression pattern of PvAKT and PvHKT genes indicated that these genes become more active under symbiosis with AMF. Probably, by increasing the solubility and availability of K+, the activity of these transporters is increased for more use K+ by the plant.
Data availability
The data generated or analyzed in this study are included in this article. Other materials that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AMF:
-
Arbuscular mycorrhiza fungi
- HKT:
-
High-affinity potassium transporters
- PvHKT:
-
Phaseolus vulgaris HKT
- PvAKT:
-
Phaseolus vulgaris AKT
- BLASTp:
-
BLAST protein
- CDS:
-
Coding DNA sequence
References
Anschütz, U., Becker, D. & Shabala, S. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 171 (9), 670–687 (2014).
Marschner, H. Marschner’s Mineral Nutrition of Higher Plants (Academic Press, 2011).
Wang, M., Zheng, Q., Shen, Q. & Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 14 (4), 7370–7390 (2013).
Hasanuzzaman, M. et al. Moumita, Fujita M: potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8 (3), 31 (2018).
Szczerba, M. W., Britto, D. T. & Kronzucker, H. J. K + transport in plants: physiology and molecular biology. J. Plant Physiol. 166 (5), 447–466 (2009).
Corratgé-Faillie, C. et al. Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell. Mol. Life Sci. 67 (15), 2511–2532 (2010).
Gierth, M. & Mäser, P. Potassium transporters in plants–involvement in K + acquisition, redistribution and homeostasis. FEBS Lett. 581 (12), 2348–2356 (2007).
Mäser, P. et al. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. 99 (9), 6428–6433 (2002).
Horie, T., Hauser, F. & Schroeder, J. I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 14 (12), 660–668 (2009).
Platten, J. D. et al. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci. 11 (8), 372–374 (2006).
Waters, S., Gilliham, M. & Hrmova, M. Plant high-affinity potassium (HKT) transporters involved in salinity tolerance: structural insights to probe differences in ion selectivity. Int. J. Mol. Sci. 14 (4), 7660–7680 (2013).
Amrutha, R. N., Sekhar, P. N., Varshney, R. K. & Kishor, P. K. Genome-wide analysis and identification of genes related to potassium transporter families in rice (Oryza sativa L). Plant Sci. 172 (4), 708–721 (2007).
Xu, J. et al. A protein kinase, interacting with two calcineurin B-like proteins, regulates K + transporter AKT1 in Arabidopsis. Cell 125 (7), 1347–1360 (2006).
Musavizadeh, Z. et al. Genome-wide analysis of potassium channel genes in rice: expression of the OsAKT and OsKAT genes under salt stress. Genes 12 (5), 784 (2021).
Hirsch, R. E., Lewis, B. D., Spalding, E. P. & Sussman, M. R. A role for the AKT1 potassium channel in plant nutrition. Science 280 (5365), 918–921 (1998).
Nieves-Cordones, M., Alemán, F., Martínez, V. & Rubio, F. K + uptake in plant roots. The systems involved, their regulation and parallels in other organisms. J. Plant Physiol. 171 (9), 688–695 (2014).
Garcia, K. & Zimmermann, S. D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 5, 337 (2014).
Lenoir, I., Fontaine, J. & Sahraoui, A. L. H. Arbuscular mycorrhizal fungal responses to abiotic stresses: a review. Phytochemistry 123, 4–15 (2016).
Smith, S. E. & Smith, F. A. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62 (1), 227–250 (2011).
Atul-Nayyar, A., Hamel, C., Hanson, K. & Germida, J. The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 19, 239–246 (2009).
Kakouridis, A. et al. Routes to roots: direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytol. 236 (1), 210–221 (2022).
Tang, H. et al. The critical role of arbuscular mycorrhizal Fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 13 (2022).
Razakatiana, A. T. E. et al. Benefits of dual inoculation with arbuscular mycorrhizal fungi and rhizobia on Phaseolus vulgaris planted in a low-fertility tropical soil. Pedobiologia 83, 150685 (2020).
Phillips, J. M. & Hayman, D. S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycological Soc. 55 (1), 158–IN118 (1970).
Bolser, D. M., Staines, D. M., Perry, E. & Kersey, P. J. Ensembl plants: integrating tools for visualizing, mining, and analyzing plant genomic data. Plant Genom. Databases Methods Protoc. 2017, 1–31.
Chen, C. et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 13 (8), 1194–1202 (2020).
Gasteiger, E. et al. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31 (13), 3784–3788 (2003).
Sievers, F. & Higgins, D. G. Clustal omega for making accurate alignments of many protein sequences. Protein Science: Publication Protein Soc. 27 (1), 135–145 (2018).
Nguyen, L-T., Schmidt, H. A., Von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32 (1), 268–274 (2015).
Blom, N., Gammeltoft, S. & Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294 (5), 1351–1362 (1999).
Lescot, M. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in Silico analysis of promoter sequences. Nucleic Acids Res. 30 (1), 325–327 (2002).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego Calif). 25 (4), 402–408 (2001).
Mulet, J. M., Porcel, R. & Yenush, L. Modulation of potassium transport to increase abiotic stress tolerance in plants. J. Exp. Bot. 74 (19), 5989–6005 (2023).
Liu, J. et al. The potassium transporter SlHAK10 is involved in mycorrhizal potassium uptake. Plant Physiol. 180 (1), 465–479 (2019).
Perner, H., Schwarz, D., Bruns, C., Mäder, P. & George, E. Effect of arbuscular mycorrhizal colonization and two levels of compost supply on nutrient uptake and flowering of pelargonium plants. Mycorrhiza 17, 469–474 (2007).
Baslam, M., Garmendia, I. & Goicoechea, N. The arbuscular mycorrhizal symbiosis can overcome reductions in yield and nutritional quality in greenhouse-lettuces cultivated at inappropriate growing seasons. Sci. Hort. 164, 145–154 (2013).
Hashemipetroudi, S. H., Arab, M., Heidari, P. & Kuhlmann, M. Genome-wide analysis of the laccase (LAC) gene family in Aeluropus Littoralis: A focus on identification, evolution and expression patterns in response to abiotic stresses and ABA treatment. Front. Plant. Sci. 14 (2023).
Yaghobi, M. & Heidari, P. Genome-Wide analysis of Aquaporin gene family in Triticum turgidum and its expression profile in response to salt stress. Genes 14 (1), 202 (2023).
Zeng, L. R., Park, C. H., Venu, R. C., Gough, J. & Wang, G. L. Classification, expression pattern, and E3 ligase activity assay of rice U-box-containing proteins. Mol. Plant. 1 (5), 800–815 (2008).
Liu, S. et al. Genome-wide analysis of OPR family genes in cotton identified a role for GhOPR9 in verticillium dahliae resistance. Genes 11 (10) (2020).
Cai, K., Zeng, F., Wang, J. & Zhang, G. Identification and characterization of HAK/KUP/KT potassium transporter gene family in barley and their expression under abiotic stress. BMC Genom. 22 (1), 317 (2021).
Rubio, F., Alemán, F., Nieves-Cordones, M. & Martínez, V. Studies on Arabidopsis athak5, atakt1 double mutants disclose the range of concentrations at which AtHAK5, AtAKT1 and unknown systems mediate K + uptake. Physiol. Plant. 139 (2), 220–228 (2010).
Lagarde, D. et al. Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K + nutrition. Plant J. 9 (2), 195–203 (1996).
Author information
Authors and Affiliations
Contributions
P.H. and H.R.A. conceived and design the study. Z.A. and P. H. organized and performed the experiments. Z.A., P.H. and H.R.A. were involved in data interpretation. : P.H. and H.R.A. wrote the manuscript. P.H. planned and supervised the study and edited the final version of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alizadeh, Z., Heidari, P. & Asghari, H.R. Exploring the influence of symbiosis between arbuscular mycorrhizal fungi and beans on potassium uptake and the activity of AKT and HKT genes. Sci Rep 15, 19169 (2025). https://doi.org/10.1038/s41598-025-04385-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04385-7