Abstract
Cadmium (Cd) and antimony (Sb) coexistence in industrial effluents poses significant threats to environmental safety and human health. Consequently, developing effective methods for the simultaneous removal of Cd(II) and Sb(V) from aqueous solutions is critically important. In this study, the adsorption performance of a birnessite (BS) and fulvic acid (FA) composite (BS-FA) for the simultaneous removal of Cd(II) and Sb(V) was optimized using response surface methodology (RSM) in combination with machine learning (ML) techniques, including the genetic algorithm-back propagation neural network (GABP) and random forest (RF) models. The RF model demonstrated superior predictive accuracy (R² = 0.8037, RMSE = 0.0625) compared to the RSM and GABP models. Under the optimized conditions (pH = 6, adsorbent dosage = 0.87 g L− 1, adsorption time = 4 h, ionic strength = 0.01 mol L⁻¹, initial concentration = 25.5 mg L⁻¹), the removal efficiencies of Cd(II) and Sb(V) were 96.9% and 70.2%, respectively. Microscopic and mechanistic analyses revealed that Cd(II) and Sb(V) interacted with the Mn–O bonds in BS and the oxygen-containing functional groups (C–OH and –COOH) in FA, forming stable complexes within the Cd-Sb coexistence system. This study successfully integrates ML models and RSM to optimize and predict the adsorption process, offering valuable insights for mitigating the environmental and health risks associated with Cd and Sb contamination in water treatment.
Similar content being viewed by others
Introduction
Cadmium (Cd) and antimony (Sb) are classified as priority-controlled pollutants due to their inherent toxicity and bioaccumulation potential, posing significant risks to ecological systems and human health1. China was the world’s largest reserve and producer of Sb and Cd; they were mainly mined and refined for the industry of textile catalysts, flame retardants, electronics, and brake lining2. Wastewater discharge from textile printing and mining industries has led to the frequent coexistence of Cd and Sb in wastewater effluents3,4. Efficient removal of these metals from aqueous systems is crucial for mitigating ecological risks and ensuring water safety.
Manganese oxide minerals (MnOs) are highly effective adsorbents and immobilizing agents for Cd4. They reduce Cd solubility, mobility, and bioavailability through ion exchange, complexation with Mn–O bonds, and the formation of carbonate precipitates5,6. Furthermore, MnOs enhance Cd sequestration by maintaining a mildly alkaline pH and delaying reductions in sediment redox potential5. However, the aggregation of MnO particles and changes in crystallinity significantly hinder their adsorption capacity and long-term stability6. Fortunately, fulvic acid (FA), a ubiquitous component of natural organic matter (OM), can adsorb onto MnOs and enhance their dispersion and stability in aqueous media primarily via electrostatic repulsions6. Compared to pure MnO aggregates, dispersed MnOs exhibit a larger specific surface area (SSA) and more available Cd(II) adsorption sites, resulting in higher binding stability and reduced susceptibility to extraction by low-molecular-weight organic acids. This combination exposes numerous binding sites within MnO surfaces, inter-flakes, and microchannels7enabling metal cations (e.g., Cd) and anions (e.g., Sb) to form complexes with the MnO-FA composite. Despite these advancements, a quantitative understanding of the effectiveness of MnO-FA composites in removing multiple heavy metals (HMs), particularly Cd and Sb, which are commonly associated with Mn minerals in aqueous systems, remains limited. Furthermore, the binding mechanisms of coexisting Cd(II) and Sb(V) on MnO-FA composites are unclear.
Numerous factors influence the adsorption performance, including adsorbent dosage, contact time, temperature, pH, and background ion concentration8. Typically, experimental conditions are optimized through single-factor (SF) experiments. However, when multiple variables are involved, SF experiments become time-consuming and often fail to identify optimal conditions9. Moreover, the adsorption capacity is often influenced by the simultaneous action of multiple factors, with complex interrelationships among them10. As a result, traditional SF analyses are insufficient for capturing the intricate relationships between variables and responses. To address the limitations of conventional adsorption models, response surface methodology (RSM) and machine learning (ML) have gained increasing attention11. RSM uses designed experiments to generate response data and constructs polynomial models that quantify the relationship between variables and their effects10. As a powerful statistical tool for optimization, RSM has found widespread application in modeling Cd adsorption by biochar12, chitosan biopolymer13, and microorganisms14. These studies have demonstrated that second-order relationships are effective in approximating the complex behavior of Cd adsorption systems. However, existing methodologies have limitations, underscoring the necessity for more advanced and interpretable data-driven predictive models. ML techniques have emerged as robust tools for multivariate modeling, adept at capturing intricate nonlinear interactions15. These models offer a deeper understanding of how individual factors contribute to and interact within the overall adsorption process, thus enriching our comprehension of complex phenomena16. Various ML algorithms, including support vector regression, linear regression, random forest (RF), and artificial neural networks (ANN), have been applied in studies of pollutant adsorption15,17,18. Among them, ANN is the most widely used, though selecting the most suitable model remains a challenge. The integration of ML with RSM represents a novel approach that not only refines parameter optimization but also enables a more comprehensive exploration of adsorption mechanisms8,10. This hybrid strategy enhances predictive reliability and provides a flexible framework for mapping complex input-output relationships in adsorption processes. More importantly, it offers a innovative advancement for environmental applications, particularly in water purification by removing contaminants such as HMs and organic pollutants11,14,15, where optimizing contaminant removal efficiency is critical. In light of these advancements, we hypothesize that integrating ML with RSM will significantly enhance the reliability and accuracy of predictive models for Cd(II) and Sb(V) removal.
Based on the hypothesis, this study aims to: (i) characterize and explore the adsorption mechanisms of Cd(II) and Sb(V) by MnO-FA composites using TEM, XPS, FTIR; (ii) investigate and optimize the key parameters affecting batch adsorption via RSM; and (iii) integrate ML (RF, and genetic algorithm-backpropagation neural network (GABP)) and RSM models to evaluate the reliability and effectiveness of predictive models using experimental adsorption data. Our findings offer valuable insights into the aqueous-phase adsorption mechanisms of Cd(II) and Sb(V) in the presence of OM and Mn mineral, and underscore the potential of MnO-FA composites for practical applications in HM removal from contaminated water systems.
Materials and methods
Characterization of adsorption mechanisms
The birnessite-fulvic acid composite (BS-FA) was synthesized using the coprecipitation method following the procedure described in our previous research6. Briefly, 200 mL of 50 mM KMnO4 was first mixed with 400 mL of 50 mM NaOH solution with magnetic stirring to create a homogeneous solution. Then, 300 mL solutions containing 50 mM MnCl2 and 3.07 g L-1 FA were added dropwise into the aforementioned mixed solution, followed by 12 h of magnetic stirring to form the BS-FA suspension. The resulting BS-FA mixtures were then washed with deionized water several times until the conductivity of suspension was below 10 µS cm− 1. Finally, the purified BS-FA product was freeze-dried for further analysis. FA was selected at a mass concentration of 5%, reflecting the natural OM content found in soils and sediments19. Stock solutions of Cd(II) and Sb(V) were prepared using CdCl2⋅2.5H2O, C4H4KO7Sb·0.5H2O, and deionized water. All reagents were of analytical grade.
The mining and textile printing industries contribute significantly to the co-contamination of aqueous systems with Sb and Cd. For instance, in Sb mining areas, Sb concentrations in wastewater typically range from 4.58 × 10³ to 9.6 × 10⁴ µg L⁻¹20. The Cd concentration in acidic mine wastewaters was up to 100 mg L-121,22. Therefore, concentrations of Cd(II) and Sb(V) were set at 25.5 mg L- 1 in this study to simulate realistic environmental conditions and to allow for a comprehensive assessment of the material’s adsorption mechanism in removing both metals simultaneously. For the adsorption experiments, 0.025 g of BS-FA was placed into a 50 mL polypropylene centrifuge tube, followed by the addition of 20 mL of 0.05 M NaCl solution to maintain a constant background ionic strength. Subsequently, 0.51 mL of 1 g L− 1 Cd(II) and 0.51 mL of 1 g L− 1 Sb(V) stocked solution were added to each tube, resulting in initial concentrations of 25.5 mg L⁻¹ for both Cd(II) and Sb(V). The pH was adjusted to 6.5 using 0.05 M HCl or NaOH, simulating typical soil conditions6. The mixtures were stirred at 190 rpm at 30 °C for 24 h. Afterward, the solutions were centrifuged at 6000 rpm for 30 m, and the precipitates were collected and dried in a vacuum oven at 40 °C to yield the BS-FA-Cd&Sb complexes. X-ray photoelectron spectroscopy (XPS, KAlpha, Thermo Scientific, USA) and Fourier-transform infrared spectroscopy (FTIR, Nicolet iS20, Thermo Scientific, USA) were employed to analyze the elemental distribution and functional group variations in BS-FA before and after adsorption of Cd(II) and Sb(V). Additionally, energy dispersive X-ray spectroscopy (EDS) mapping and elemental line scans were used to investigate the distribution of Mn, C, O, Cd, and Sb in the BS-FA after adsorption using transmission electron microscopy (TEM, FEI Talos F200S, USA).
Single-factor (SF) experiments
The SF experiments were conducted in 50 mL centrifuge tubes to investigate the adsorption efficiency of Cd(II) and Sb(V) by the BS-FA composite. Various parameters were examined, including adsorbent mass (0.125–2.5 g L⁻¹), initial concentrations of Cd(II) and Sb(V) (1–100 mg L⁻¹), pH (2–9), adsorption times (10–1440 min), temperatures (10–40 °C), and ionic strengths (0–0.2 mol L⁻¹). Research has shown that selecting HM ion concentrations in the range of 1–200 mg L-1 during experiments allows for a deeper understanding of the adsorption and removal processes, a more comprehensive study of the kinetic and thermodynamic properties of HM ions in solution, and enhances the accuracy of data analysis23,24,25,26. Therefore, for batch experiments, Cd(II) and Sb(V) concentrations were selected within the range of 1–100 mg L- 1. After equilibration, the solutions were centrifuged and supernatant Cd(II) and Sb(V) concentrations were determined using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7700x, USA). All trials were conducted in triplicate. The removal efficiency (R, %) and adsorption capacity (Q, mg g⁻¹) were calculated using the following equations:
Where C0 and Ce (mg L-1) are initial and equilibrium concentrations of Cd(II) and Sb(V), respectively; V (mL) is the solution volume; m (g) is the adsorbent mass.
Optimization of process parameters using central composite design-response surface methodology (CCD-RSM)
Based on the results from SF experiments. the independent variables pH (X1), initial concentration (X2), adsorption time (X3), adsorbent dosage (X4), and ionic strength (X5) were selected for further optimization. Each parameter was coded at five levels: -α, -1, 0, + 1, and + α (Table S1). The axial distance α representing the distance of the star points from the center of the design, was calculated as 2.38 for a central composite design (k < 6)10where k is the number of factors (= 5). NaCl was used to adjust the ionic strength in each tube. The pH values of the mixed suspension were adjusted to desired values using either 0.1 M HCl or 0.1 M NaOH solutions. The total volumes of HNO3/NaOH added were less than 0.05 mL. In the CCD-RSM, the relationship between the dependent variable (the removal efficiency of Cd(II) as response) and the five factors (pH, initial concentrations of Cd(II) and Sb(V), adsorption time, adsorbent dosage, and ionic strength) was investigated. The significance of the model was assessed using analysis of variance (ANOVA), which provided corresponding p and F values. To validate the model, experimental tests were conducted to confirm the accuracy of the predictions. All analyses were performed by Design-Expert 12.0.
Machine learning models for prediction of adsorption efficiency
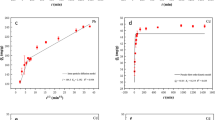
A three-layer genetic algorithm-back propagation neural network (GABP) was constructed (Fig. 1(a) and (b)), with detailed procedures provided in the Supplementary Material.
Random Forest (RF) is a nonparametric ensemble learning technique that builds a multitude of decision trees to uncover intricate relationships between variables (Fig. 1(c)), while remaining robust to multicollinearity15,18. In developing the RF model, the dataset for each Cd(II) adsorption set by BS-FA was randomly divided into the training set and the validation set wherein pH, initial concentration, time, adsorbent dosage, and ionic strength were designated as input variables whereas Cd(II) adsorption efficiency was the output variable. The optimal parameters for the RF model were selected using the randomized grid search algorithm27. Table S2 shows the different ranges of the parameters.
Model comparison
The dataset was split into training and test sets in a 70:30 ratio, which is commonly used in machine learning to ensure model reliability11. Model performance of the RSM, GABP, and RF was evaluated using several statistical metrics, including the coefficient of determination (R²), root mean square error (RMSE), mean squared error (MSE), mean absolute deviation (MAD), and mean absolute percentage error (MAPE). The formulas used for error analysis can be found in our previous study10.
Results and discussion
Adsorption characterization
The distribution of Cd(II) and Sb(V) on the BS-FA composite was comprehensively analyzed using TEM, EDS mapping, and EELS line scans. The morphology of the synthesized BS-FA composite revealed a nanoflower-like structure, with an approximate size of 10–20 nm (Fig. S1). These nanostructures, consisting of intergrown flakes, were consistent with previous reports6,7. Notably, the addition of FA effectively inhibited the aggregation of BS particles, resulting in an increase in SSA from 161.32 m² g-1 to 224.88 m² g-1, as demonstrated in our earlier study6. This increase in surface area and the enhanced particle dispersion likely contribute to the improved adsorption capacity for multiple HMs. EDS mapping further confirmed the adsorption of Cd and Sb, showing overlapping signals of Cd and Sb with O and Mn on the surface of the composite (Fig. S2). Several published works have shown the manganese (oxy)hydroxide–Cd–As ternary complex28,29. Because of the similarity between As(V) oxyanions and Sb(V), the BS-Cd-Sb ternary complex is a theoretical possibility on BS-FA composite. Quantitative analysis revealed average adsorption percentages of 3.15% for Cd and 1.32% for Sb, indicating their successful incorporation into the BS-FA. Further EDS line scans within small BS-FA aggregates (approximately ten of nanometers) demonstrated a strong correlation (R² = 0.95) between Mn and O (Fig. 2), suggesting a close association between BS and the oxygen-containing groups in FA. Additionally, significant correlations were observed between Cd and O (R² = 0.76), Sb and O (R² = 0.70), Mn and Cd (R² = 0.75), and Mn and Sb (R² = 0.68), highlighting that Cd(II) and Sb(V) primarily bind to oxygen-containing groups in FA and Mn–O bonds.
The adsorption mechanisms were further investigated through XPS analysis (Fig. 3). Analysis of the Cd 3d signals revealed peaks at 411.28 eV (Cd 3d₃/₂) and 404.58 eV (Cd 3d₅/₂) (Fig. 3(a)), indicative of the formation of Cd–O, Cd–OH, and/or (-COOH)₂Cd complexes30. These values align well with reported binding energies for Cd(II) in surface complexes and microprecipitates on Mn oxide-based materials, which typically range from 404.4 to 405.0 eV for Cd 3d₅⁄₂ and 411.2-412.2 for Cd 3d₃/₂5,26,30. This supports a mixed mechanism of surface complexation and possible precipitation. Similarly, the Sb 3d signals at 539.38 eV and 529.48 eV confirmed the oxidation state of Sb(V), and suggest chemical bonding with functional groups including phenolic hydroxyl, carboxyl, and Mn–O sites31. These binding energies are comparable to those reported for Sb(V) adsorbed onto Fe/Mn oxides (529.2–530.0 eV for Sb 3d₅⁄₂)1,3,23,31suggesting that the immobilization occurred mainly via inner-sphere complexation.
In the O1s spectrum (Fig. 3(b)), binding energies at 529.5 eV, 530.7–530.8 eV, and 531.3 eV corresponded to metal-bonded oxygen (M–O), oxygen in the BS crystal lattice (O²⁻), and surface oxygen (Mn–OH and C–OH bonds), respectively5,8. After adsorption, the proportion of surface oxygen species in BS-FA decreased from 33.18 to 24.87%, suggesting the involvement of Mn–OH and C–OH bonds in the adsorption of Cd(II) and Sb(V). This reduction is likely attributed to the binding of Cd²⁺ to > OH groups, forming > OCd⁺ complexes through ion exchange10and Sb(OH)₆⁻ binding to > OH groups, resulting in the formation of H[Sb(OH)6] complexes3. The C1s spectrum (Fig. 3(c)) was deconvoluted into three subpeaks: C–C (284.2 eV), C–O (285.7 eV), and C = O (287.9–288.4 eV)32. After Cd(II) and Sb(V) adsorption, the atomic ratio of C–O groups decreased from 22.14 to 19.78%. These findings proved that C − O bonds in FA participated in Cd(II) and Sb(V) adsorption by complexation, in agreement with previous reports26,29,30. The Mn2p spectrum (Fig. 3(d)) showed subpeaks corresponding to Mn(II) (641.6 eV), Mn(III) (642.7–642.9 eV), and Mn(IV) (643.9 eV)6,8,26. The atomic ratios of Mn(II): Mn(III): Mn(IV) before and after adsorption were 35:16:49 and 35:17:48, respectively, indicating that the BS crystalline structure remained stable with no significant redox transformation during metal binding.
FTIR analysis further supported the proposed mechanisms (Fig. S3). The O–H stretching band shifted from 3379 to 3343 cm⁻¹ after adsorption, indicating the participation of surface hydroxyl groups. The C = O peak at 1621 cm⁻¹ shifted, further confirming the involvement of oxygen-containing groups in FA, such as C–OH and –COOH29in the adsorption process. Additionally, the Mn–O bond vibrations sharpened and shifted from 517 cm⁻¹ to 511 cm⁻¹30indicating that Cd(II) and Sb(V) formed stable complexes with Mn–O bonds during the adsorption process.
Results of SF experiments
As the dosage of BS-FA increases from 0 to 0.0125 g, the removal rates for Cd(II) and Sb(V) reach 95.59% and 48.73%, respectively (Fig. 4(a)). At a dosage of 0.025 g, the removal rates stabilize. Given the cost considerations associated with BS-FA in adsorption studies, 0.025 g is selected as the optimal dosage.
The removal efficiencies of Cd(II) and Sb(V) by BS-FA are notably high within the pH range of 4 to 8 (Fig. 4(b)). For Cd(II), the removal efficiency remains consistently high, ranging from 95.67 to 98.87% between pH values of 5 and 8. Conversely, the maximum removal efficiency for Sb(V) is observed at pH 4, reaching 83.35%, while efficiencies stay above 60% within the pH range of 5 to 8. Thus, considering both the effectiveness and cost, the optimal pH range for adsorption is determined to be between 4 and 8.
As shown in Fig. 4(c), the removal efficiencies for Cd(II) and Sb(V) by BS-FA remain relatively constant as the temperature increases from 10 to 40 °C. This indicates that temperature has little impact on the adsorption process, allowing subsequent experiments to be conducted at room temperature.
Figure 4(d) illustrates that as the initial concentration increases, the removal rates for both Cd(II) and Sb(V) decrease. BS-FA exhibits high removal efficiency at lower concentrations due to the abundance of unoccupied active sites on the adsorbent surface. However, as the initial concentration rises, these active sites become increasingly occupied19, reducing removal efficiency. Therefore, an initial concentration of 25.5 mg L-1 was optimal, providing a balance between cost and efficiency.
Initially, both Cd(II) and Sb(V) are adsorbed rapidly (Fig. 4(e)), with significant increases in removal efficiency observed within the first 4 h, attributed to the availability of active sites. Following this period, the removal rate for Cd(II) stabilizes, whereas the removal rate for Sb(V) starts to decline. This decline is due to the saturation of the adsorbent, at which point both adsorption and ionic exchange equilibrium are reached19. Consequently, an optimal adsorption time of 4 h is identified.
As shown in Fig. 4(f), increasing the ionic strength from 0 to 0.2 mol L-1 initially enhances the removal efficiency of Cd(II); however, a decrease is observed once the ionic strength exceeds 0.05 mol L-1, likely due to increased competition between Cd(II) and background ions10. In contrast, the adsorption efficiency of Sb(V) exhibits a continuous upward trend with increasing ionic strength, suggesting a different adsorption mechanism. To ensure the effective removal of Cd(II) while maintaining favorable conditions for Sb(V) adsorption, 0.05 mol L⁻¹ is identified as the optimal ionic strength for the adsorption process.
Optimization of BS-FA adsorption performance using CCD-RSM
Based on the results from the SF experiments, the optimal value for each factor from the single-factor experiments was selected as the center point14. Two levels, representing the upper and lower regions, were chosen for each factor to define the experimental design. Thus, a 4-factor, 5-level CCD-RSM was established (Table S1). In total, 50 experimental runs were conducted, systematically assessing five independent variables: pH, initial concentration, adsorption time, adsorbent dosage, and ionic strength, which are denoted as X1, X2, X3, X4, and X5, respectively. Additionally, since the response variable of Cd(II) adsorption efficiency showed a more pronounced effect in the CCD-RSM framework, the removal efficiency of Cd(II) was selected as the dependent variable. The experimental setup and predicted results are outlined in Table S3.
To establish the relationship between the independent variables and Cd(II) removal efficiency, a polynomial regression analysis was conducted on the data presented in Table S4, using the Design-Expert statistical software. The resulting quadratic regression equation, which models the Cd(II) adsorption efficiency as a function of independent variables, is expressed as follows:
The F-values in Table S4 indicate that the factors influencing Cd(II) adsorption can be ranked in the following order: adsorbent dosage > ionic strength > initial concentration > pH > adsorption time. Furthermore, the F-value of the model was 5.12, with a p-value < 0.0001, implying that the model is highly significant and provides a robust fit to the regression area8,13. The F-value for “lack of fit” was 1.85, with a p-value of 0.205 > 0.05, indicating that the model aligns well with the experimental data (R2 = 0.7794). These results confirm that the model exhibits a high degree of accuracy with minimal error, making it a reliable tool for investigating the Cd(II) adsorption by BS-FA.
Optimal adsorption conditions were determined by selecting the “in-range” values for all treatment parameters, aiming to maximize Cd(II) adsorption capacity. Based on the regression analysis and model fitting, the optimal experimental conditions were identified as follows: X1(pH) = 6.84, X2 (initial concentration) = 15.2 mg L-1, X3 (adsorption time) = 1.51 h, X4 (adsorbent dosage) = 0.036 g, and X5 (ionic strength) = 0.03 mol L-1. Under these conditions, the Cd(II) removal rate reached 95.54%.
Machine learning models for prediction of adsorption efficiency
To apply the GABP model, the experimental data for training (70%) and validation (30%) sets11 matched those used in the RSM. The training used a three-layer neural network (5:3:1) with the tansig and purelin transfer functions for the hidden and output layers31respectively. After optimizing the number of hidden layer nodes, the model achieved an optimal RMSE of 0.032 with 3 hidden nodes. The optimal parameters for the GABP model are summarized in Table S5. The fitting results are presented in Table S3.
For the RF model, the dataset was also split into 70% training and 30% testing sets. This division was consistent with the approach used in the RSM analysis. The optimal hyperparameters were identified as ntrees = 200, max_depth = 10, and min_samples_leaf = 2.
Comparison of RSM with ML models
Figure 5 presents a correlation plot comparing the predicted and actual Cd(II) adsorption efficiencies for the RSM, GABP, and RF models. In the training set, the R² values for the RSM, GABP, and RF models were 0.737, 0.9337, and 0.84, respectively, indicating strong correlations between predicted and observed values. However, in the validation set, the R² values decreased to 0.7248, 0.4825, and 0.8037, respectively. The RF model demonstrated strong consistency in its performance, with good fitting results for both the training set (R² = 0.84, RMSE = 0.0623) and the validation set (R² = 0.804, RMSE = 0.0625) for adsorption data (Fig. 5 and Table S6). These results indicate that the RF model is robust and generalizes well despite the relatively limited dataset. In contrast, the GABP model exhibited significant discrepancies between the training set (R² = 0.934, RMSE = 0.0302) and the validation set (R² = 0.483, RMSE = 0.1207), which is attributed to the model’s inherent requirement for a larger number of data points (several hundred to thousands) to ensure effective learning and generalization. Given the smaller dataset in our study, the GABP model’s performance was less reliable during validation, suggesting potential overfitting26,32. Furthermore, the RF model outperformed both the RSM and GABP models in terms of error metrics, as evidenced by its significantly lower MSE, MAD, and MAPE values in both the training and prediction sets (Table S6). These results underscore the robustness of the RF model and its ability to effectively capture non-linear relationships between input factors and response variables16.
The RSM model generally excels in optimizing process parameters by establishing linear relationships between input factors and adsorption efficiency. However, it faces limitations in capturing complex non-linear interactions31. The GABP model is well-suited for addressing non-linear problems but requires a large dataset to achieve optimal performance32. In contrast, the RF model performs more reliably with smaller datasets27, offering both stability and enhanced interpretability, making it a valuable tool for modeling non-linear adsorption processes. The predicted optimal process conditions by the RF model were: pH = 6, adsorbent dosage = 0.026 g, adsorption time = 4 h, ionic strength = 0.01 mol L-1, initial concentration = 25.5 mg L-1.
To assess the accuracy and reliability of the optimization outcomes, three replicate experiments were conducted, each utilizing the optimal adsorption conditions forecasted by the RSM, GABP, and RF models (Table 1). The experimental findings revealed that, under the conditions predicted by the RF model, the adsorption capacities of Cd(II) and Sb(V) by BS-FA were 28.5 mg g⁻¹ and 20.66 mg g⁻¹, respectively. Under the GABP model’s predicted conditions, the adsorption capacities were 12.27 mg g⁻¹ for Cd(II) and 8.06 mg g⁻¹ for Sb(V), while under the RSM model’s predicted conditions, they were 12.1 mg g⁻¹ and 7 mg g⁻¹, respectively. The relative error between the predicted and actual values was found to be 0.836% for the RF model, which was smaller than the corresponding relative errors for both the RSM (1.032%) and GABP (22.98%) models. These results highlight that the RF model exhibited higher accuracy and superior predictive capability than the RSM and GABP models. Additionally, the optimal adsorption conditions predicted by the RF model resulted in Cd(II) and Sb(V) removal efficiencies of 96.9% and 70.2%, respectively, further supporting the robustness and effectiveness of the RF model in predicting optimal adsorption conditions.
Environmental implication
The co-occurrence of Cd and Sb in industrial effluents presents serious environmental and public health challenges due to the toxicity and differing geochemical behaviors of these HMs33,34. Cd(II), a divalent cation, and Sb(V), a pentavalent anion, respond differently to environmental factors such as pH and ionic strength, making their simultaneous remediation particularly complex. The widespread occurrence of Mn oxides and natural OM in these soils plays a key role in influencing the mobility, transformation, and long-term stability of such contaminants.
This study demonstrates that the indigenous BS-FA composite can effectively and simultaneously removal of Cd(II) and Sb(V) from the aqueous phase, thereby reducing their bioavailability and environmental risks in natural soils. Although Cd(II) and Sb(V) may exhibit distinct adsorption mechanisms—Cd(II) primarily binds with Mn–O sites via inner-sphere complexation and precipitation5,6,26while Sb(V) forms surface complexes with oxygen-containing functional groups such as hydroxyl and carboxyl in FA2,3,31—the composite still achieved notable co-removal efficiency under a unified set of optimized conditions based on Cd(II)-oriented modeling. These results highlight the potential of BS–FA as a versatile amendment for addressing complex mixed-contaminant scenarios.
Table S7 presents a comparative evaluation of Cd(II) and Sb(V) adsorption capacities across various MnO-based composites, revealing notable differences in performance. Compared to pure MnO, the BS-FA composite showed enhanced adsorption due to the dispersion of MnO aggregates, which increased the SSA and available adsorption sites6. The table also suggests that the effectiveness of Cd(II) and Sb(V) adsorption is highly dependent on the reaction conditions. These factors play a critical role in determining the adsorption capacity and selectivity, highlighting the complexity of optimizing adsorption processes for real-world applications16. Future research should focus on real wastewater matrices, long-term stability, and the scalability of such composite adsorbents for practical water treatment scenarios.
Conclusion
This study demonstrated that the birnessite–fulvic acid (BS-FA) composite has potential merit for the simultaneous removal of Cd(II) and Sb(V) from aqueous solutions for the first time. The coexisting Cd(II) and Sb(V) were primarily complexed with Mn–O bonds in birnessite (BS) and oxygen-containing functional groups in fulvic acid (FA), such as C–OH and –COOH. Additionally, the response surface methodology (RSM) model successfully optimized the conditions for achieving maximum removal efficiency, although it did not fully capture the nonlinear dynamics of the adsorption process. By integrating the random forest (RF) model with RSM, the predictive accuracy and reliability of the adsorption process were significantly enhanced. These findings highlight the potential of BS-FA as a promising adsorbent for water treatment applications involving mixed metal contamination. Future studies should extend to natural waters or contaminated soils to validate environmental applicability under more complex conditions.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Liu, R. et al. Simultaneous removal of Cd(II) and Sb(V) by Fe–Mn binary oxide: positive effects of Cd(II) on Sb(V) adsorption. J. Hazard. Mater. 300, 847–854. https://doi.org/10.1016/j.jhazmat.2015.08.020 (2015).
Wang, Y. et al. Simultaneous alleviation of Sb and cd availability in contaminated soil and accumulation in Lolium multiflorum lam. After amendment with Fe–Mn-Modified Biochar. J. Clean. Prod. 231, 556–564. https://doi.org/10.1016/j.jclepro.2019.04.407 (2019).
Li, J. et al. Pristine and Fe-functionalized Biochar for the simultaneous immobilization of arsenic and antimony in a contaminated mining soil. J. Hazard. Mater. 469, 133937. https://doi.org/10.1016/j.jhazmat.2024.133937 (2024).
Islam, M. A., Morton, D. W., Johnson, B. B., Mainali, B. & Angove, M. J. Manganese oxides and their application to metal ion and contaminant removal from wastewater. J. Water Process. Eng. 26, 264–280. https://doi.org/10.1016/j.jwpe.2018.10.018 (2018).
Jin, C. et al. Cadmium immobilization in lake sediment using different crystallographic manganese oxides: performance and mechanism. J. Environ. Manage. 313, 114995. https://doi.org/10.1016/j.jenvman.2022.114995 (2022).
Jin, C. et al. Binding of Cd(II) to birnessite and fulvic acid organo-mineral composites and controls on Cd(II) availability. Chemosphere 329, 138624. https://doi.org/10.1016/j.chemosphere.2023.138624 (2023).
Ding, Z. et al. Coupled sorption and oxidation of soil dissolved organic matter on manganese oxides: Nano/sub-nanoscale distribution and molecular transformation. Environ. Sci. Technol. 56 (4), 2783–2793. https://doi.org/10.1021/acs.est.1c07520 (2022).
Lu, M. et al. Optimization of adsorption performance of cerium-loaded intercalated bentonite by CCD-RSM and GA-BPNN and its application in simultaneous removal of phosphorus and ammonia nitrogen. Chemosphere 336, 139241. https://doi.org/10.1016/j.chemosphere.2023.139241 (2023).
Jin, C., Deng, R., Ren, B., Hou, B. & Hursthouse, A. S. Enhanced biosorption of Sb(III) onto living Rhodotorula mucilaginosa strain DJHN070401: optimization and mechanism. Curr. Microbiol. 77, 2071–2083. https://doi.org/10.1007/s00284-020-02025-z (2020).
Jin, C. et al. Laboratory and simulation study on the Cd(II) adsorption by lake sediment: mechanism and influencing factors. Environ. Res. 197, 111138. https://doi.org/10.1016/j.envres.2021.111138 (2021).
Mahanty, B., Saawarn, B., Mahto, B., Hussain, S. & Hait, S. Efficient removal of perfluorooctanoic acid from aqueous matrices using cationic surfactant functionalized graphene oxide nanocomposite: RSM and ANN modeling, and adsorption behaviour. J. Water Process. Eng. 68, 106448. https://doi.org/10.1016/j.jwpe.2024.106448 (2024).
Xiang, X., Li, X., Yang, T., Cao, J. & Yang, Z. A novel feca composite modified Biochar prepared for enhanced cd adsorption: experimental, DFT calculation and mechanism unveiling. J. Water Process. Eng. 68, 106565. https://doi.org/10.1016/j.jwpe.2024.106565 (2024).
Kavisri, M. et al. Adsorption isotherm, kinetics and response surface methodology optimization of cadmium (Cd) removal from aqueous solution by Chitosan biopolymers from cephalopod waste. J. Environ. Manage. 335, 117484. https://doi.org/10.1016/j.jenvman.2023.117484 (2023).
Yuan, W. et al. Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol. Environ. Saf. 175, 138–147. https://doi.org/10.1016/j.ecoenv.2019.03.057 (2019).
Liang, C. et al. Simulation, prediction and optimization for synthesis and heavy metals adsorption of schwertmannite by machine learning. Environ. Res. 265, 120471. https://doi.org/10.1016/j.envres.2024.120471 (2025).
He, M. et al. A review of hydroxyapatite synthesis for heavy metal adsorption assisted by machine learning. J. Hazard. Mater. 481, 136525. https://doi.org/10.1016/j.jhazmat.2024.136525 (2025).
Shi, S. et al. Interpreting machine learning predictions of Pb2+ adsorption onto biochars produced by a fluidized bed system. J. Clean. Prod. 486, 144551. https://doi.org/10.1016/j.jclepro.2024.144551 (2025).
Xu, Z., Ding, Y., Han, S. C. & Zhang, C. Predicting the performance of lithium adsorption and recovery from unconventional water sources with machine learning. Water Res. 266, 122374. https://doi.org/10.1016/j.watres.2024.122374 (2024).
Du, H. et al. Ferrihydrite–organo composites are a suitable analog for predicting Cd(II)–As(V) coexistence behaviors at the soil solid-liquid interfaces. Environ. Pollut. 290, 118040. https://doi.org/10.1016/j.envpol.2021.118040 (2021).
He, M., Wang, X., Wu, F. & Fu, Z. Antimony pollution in China. Sci. Total Environ. 421–422. https://doi.org/10.1016/j.scitotenv.2011.06.009 (2012).
Kuang, X. et al. Application of biological soil crusts for efficient cadmium removal from acidic mine wastewater. J. Hazard. Mater. 465, 133524. https://doi.org/10.1016/j.jhazmat.2024.133524 (2024).
Xia, S., Song, Z., Zhao, X. & Li, J. Review of the recent advances in the prevention, treatment, and resource recovery of acid mine wastewater discharged in coal mines. J. Water Process. Eng. 52, 103555. https://doi.org/10.1016/j.jwpe.2023.103555 (2023).
Deng, S. et al. Study on the adsorption performance of carbon-magnetic modified sepiolite nanocomposite for Sb(V), Cd(II), Pb(II), and Zn(II): optimal conditions, mechanisms, and practical applications in mining areas. J. Hazard. Mater. 487, 137129. https://doi.org/10.1016/j.jhazmat.2025.137129 (2025).
Xu, H. et al. Cadmium(II) adsorption by recyclable Zeolite-Loaded hydrogel: extension to the removal of Cadmium(II) from contaminated soil. Chem. Eng. J. 492, 151842. https://doi.org/10.1016/j.cej.2024.151842 (2024).
Yang, Z., Xu, Z., Geng, L., Shu, W. & Zhu, T. Effect of multi-walled carbon nanotubes on extractability of Sb and cd in contaminated soil. Ecotoxicol. Environ. Saf. 205, 111316. https://doi.org/10.1016/j.ecoenv.2020.111316 (2020).
Yin, G. et al. Novel Fe-Mn binary oxide-biochar as an adsorbent for removing Cd(II) from aqueous solutions. Chem. Eng. J. 389, 124465. https://doi.org/10.1016/j.cej.2020.124465 (2020).
Abd El-Ghany, S. & El-Aziz, A. A robust tuned random forest classifier using randomized grid search to predict coronary artery diseases. CMC-Comput Mater. Con. 75, 4633–4648. https://doi.org/10.32604/cmc.2023.035779 (2023).
Zeng, W. et al. Simultaneous removal of Cd(II) and As(V) by ferrihydrite-biochar composite: enhanced effects of As(V) on Cd(II) adsorption. J. Environ. Sci. 139, 267–280. https://doi.org/10.1016/j.jes.2023.04.020 (2024).
Khan, Z. H. et al. Simultaneous and efficient removal of Cd(II) and As(III) by a magnesium-manganese codoped Biochar composite: sorption performance and governing mechanisms. J. Environ. Chem. Eng. 11 (3), 109919. https://doi.org/10.1016/j.jece.2023.109919 (2023).
Yin, G. et al. Co-adsorption mechanisms of Cd(II) and As(III) by an Fe-Mn binary oxide Biochar in aqueous solution. Chem. Eng. J. 466, 143199. https://doi.org/10.1016/j.cej.2023.143199 (2023).
Duan, Y. et al. Adsorption of Sb(V) in wastewater by fe/mn binary oxides loaded sludge derived activated carbon: performance, mechanisms and applicability. Environ. Technol. Inno. 36, 103881. https://doi.org/10.1016/j.eti.2024.103881 (2024).
Yang, H., Liu, X., Liu, Y., Cui, J. & Xiao, Y. Revolutionizing Biochar synthesis for enhanced heavy metal adsorption: Harnessing machine learning and bayesian optimization. J. Environ. Chemi Eng. 11, 110593. https://doi.org/10.1016/j.jece.2023.110593 (2023).
Yousefi, M. et al. Adsorption of Diazinon from aqueous solution using metal organic framework and functionalized graphene: comparison of BBD, ANN models. Chemosphere 351, 141222. https://doi.org/10.1016/j.chemosphere.2024.141222 (2024).
Xiang, X. et al. Cd adsorption prediction of Fe mono/composite modified Biochar based on machine learning: application for controllable Preparation. Environ. Res. 265, 120466. https://doi.org/10.1016/j.envres.2024.120466 (2025).
Acknowledgements
This research was funded by National Natural Science Foundation of China, grant number 42407518.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.J. and Y.L.; methodology, Y.L.; software, Y.L.; validation, C.J. and Y.L.; formal analysis, C.J.; investigation, J.L. and Y.G.; resources, C.J.; data curation, B.H.; writing—original draft preparation, C.J.; writing—review and editing, Y.L.; visualization, Y.L.; supervision, B.H.; project administration, B.H.; funding acquisition, C.J. All authors have read and agreed to the published version of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
We confirm that the manuscript has been read and approved by all named authors. We further confirm and understand that the corresponding author is the sole contact for the editorial process.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, C., Lu, J., Gao, Y. et al. Mechanisms and optimization for simultaneous removal of Cd(II) and Sb(V) from aqueous solutions using birnessite and fulvic acid composite. Sci Rep 15, 19502 (2025). https://doi.org/10.1038/s41598-025-04527-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04527-x