Abstract
Visual hallucinations (VH) are common in Parkinson’s disease (PD), yet their mechanisms remain poorly understood. Several studies have investigated structural brain correlates of Parkinsonian VH, but critical gaps in knowledge persist. An inverse relationship between auditory hallucinations and paracingulate sulcus (PCS) length, associated with reality-monitoring mechanisms, has been reported. This study examines the relationship between PCS length and VH in PD patients. Sixty-five PD patients (aged 48–81 years) meeting diagnostic criteria were included. The University of Miami Parkinson’s Disease Hallucinations Questionnaire was used to classify patients into PD with VH (PDVH, n = 32) or PD without VH (PDnonVH, n = 33) groups. PCS length was measured using sagittal T1-weighted MRI scans, and total intracranial volume was calculated. Clinical and neuropsychometric assessments were also performed. No significant demographic or clinical differences were found between groups. Total PCS length was significantly shorter in the PDVH group (68.56 ± 38.03 mm) compared to the PDnonVH group (106.36 ± 48.77 mm; p < .01). Right and left PCS lengths were also shorter in the PDVH group (p < .01). Visual immediate and long-term memory scores were significantly lower in the PDVH group (p < .01, p < .05, respectively), while Spatial Boundaries Subtest recognition scores were higher (p < .05). In the PDVH group, semantic fluency scores positively correlated with PCS length (p < .05). Reduced PCS length increased the likelihood of VH (β =−0.020, Odds Ratio = 0.980, p < .01). PCS length may serve as a biomarker indicative of anatomical structures associated with reality-monitoring mechanisms and biological predisposition to hallucinations in patients with PD.
Similar content being viewed by others
Introduction
Visual hallucinations (VH) are the most common type of hallucination observed in Parkinson’s disease (PD)1 Despite their prevalence, the causes and mechanisms of VH in PD remain poorly understood2,3,4,5 Initially, it was hypothesized that VH in PD was primarily linked to dopaminergic dysregulation6 However, subsequent studies employing structural, metabolic, and functional imaging techniques have identified cortical atrophy and dysfunction models, supporting the hypothesis that hallucinations arise from disease-related brain changes5 Nevertheless, the structural predispositions of the brain that might predict Parkinsonian VH remain largely unexplored.
Conversely, in schizophrenia, a significant inverse relationship has been demonstrated between auditory hallucinations and the length of the paracingulate sulcus (PCS), a structure located in the medial prefrontal cortex. This relationship has also been observed in VH associated with schizophrenia7.
The PCS is a sulcus running dorsal and parallel to the cingulate sulcus, located on the medial wall of the frontal lobes, dorsal to the corpus callosum8,9 First described by Elliot Smith in 1907, the PCS has gained clinical significance due to its association with reality-monitoring mechanisms and the specific sulcal morphologies implicated in psychiatric disorders10,11,12 Studies in this domain have linked the length of the PCS to its surrounding medial frontal and anterior cingulate structures, which are thought to underpin reality-monitoring mechanisms13,14 Reality monitoring refers to the ability to distinguish between internally generated and externally presented information, enabling individuals to differentiate between thoughts or imagined events and real-world occurrences13,15,16.
Buda et al. proposed that variability in PCS dimensions, particularly a larger prefrontal cortex (PFC) surface area, might correlate with individual differences in reality-monitoring abilities in healthy populations17 This finding suggests that specific structural variations in the PFC could underlie individual differences in introspective abilities, such as reality monitoring.
Impairments in reality monitoring have been attributed to the deficits observed in visual imagery and memory tasks among PD patients experiencing VH18 Collerton and colleagues proposed a model to explain the mechanisms of complex visual hallucinations, with most theories pointing directly19 or indirectly20,21,22 to dysfunctions in reality-monitoring mechanisms in Parkinsonian hallucinations23.
We hypothesize that the occurrence of VH in PD patients may be associated with shorter PCS length. Specifically, we propose that PCS length could serve as a neuroanatomical biomarker for biological predisposition to VH and its severity in PD. This study investigates the relationship between PCS length and VH in PD patients with and without hallucinations. Furthermore, we aim to explore the potential association between PCS length and neuropsychometric test scores, shedding light on the role of reality-monitoring dysfunction in the development of visual hallucinations.
Materials and methods
Study design and participants
This retrospective cross-sectional study utilized anonymized cranial magnetic resonance imaging (MRI) data acquired with a 3 T MRI scanner, along with relevant clinical information from the Functional Imaging Archive of Istanbul Medipol University. Data were collected from patients followed at the neurology clinic of Medipol Mega Hospital (Bağcılar, Istanbul). Ethical approval for the study was obtained from the Istanbul Medipol University Ethics Committee (Approval No. E6229477 × 8). As this study is retrospective in nature and involved the use of anonymized data, the requirement for informed consent was waived by the Institutional Review Board (IRB) in accordance with national regulations and the Declaration of Helsinki.
A total of 65 individuals aged between 48 and 81 years who met the diagnostic criteria for Parkinson’s disease (PD) were included in the study. The required sample size was determined a priori using G*Power 3.1, based on a large effect size assumption, with an alpha level of 0.05 and a desired power of 0.80. The diagnosis of PD was confirmed by an expert clinician (LH) according to the Movement Disorder Society’s diagnostic criteria24 Exclusion criteria included a history of any current or previous neurological disorders other than PD, psychiatric illness, head trauma, irreversible auditory or visual impairments, poorly controlled comorbidities (e.g., diabetes mellitus, hypertension), unstable medical treatment, the presence of pyramidal signs, cerebellar involvement, gaze palsy, or autonomic dysfunction.
To evaluate hallucinations, the University of Miami Parkinson’s Disease Hallucination Questionnaire (UM-PDHQ), a 20-item screening tool, was administered.²⁵ Although the UM-PDHQ assesses hallucinations across multiple sensory modalities, in the current study it was used to screen for the presence of visual hallucinations specifically. Only patients who reported visual hallucinations on the UM-PDHQ and whose symptoms were clinically confirmed were included in the PD with VH (PDVH) group (n = 32). Patients who did not report any hallucinations were classified into the PD without VH (PDnonVH) group (n = 33). Data analysis was conducted on the full sample of 65 participants (Fig. 1).
Clinical and neuropsychometric assessment
Clinical features of PD were assessed using the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS-III)25 and the Hoehn-Yahr scale to determine disease stage26 Total daily levodopa equivalent doses (LED) were calculated based on patients’ medication regimens27 Cognitive evaluation included the Standardized Mini-Mental State Examination (MMSE)28 for general cognition. Memory was assessed using the Oktem Verbal Memory Processes Test (VMPT)29 and the Visual Subtest of the Wechsler Memory Scale30Executive functions were evaluated using the Stroop Test31, the Clock Drawing Test32, and the Verbal Fluency Test33 For visuospatial functions, the Benton Facial Recognition Test (BFRT)34 was employed. Neuropsychometric tests were conducted by researchers blinded to group assignments (PDVH or PDnonVH).
Measurement of the PCS
The length of the PCS in the medial prefrontal cortex was measured manually using the Freesurfer software. The measurement protocol was based on Garrison et al., whose study established significant findings related to auditory hallucinations7 To ensure consistency, all measurements were performed by a single researcher, minimizing inter-rater variability. To account for individual differences in brain size, PCS length was normalized by intracranial volume (ICV), allowing for standardized comparisons across participants. This normalization was performed using the ratio of PCS length to ICV, following previously established methodologies. In addition, both intra-rater and inter-rater reliability were assessed using Intraclass Correlation Coefficient (ICC)s based on a two-way mixed-effects model with absolute agreement. Intra-rater reliability, evaluated in 18 participants, yielded perfect agreement. Inter-rater reliability, assessed in 22 participants by an independent rater, also demonstrated excellent consistency (ICCs ≥ 0.999).
MRI data were analyzed in Freesurfer’s (version 6.0.0) native space using the Multimodality Viewer to examine sagittal T1-weighted images. The anterior commissure (AC) and posterior commissure (PC) were identified and marked. The images were then aligned horizontally along the AC-PC plane, and the origin was set to the level of the anterior commissure.
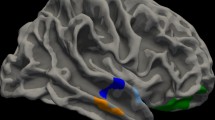
The cingulate sulcus, defined as the first major sulcus located dorsally to the corpus callosum, was identified. The paracingulate sulcus was identified as a distinct sulcus running parallel, horizontally, and dorsally to the cingulate sulcus (Fig. 2). Manual tracings were performed using the line-polyline option to measure PCS length. All measurements were conducted by the same researcher to ensure consistency, and intracranial volume comparisons were used for standardization following Garrison et al.7.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY). The primary aim of the analysis was to evaluate whether the length of the PCS could predict the presence of VH in PD patients. This was assessed using a binary logistic regression model. Additionally, preliminary analyses were conducted to examine group differences and variable relationships to support the main hypothesis.
Descriptive statistics were calculated to summarize the demographic and clinical characteristics of the participants. To assess the normality of continuous variables, skewness and kurtosis values were examined, and histogram plots were visually inspected. Independent samples t-tests were used to compare continuous variables, such as PCS lengths, between the PDVH (PD with VH) and PDnonVH (PD without VH) groups. To further investigate PCS asymmetry and potential lateralization effects, a two-way mixed analysis of variance (ANOVA) was conducted with laterality (left vs. right PCS length) as the within-subjects factor and group (PDVH vs. PDnonVH) as the between-subjects factor. For categorical variables, such as gender distribution and disease stage, Chi-square (χ²) tests were conducted to assess group associations.
To evaluate the reliability of manual PCS measurements, both intra-rater and inter-rater reliability analyses were performed using the ICC, calculated with a two-way mixed-effects model assuming absolute agreement.
The relationship between PCS lengths and neuropsychometric test scores was explored using Pearson’s correlation analysis to identify potential linear associations. This analysis aimed to determine whether variations in PCS length were related to differences in cognitive performance.
To test the main hypothesis, a binary logistic regression model was employed to evaluate whether PCS length could significantly predict the presence of visual hallucinations. Visual hallucination status (presence vs. absence) was entered as the binary dependent variable, and PCS length was included as the independent variable. The enter method was used in the regression model to ensure a comprehensive assessment of the independent variable’s impact. A p-value of < 0.05 was considered statistically significant for all analyses.
Results
The PDVH and PDnonVH groups did not differ significantly in terms of age, gender, education, global cognition, motor impairment severity, levodopa equivalent doses, or intracranial volumes measured by MRI volumetry. The only statistically significant difference was observed in the UM-PDHQ scores, which reflect the hallucination experience parameter (p <.001) (Table 1).
When the total, right, and left PCS lengths were evaluated, the PDVH group exhibited significantly shorter mean lengths for total PCS, right PCS, and left PCS compared to the PDnonVH group (p <.01, p <.01, p <.001, respectively) (Table 2; Fig. 3).
To investigate PCS asymmetry (left vs. right) and its potential lateralization across groups, a two-way ANOVA was conducted with laterality (left vs. right PCS length) as the within-subjects factor and group (PDVH vs. PDnonVH) as the between-subjects factor. The main effect of laterality was not statistically significant (F(1, 39) = 2.770, p =.104, \(\eta_\text{p}^2\) = 0.066), indicating no overall difference between left and right PCS lengths across all participants. The interaction between laterality and group was also not significant (F(1, 39) = 0.028, p =.867, \(\eta_\text{p}^2\) = 0.001), suggesting that PCS asymmetry did not differ between the PDVH and PDnonVH groups. However, a significant main effect of group was observed (F(1, 39) = 16.546, p <.001, \(\eta_\text{p}^2\) = 0.298), with the PDnonVH group exhibiting greater overall PCS length compared to the PDVH group (95% CI [11.63, 34.64], p <.001, Bonferroni-corrected).
When the neuropsychometric subtest performance of the PDVH and PDnonVH groups was compared, the PDVH group demonstrated significantly poorer scores on the WMS Visual Immediate Memory (p <.05) and the Visual Delayed Memory (p <.05) Conversely, the VMPT recognition scores of the PDVH group were significantly higher than those of the PDnonVH group (p <.05) (Table 3).
Additionally, according to the correlation analysis between PCS lengths and neuropsychometric variables in the PDVH group, semantic fluency showed a moderate positive correlation with right PCS length (r =.503, p <.05), a weak positive correlation with left PCS length (r =.442, p <.05), and a strong positive correlation with total PCS length (r =.506, p <.05). These findings suggest that longer PCS lengths, particularly in the right hemisphere and overall, are associated with better semantic fluency performance. However, no significant correlations were observed between PCS lengths and neuropsychometric variables in the PDnonVH group.
Furthermore, across the entire sample, significant negative correlations were observed between PCS lengths and hallucinatory burden, as measured by the total score of the UM-PDHQ. Specifically, UM-PDHQ scores were negatively correlated with right PCS length (r = –.483, p <.01), left PCS length (r = –.472, p <.01), and total PCS length (r = –.393, p =.001). These results indicate that shorter PCS length is associated with greater hallucinatory burden in PD.
Binary logistic regression analysis was conducted to examine the effect of PCS length (mm) on the presence of visual hallucinations (PDVH vs. PDnonVH). The overall model was statistically significant (χ²(1) = 11.238, p =.001) and demonstrated a good fit to the data, as indicated by the Hosmer-Lemeshow goodness-of-fit test (χ²(7) = 4.015, p =.778). PCS length was a significant predictor of visual hallucinations (β = −0.020, SE = 0.007, Wald = 9.072, p =.003, OR = 0.980, 95% CI [0.968, 0.993]). For each unit decrease in PCS length, the odds of experiencing visual hallucinations increased by a factor of 1.020, corresponding to a 2% increase in odds. The model explained 21.2% of the variance in the presence of visual hallucinations (Nagelkerke R² = 0.212) and correctly classified 67.7% of cases.
Discussion
Our study revealed that the total, right, and left PCS lengths were significantly shorter in the PD with VH (PDVH) group compared to the PD without VH (PDnonVH) group. Neuropsychometric tests showed that the PDVH group performed significantly worse on tests of visual immediate memory and visual long-term memory, while their recognition scores on the Verbal Memory Processes Test were significantly higher than those of the PDnonVH group. Correlation analysis demonstrated a significant positive relationship between semantic fluency scores and total PCS length. Furthermore, binary logistic regression analysis indicated that each unit reduction in PCS length was associated with a 0.980-fold increase in the likelihood of experiencing visual hallucinations.
Models of cortical atrophy and dysfunction have been proposed to explain the emergence of parkinsonian visual hallucinations. Within this framework, the hypothesis that hallucinations result from the progression of disease-related brain changes in combination with the use of dopaminergic medications remains current5 However, the aim and emphasis of our study is to investigate a potential biological predisposition that may underlie, or even precede, the findings reported in these previously established studies.
A remarkable feature of human cognition and behavior is the notable variability among individuals, which is intriguingly linked to differences in brain structure35 Developmental disorders, such as neuropsychiatric and neurodevelopmental conditions, as well as specific learning disorders, have been associated with brain asymmetries36 A recent study even identified a strong correlation between the morphology of the intraparietal sulcus and performance on memory and language tasks37.
Recent evidence has increasingly linked the PCS to reality-monitoring processes, not only in pathological conditions like schizophrenia but also in the general population. Reality-monitoring, broadly defined, refers to the ability to distinguish between real and imagined information. This process has been shown to be impaired in patients with schizophrenia who experience hallucinations7,17,38 The main finding of our study that the PCS is significantly shorter in PD patients with hallucinations represents, to our knowledge, the first report of its kind. Our results align with findings reported in patients with schizophrenia experiencing auditory hallucinations7,14,39 The question of how cognitive abilities are influenced by underlying biological parameters or structural variability in pathological contexts is, in our view, a fascinating area of inquiry. Examining Parkinsonian hallucinations from this perspective offers a promising avenue for predicting clinical outcomes.
In studies examining normal populations, PCS variations and their associations with cognitive functions have been a topic of interest. Wei et al. demonstrated that the PCS is lateralized to the left hemisphere in individuals from various racial backgrounds, with longer and more pronounced PCS morphology observed in the left hemisphere9 This asymmetry was found to be independent of racial differences. In our study, a similar trend was observed, with the left PCS length being greater than the right PCS length in both PDVH and PDnonVH groups. However, a two-way mixed ANOVA revealed that this asymmetry was not statistically significant, nor did the pattern of asymmetry differ between the PDVH and PDnonVH groups. This suggests that PCS asymmetry is unlikely to play a significant role in the predisposition to visual hallucinations in PD, although the observed trend may warrant further investigation in larger samples.
Early studies by Fornito et al. suggested that PCS length correlates with structures forming its superior and inferior boundaries, such as the superior frontal gyrus and the cingulate gyrus40 They reported that the presence of the PCS is associated with an approximately 88% increase in paralimbic volume and a 39% decrease in anterior cingulate cortex (ACC) volume. Thus, morphological findings related to the PCS may be linked to functions associated with the inferior frontal and cingulate gyri40 More recent studies have further shown that the presence of the PCS in healthy adults is associated with distinct structural and functional connectivity characteristics41.
In the literature, variations in PCS morphology, including asymmetry, have been associated with individual differences in cognitive abilities40,42 For instance, Fornito and colleagues found that leftward PCS asymmetry was associated with better performance in verbal and nonverbal executive functions42 Similarly, PCS presence was linked to increased paracingulate cortical volume and decreased anterior cingulate cortical gray matter volume in the same hemisphere. Leftward asymmetry was also associated with superior spatial working memory performance43 In pathological contexts, Park et al. demonstrated reduced leftward PCS asymmetry in individuals with genetic risk for psychosis and in patients with schizophrenia, even in the absence of psychotic symptoms. This reduction in asymmetry was associated with poorer working memory performance44.
In our study, we identified a significant positive correlation between PCS length and semantic fluency scores, reflecting executive function performance. While it may not be entirely appropriate to compare neurodegenerative conditions like Parkinson’s disease with populations at high genetic risk for schizophrenia, this finding may indicate an underlying biological predisposition in PD.
The “source monitoring framework,” which includes reality-monitoring, suggests that distinguishing the origins of information whether generated internally by cognitive functions (e.g., thought, imagination) or derived externally through sensory perception involves complex decision-making processes45 Determining whether a sensory experience originates externally or internally is prone to error in both healthy and pathological conditions.
Buda et al. investigated the relationship between PCS and reality-monitoring processes by categorizing healthy participants into four groups based on the presence or absence of the PCS in the left or right hemisphere17 Participants lacking the PCS in both hemispheres exhibited significant impairments in reality-monitoring performance and introspective metacognitive accuracy compared to other groups. Using voxel-based morphometry, the study also reported a significant negative correlation between anterior medial prefrontal cortex (amPFC) gray matter volume and reality-monitoring performance. These findings suggest a distinct neuroanatomical basis for individual differences in reality-monitoring ability, potentially mediated by structural variability in the PFC17.
Similar evidence has emerged from schizophrenia research, where PCS and amPFC are implicated in perceptual reality monitoring. PCS morphology has been shown to differentiate schizophrenia patients from healthy controls40 Schizophrenia patients with hallucinations, regardless of modality (e.g., auditory, visual, tactile), exhibit shorter PCS lengths compared to those without hallucinations7 Additionally, Garrison et al. demonstrated reduced activation of the left amPFC, associated with the PCS, during a reality-monitoring task in schizophrenia patients16 Perret et al. further reported that impaired reality-monitoring abilities in schizophrenia patients with auditory hallucinations were significantly associated with reduced right PCS length and decreased gray matter volume in the temporal and parietal regions of the reality-monitoring network13 Powers et al. extended these findings to nonclinical populations, showing reduced right PCS length in participants experiencing auditory hallucinations compared to those without hallucinations46 These findings collectively support a growing body of evidence linking the PCS to hallucinations and reality-monitoring processes, particularly in schizophrenia.
The neuroscience literature provides strong support for the association between the morphological characteristics of the PCS and deficits in reality-monitoring. A recent meta-analysis by Lavallé et al. demonstrated a significant relationship between reduced PCS length and impaired reality-monitoring47 This association appears to be grounded in evidence from functional neuroimaging studies, particularly regarding the role of the medial prefrontal cortex (mPFC), of which the PCS is a structural component. The mPFC plays a critical role in the metacognitive evaluation of sensory signals, thereby contributing not only to perceptual and cognitive control aspects of reality-monitoring but also to self-referential processing an essential mechanism for accurate source attribution. Findings from neuroimaging studies have consistently highlighted the role of the mPFC, especially its anterior region (amPFC), in distinguishing whether a given signal originates from internal imagination or external perception45 This supports the hypothesis that the amPFC serves a higher-order function in evaluating low-level sensory inputs as well as the cognitive control aspects of both perception and imagination for the purpose of accurate source monitoring38 As illustrated, neuroimaging findings suggest a compelling link between reality-monitoring function and PCS morphology, particularly in terms of PCS length as a structural marker.
Impairments in reality-monitoring have also been proposed as a mechanism underlying hallucinations in PD18,23,5,48 Studies investigating cognitive patterns associated with Parkinsonian visual hallucinations frequently report deficits in visuospatial functions, memory, and frontal functions, all of which may relate to reality-monitoring impairments18,49,50 Consistent with these findings, our previous research suggested that visual-perceptual impairments may not be the primary source of hallucinations in PD, but rather deficits in reality-monitoring18 Similarly, our current study demonstrated that PDVH patients scored significantly lower on visual memory tests. This pattern, coupled with compensatory differences in verbal memory recognition, may reflect frontal dysfunction in PD51.
In addition to the role of the PCS and mPFC, reality-monitoring has been associated with a broader neural network. Metzak et al. demonstrated that anticorrelation between the mPFC and the default mode network (DMN) decreases during reality-monitoring tasks, resulting in increased DMN activity52 This supports earlier findings suggesting that the mPFC plays a key role in self-referential thought during reality-monitoring. Perret et al. further proposed that reality-monitoring involves a broad brain network, including the medial temporal, temporoparietal, and parietal cortices, alongside the PFC13.
There is strong alignment between this framework and the network disruptions observed in PD hallucinations. From the earliest studies, functional MRI data have suggested that Parkinsonian hallucinations result from mechanisms such as reduced bottom-up sensory input, impairments in attentional networks, and an overemphasis on top-down signals22,53 Consequently, functional neuroimaging studies indicate that the features of attention networks associated with reality-monitoring are implicated in PD visual hallucinations. These studies suggest that PD hallucinations are associated with a failure in top-down attentional processing, abnormal coupling between the DMN and visual networks, and disrupted connectivity between the thalamus and posterior brain regions. This disruption leads to abnormal disinhibition of the DMN, which is known to be involved in self-referential thought, mental imagery, and episodic memory retrieval5,54,55.
At this point, we return to the question raised at the beginning of the discussion: What is the nature of the relationship between genetically determined PCS length and the emergence of visual hallucinations observed in neurodegenerative diseases such as PD? Could PCS length serve as a neurobiological predisposition factor? Clearly, many more studies are needed to answer this question. Some authors have noted that hallucinations observed in Parkinson’s disease appear to follow a continuum, emerging even in the earliest stages of the disease. This continuum ranges from vivid dreams and minor hallucinations to fully formed visual hallucinations and delusions5 Additionally, the hypothesis that a higher-level control mechanism operates through the amPFC involved in distinguishing between perceived and imagined stimuli has been suggested as relevant to visual hallucinations in Parkinson’s disease38 This mechanism could explain the graded emergence of hallucinations, beginning with vivid dreams and REM sleep behavior disorder and progressing to elementary hallucinations, illusions, formed hallucinations, and eventually delusions. In this context, we propose that the emergence of Parkinsonian visual hallucinations may be linked to an inherent weakness in reality-monitoring, potentially rooted in PCS length. This predisposition may contribute to an increasingly insufficient reality-monitoring system as the disease progresses.
In our study, several limitations should be noted, including the relatively small sample size, the absence of a healthy control group, and the lack of volumetric and functional network analyses of PCS and reality-monitoring related structures. These limitations hinder our ability to fully interpret the findings within this discussion. However, it is important to highlight the significance of the observed PCS differences among patient groups and the fact that our study represents the first report on this topic.
Conclusion
In conclusion, our study found that PCS length was shorter in Parkinson’s patients with visual hallucinations compared to those without hallucinations. These findings are consistent with previous research examining the relationship between hallucinations, PCS, and reality-monitoring. As the first study conducted with Parkinson’s patients in this context, our results are significant. The key contribution of our study is the suggestion that PCS length may represent a candidate biomarker for Parkinsonian visual hallucinations, similar to its role in reality-monitoring mechanisms observed in schizophrenic auditory hallucinations. PCS length may provide insights into the anatomical structures involved in reality-monitoring and indicate a biological predisposition to hallucinations.
This study underscores the importance of further research into biological predispositions and highlights the need to advance our understanding of the complex nature of hallucinations. Emerging perspectives on predispositions, such as pareidolia and aphantasia, and their investigation through potential anatomical and biological markers, could contribute to our understanding of how internally and externally constructed images are perceived as reality in the human mind. Future research is needed to evaluate the potential of PCS as a biomarker for hallucinations in greater detail.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Frei, K. & Truong, D. D. Hallucinations and the spectrum of psychosis in Parkinson’s disease. J. Neurol. Sci. 374, 56–62. https://doi.org/10.1016/J.JNS.2017.01.014 (2017).
Fénelon, G., Mahieux, F., Huon, R. & Ziégler, M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 123 (Pt 4), 733–745. https://doi.org/10.1093/BRAIN/123.4.733 (2000).
Aarsland, D. et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J. Neurol. Neurosurg. Psychiatry. 78 (1), 36–42. https://doi.org/10.1136/JNNP.2005.083113 (2007).
Powell, A., Ireland, C. & Lewis, S. J. G. Visual hallucinations and the role of medications in Parkinson’s disease: triggers, pathophysiology, and management. J. Neuropsychiatry Clin. Neurosci. 32 (4), 334–343. https://doi.org/10.1176/APPI.NEUROPSYCH.19110316 (2020).
Pagonabarraga, J., Bejr-Kasem, H., Martinez-Horta, S. & Kulisevsky, J. Parkinson disease psychosis: from phenomenology to Neurobiological mechanisms. Nat. Rev. Neurol. 20 (3), 135–150. https://doi.org/10.1038/S41582-023-00918-8 (2024).
Banerjee, A. K., Falkai, P. G. & Savidge, M. Visual hallucinations in the elderly associated with the use of Levodopa. Postgrad. Med. J. 65 (764), 358–361. https://doi.org/10.1136/PGMJ.65.764.358 (1989).
Garrison, J. R. et al. Paracingulate sulcus morphology is associated with hallucinations in the human brain. Nat. Commun. 6 https://doi.org/10.1038/NCOMMS9956 (2015).
Le Provost, J. B. et al. Paracingulate sulcus morphology in men with early-onset schizophrenia. Br. J. Psychiatry. 182 (MAR.), 228–232. https://doi.org/10.1192/BJP.182.3.228 (2003).
Wei, X. et al. Paracingulate sulcus asymmetry in the human brain: effects of sex, handedness, and race. Sci. Rep. 7 https://doi.org/10.1038/SREP42033 (2017).
SmithGE A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J. Anat. Physiol. 41 (Pt 4), 237 (1907). https://pmc.ncbi.nlm.nih.gov/articles/PMC1289123/ Accessed December 19, 2024.
Allen, P., Larøi, F., McGuire, P. K. & Aleman, A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav Rev. 32 (1), 175–191. https://doi.org/10.1016/J.NEUBIOREV.2007.07.012 (2008).
Loh, K. K., Petrides, M., Hopkins, W. D., Procyk, E. & Amiez, C. Cognitive control of vocalizations in the primate ventrolateral-dorsomedial frontal (VLF-DMF) brain network. Neurosci. Biobehav Rev. 82, 32–44. https://doi.org/10.1016/J.NEUBIOREV.2016.12.001 (2017).
Perret, M., Neige, C., Brunelin, J. & Mondino, M. Unraveling the brain mechanisms of source monitoring with non-invasive brain stimulation: A systematic review. Int. J. Clin. Health Psychol. 24 (2). https://doi.org/10.1016/J.IJCHP.2024.100449 (2024).
Curcic-Blak, B. et al. Paracingulate sulcus length and cortical thickness in schizophrenia patients with and without a lifetime history of auditory hallucinations. Schizophr Bull. 49 (12 Suppl 2), S48–S57. https://doi.org/10.1093/SCHBUL/SBAC072 (2023).
MK, J. Source monitoring. Psychol. Bull. 114 (1), 1063–1065. https://doi.org/10.1037/0033-2909.114.1.3 (1993).
Garrison, J. R., Fernandez-Egea, E., Zaman, R., Agius, M. & Simons, J. S. Reality monitoring impairment in schizophrenia reflects specific prefrontal cortex dysfunction. Neuroimage Clin. 14, 260–268. https://doi.org/10.1016/J.NICL.2017.01.028 (2017).
Buda, M., Fornito, A., Bergström, Z. M. & Simons, J. S. A specific brain structural basis for individual differences in reality monitoring. J. Neurosci. 31 (40), 14308–14313. https://doi.org/10.1523/JNEUROSCI.3595-11.2011 (2011).
Ozer, F. et al. Cognitive impairment patterns in Parkinson’s disease with visual hallucinations. J. Clin. Neurosci. 14 (8), 742–746. https://doi.org/10.1016/J.JOCN.2006.05.006 (2007).
Barnes, J., Boubert, L., Harris, J., Lee, A. & David, A. S. Reality monitoring and visual hallucinations in Parkinson’s disease. Neuropsychologia 41 (5), 565–574. https://doi.org/10.1016/S0028-3932(02)00182-3 (2003).
Collerton, D., Perry, E. & McKeith, I. Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 28 (6), 737–757. https://doi.org/10.1017/S0140525X05000130 (2005).
Diederich, N. J., Goetz, C. G. & Stebbins, G. T. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov. Disord. 20 (2), 130–140. https://doi.org/10.1002/MDS.20308 (2005).
Shine, J. M. et al. Imagine that: elevated sensory strength of mental imagery in individuals with Parkinson’s disease and visual hallucinations. Proc. Biol. Sci. 282 (1798). https://doi.org/10.1098/RSPB.2014.2047 (2015).
Collerton, D. et al. Understanding visual hallucinations: A new synthesis. Neurosci. Biobehav Rev. 150 https://doi.org/10.1016/J.NEUBIOREV.2023.105208 (2023).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30 (12), 1591–1601. https://doi.org/10.1002/MDS.26424 (2015).
Goetz, C. G. et al. Movement disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23 (15), 2129–2170. https://doi.org/10.1002/MDS.22340 (2008).
Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression and mortality. Neurology 17 (5), 427–442. https://doi.org/10.1212/WNL.17.5.427 (1967).
Tomlinson, C. L. et al. Systematic review of Levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25 (15), 2649–2653. https://doi.org/10.1002/MDS.23429 (2010).
Güngen, C., Ertan, T., Eker, E., Yaşar, R. & Engin, F. [Reliability and validity of the standardized Mini Mental State Examination in the diagnosis of mild dementia in Turkish population] - PubMed. Turk Psikiyatri Derg. ;13(4):273–281. Accessed July 12, 2022. (2002). https://pubmed.ncbi.nlm.nih.gov/12794644/
Oktem, O. A verbal test of memory processes: a preliminary study. Arch. Neuropsychatry. 29, 196–206 (1992).
Wechsler David. WMS-R: Wechsler Memory Scale–Revised : manual. Psychological Corporation. Published online 1987:150. Accessed December 19, 2024. https://books.google.com/books/about/WMS_R.html?id=Q2RIPwAACAAJ
Karakaş S, Erdoğan E, Soysal Ş, Ulusoy T, Yüceyurt Ulusoy İ, Alkan S. Stroop Test TBAG Form: Standardisation for Turkish Culture, Reliability and Validity. Turkısh Journal Clınıcal Psychıatry. 2(2):75–88 (1999).
Brodaty, H. & Moore, C. M. The clock drawing test for dementia of the Alzheimer’s type: A comparison of three scoring methods in a memory disorders clinic. Nt J. Geriatr. Psychiatry. 12 (6), 619–627. https://doi.org/10.1002/(SICI)1099-1166(199706)12:6 (1997).
Crawford, J. R., Moore, J. W. & Cameron, I. M. Verbal fluency: a NART-based equation for the Estimation of premorbid performance. Br. J. Clin. Psychol. 31 (3), 327–329. https://doi.org/10.1111/J.2044-8260.1992.TB00999.X (1992).
Benton, A. Facial recognition 1990. Cortex 26 (4), 491–499. https://doi.org/10.1016/S0010-9452(13)80299-7 (1990).
Genon, S., Eickhoff, S. B. & Kharabian, S. Linking interindividual variability in brain structure to behaviour. Nat. Rev. Neurosci. 23 (5), 307–318. https://doi.org/10.1038/S41583-022-00584-7 (2022).
Ocklenburg, S. & Guo, Z. V. Cross-hemispheric communication: insights on lateralized brain functions. Neuron 112 (8), 1222–1234. https://doi.org/10.1016/J.NEURON.2024.02.010 (2024).
Santacroce, F. et al. Human intraparietal sulcal morphology relates to individual differences in Language and memory performance. Commun. Biol. 7 (1). https://doi.org/10.1038/S42003-024-06175-9 (2024).
Dijkstra, N., Kok, P. & Fleming, S. M. Perceptual reality monitoring: neural mechanisms dissociating imagination from reality. Neurosci. Biobehav Rev. 135 https://doi.org/10.1016/J.NEUBIOREV.2022.104557 (2022).
Garrison, J. R., Fernyhough, C., McCarthy-Jones, S., Simons, J. S. & Sommer, I. E. C. Paracingulate sulcus morphology and hallucinations in clinical and nonclinical groups. Schizophr Bull. 45 (4), 733–741. https://doi.org/10.1093/SCHBUL/SBY157 (2019).
Fornito, A. et al. Morphology of the paracingulate sulcus and executive cognition in schizophrenia. Schizophr Res. 88 (1–3), 192–197. https://doi.org/10.1016/J.SCHRES.2006.06.034 (2006).
Harper, L. et al. Structural and functional connectivity associations with anterior cingulate sulcal variability. Brain Struct. Funct. 229 (7), 1561–1576. https://doi.org/10.1007/S00429-024-02812-5 (2024).
Fornito, A. et al. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb. Cortex. 14 (4), 424–431. https://doi.org/10.1093/CERCOR/BHH004 (2004).
Fornito, A. et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum. Brain Mapp. 29 (2), 222–236. https://doi.org/10.1002/HBM.20381 (2008).
Park, H. Y. et al. Altered asymmetry of the anterior cingulate cortex in subjects at genetic high risk for psychosis. Schizophr Res. 150 (2–3), 512–518. https://doi.org/10.1016/J.SCHRES.2013.08.027 (2013).
Simons, J. S., Garrison, J. R. & Johnson, M. K. Brain mechanisms of reality monitoring. Trends Cogn. Sci. 21 (6), 462–473. https://doi.org/10.1016/J.TICS.2017.03.012 (2017).
Powers, A. R., Van Dyck, L. I., Garrison, J. R. & Corlett, P. R. Paracingulate sulcus length is shorter in Voice-Hearers regardless of need for care. Schizophr Bull. 46 (6), 1520–1523. https://doi.org/10.1093/SCHBUL/SBAA067 (2020).
Lavallé, L., Brunelin, J., Jardri, R., Haesebaert, F. & Mondino, M. The neural signature of reality-monitoring: A meta-analysis of functional neuroimaging studies. Hum. Brain Mapp. 44 (11), 4372–4389. https://doi.org/10.1002/HBM.26387 (2023).
Muller, A. J., Shine, J. M., Halliday, G. M. & Lewis, S. J. G. Visual hallucinations in Parkinson’s disease: theoretical models. Mov. Disord. 29 (13), 1591–1598. https://doi.org/10.1002/MDS.26004 (2014).
Pezzoli, S. et al. Neuroanatomical and cognitive correlates of visual hallucinations in Parkinson’s disease and dementia with lewy bodies: Voxel-based morphometry and neuropsychological meta-analysis. Neurosci. Biobehav Rev. 128, 367–382. https://doi.org/10.1016/J.NEUBIOREV.2021.06.030 (2021).
Montagnese, M. et al. A review of multimodal hallucinations: categorization, assessment, theoretical perspectives, and clinical recommendations. Schizophr Bull. 47 (1), 237–248. https://doi.org/10.1093/SCHBUL/SBAA101 (2021).
Hanoğlu, L. et al. Relation between olfactory dysfunction and episodic verbal memory in early Parkinson’s disease. Noro Psikiyatr Ars. 51 (4), 389–394. https://doi.org/10.5152/NPA.2014.7353 (2014).
Metzak, P. D., Lavigne, K. M. & Woodward, T. S. Functional brain networks involved in reality monitoring. Neuropsychologia 75, 50–60. https://doi.org/10.1016/J.NEUROPSYCHOLOGIA.2015.05.014 (2015).
Lefebvre, S. et al. Hallucinations and conscious access to visual inputs in Parkinson’s disease. Sci. Rep. 6 https://doi.org/10.1038/SREP36284 (2016).
Bhome, R., Thomas, G. E. C., Zarkali, A. & Weil, R. S. Structural and functional imaging correlates of visual hallucinations in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 23 (6), 287–299. https://doi.org/10.1007/S11910-023-01267-1 (2023).
Thomas, G. E. C. et al. Changes in both top-down and bottom-up effective connectivity drive visual hallucinations in Parkinson’s disease. Brain Commun. 5 (1). https://doi.org/10.1093/BRAINCOMMS/FCAC329 (2022).
Acknowledgements
This article is based on the master’s thesis of the first author.
Author information
Authors and Affiliations
Contributions
B.K., Z.Y., and L.H. contributed to the conception and design of the study. G.E., H.A.V., A.B.S., and B.U.S. were responsible for data acquisition and analysis. All authors participated in drafting and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Karagoz, B., Temel, Z., Ertan, G. et al. The relationship between paracingulate sulcus length and visual hallucinations in Parkinson’s disease suggests a neurobiological predisposition. Sci Rep 15, 23123 (2025). https://doi.org/10.1038/s41598-025-04513-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04513-3