Abstract
Respiratory symptoms like prolonged cough and breathlessness have increased post-COVID-19, even in those with normal chest X-rays and FEV1/FVC ratios. This study assessed the benefits of Lactobacillus plantarum GCWB1001 on such symptoms in individuals without asthma or COPD. In a double-blind, randomized, placebo-controlled trial, 126 participants aged 19–70 were included. Exclusions were for asthma, COPD, abnormal chest X-rays, or recent antibiotic use. The primary outcome was the Breathlessness, Cough, and Sputum Scale (BCSS), with secondary outcomes including Visual Analogue Scale (VAS) scores for respiratory function. The total BCSS score at 12 weeks did not differ significantly between the GCWB1001 and placebo groups. Secondary endpoints such as sputum and breathlessness showed numerical improvements, particularly in males and participants over 40, but these findings were not statistically significant after correction for multiple comparisons. No serious adverse events were reported, indicating safety. Although this study did not demonstrate a clear clinical benefit, the exploratory trends suggest that additional, larger-scale trials may be needed to determine if these observations reflect a meaningful effect.
Similar content being viewed by others
Introduction
The period following the coronavirus pandemic has seen a notable increase in respiratory symptoms1. This rise is attributed to factors such as the spread of COVID-19 and more frequent exposure to allergens. Environmental issues like air pollution and fine dust further compound this trend, leading to more patients experiencing respiratory problems2. Notably, many of these patients, who don’t have asthma or chronic obstructive pulmonary disease (COPD), report prolonged respiratory symptoms like coughing, phlegm, and breathing difficulties3,4. However, their standard respiratory tests, including chest X-rays and first second of forced expiration/forced vital capacity (FEV1/FVC) ratios, often show normal results3. These patients also tend to have a limited response to usual treatments like inhaled steroids or bronchodilators. This scenario, which has become more pronounced post-pandemic, demonstrates the complex relationship between infectious diseases, environmental factors, and respiratory health concerns5,6.
Recent studies have been focusing on the microbiome, particularly prebiotics and probiotics, as potential solutions. There is growing evidence that the microbiome plays a significant role in respiratory allergic diseases. Research is looking into how changes in the gut and respiratory microbiota might affect overall respiratory health. The idea is that a well-balanced microbiome might help the immune system, possibly reducing inflammation and allergic reactions in the respiratory system. Lactobacillus plantarum, a bacterium found in fermented foods like kimchi, is drawing attention for its health benefits. This bacterium, linked to the fermentation process, has been associated with various health advantages in recent studies.
In earlier research using mice exposed to diesel exhaust particulate matter to worsen respiratory health, Lactobacillus plantarum GCWB1001 (hereafter GCWB1001) showed promising results7. It significantly reduced bronchial allergic inflammation, decreased mucus production, and improved the structure of alveoli and smooth muscles in the lungs. Lactobacillus plantarum is known for its benefits to gut health and immune function, but its effect on respiratory symptoms in patients without standard asthma or COPD diagnose is not yet fully understood. This gap presents a valuable opportunity to explore new treatments that could help patients who currently have limited relief options.
Our study aims to thoroughly investigate the effectiveness of GCWB1001 in reducing prolonged respiratory symptoms in patients with these specific health concerns. This double-blind, randomized, controlled, multicenter, prospective trial seeks to provide new insights into respiratory health management and potentially introduce innovative treatment approaches for patients not adequately helped by existing treatments for respiratory diseases.
Methods
Participants
From Aug 2022 to April 2023, a total of 126 adult subjects (19–70 years old) were voluntarily recruited from 4 study organizations, Chungbuk National University Hospital in Cheongju, Hallym University Dongtan Sacred Heart Hospital in Dongtan, Hallym University Chuncheon Sacred Heart Hospital in Chuncheon and Hanyang University Hospital in Seoul. This study was first registered on 19/09/2022 at Chungbuk National University Hospital (CBNUH-GCWB1001-00001) and also registered on ClinicalTrials.gov with the registration number NCT05544942.
The inclusion criteria were (1) FEV1/FVC ≥ 70% and (2) a Breathlessness, Cough, and Sputum Scale (BCSS) score of ≥ 3 who had two or more of the following symptoms in the past month: cough, sputum, breathlessness, or chest tightness. The random code was generated by blocked randomization and randomization was based on concealed random allocation using sealed opaque envelopes.
The study participants were excluded from the study if they had been diagnosed with asthma or COPD, had clinically significant abnormal findings on chest x-ray, or were the patients with chronic bronchitis exhibiting respiratory symptoms scoring above 9 on the BCSS. Additionally, the subjects were excluded from the study who had been taking functional foods or received antibiotics related to respiratory health, who had respiratory symptoms due to viral or bacterial infections in the past four weeks, who had taken antibiotics in the past two weeks, who had consumed antitussive, expectorant, systemic corticosteroids, or immunosuppressants in the past four weeks or who were current smokers.
All subjects provided written informed consent. All data were stored in a separate locked place and maintained security. This study was conducted in accordance with the International Conference on Harmonization—Good Clinical Practice (ICH-GCP) guidelines after a review and approval by the independent Institutional Review Board (IRB) of the trial institutions, which is Chungbuk National University Hospital (IRB No: CBNUH 2022-05-011-003), Hallym University Dongtan Sacred Heart Hospital (IRB No: HDT 2022-05-007), Hallym University Chuncheon Sacred Heart Hospital (IRB No: CHUNCHEON 2022-05-006), and Hanyang University Hospital (IRB No: HYUH 2022-05-035). All methods were performed in accordance with the relevant guidelines and regulations. The study was registered at ClinicalTrials.gov under the identifier NCT05544942. This study was conducted under CONSORT guidelines.
Study product, GCWB1001
GCWB1001 is a lactic acid bacterium of green cruciate well -being that completed the genome map through the battlefield genome detoxification. The size of the genome was analyzed at about 3.4 MB, G + C Content was 44.2% and 3,314 coding sequences (previously abbreviated as CDSs).
GCWB1001 (GREEN CROSS Wellbeing, Seoul, Korea) was seed-cultured for three rounds at 37 °C for 8–18 h, based on the culture ingredient. After the main culture was prepared using the seed culture broth at 34 °C for 8–18 h, centrifugation and filtration were performed. Subsequently, cryoprotectant was added and the mixture was freeze dried at − 40 °C. The extracted powder was sieved and used in standardization test before being packaged as the final ingredient. The participants in the experimental group were administered one capsule daily (1 × 1010 CFU/day) for 12 weeks, whereas the placebo (GCWB1001 replaced with an equal amount of maltodextrin) was administered to the control group.
Study design
This study was conducted as a multi-center, double-blind, randomized, placebo-controlled clinical trial. At visit 1 (screening), demographics, medical history, and medication history were obtained from each participant, and physical examination, laboratory tests, electrocardiogram, chest radiography, and pulmonary function tests (spirometry) were performed. The participants also completed questionnaires including BCSS, St. George’s Respiratory Questionnaire (SGRQ), and Visual Analog Scale (VAS) to assess respiratory symptoms and quality of life. Those who met the inclusion criteria were enrolled and randomly assigned to the GCWB1001 group or control group at Visit 2 (week 0), with a randomization ratio of 1:1. GCWB1001 [1 × 1010 CFU/day] or placebo [maltodextrin stick/pack] was taken orally once daily for 12 weeks. Visit 3 (week 6) and Visit 4 (week 12) occurred at 6-week intervals after Visit 2. At each visit, BCSS, SGRQ, and VAS were used to assess changes in respiratory symptoms and quality of life over time. Peripheral blood tests, including eosinophil counts, TNF-alpha, IgE, IL-6, IL-10, and IFN-gamma, were conducted at screening and Visit 4 (week 12) to evaluate inflammatory markers.
The BCSS was used to evaluate key respiratory symptoms—breathlessness, cough, and sputum—in chronic respiratory disease patients. Each symptom was scored from 0 (no symptoms) to 5 (severe symptoms), providing an overall score to track changes over time. The SGRQ assessed health-related quality of life by measuring symptoms, activity limitations, and social or emotional impacts, with scores ranging from 0 to 100, where higher scores indicate greater impairment. The VAS was employed to measure the intensity of symptoms such as pain or discomfort, allowing participants to indicate their symptom level along a continuous line from 0 (no symptom) to 10 (worst imaginable symptom). These tools collectively allowed us to comprehensively monitor both the physical symptoms and the impact of the disease on patients’ lives throughout the study.
Primary outcomes
The primary outcome of this study was the change in the total BCSS score after 12 weeks of supplementation. The BCSS is a validated tool that measures the severity of breathlessness, cough, and sputum. Permission to use the BCSS was obtained from AstraZeneca8.
Secondary outcomes
Secondary outcomes included the analysis of the individual components of the BCSS score—breathlessness, cough, and sputum scores—to assess changes in specific respiratory symptoms. Additionally, respiratory function improvements were evaluated using the VAS for breathlessness, cough, and sputum.
Safety assessment
The safety of GCWB1001 consumption was assessed based on adverse events (AEs), hematological tests, blood chemistry, urinalysis (UA), vital signs (blood pressure [BP] and pulse), and anthropometry (body weight).
Statistical analysis
The sample size was calculated based on previously established method9. The expected effect size of 1.0 was calculated based on previous reports confirming changes in the BCSS. A sample size of 45 provided 80% power to detect differences between groups at a significance level of 0.05. A total of 120 people were recruited, 60 per group, considering a 25% dropout rate.
All statistical analyses were performed using SAS® (Version 9.4, SAS Institute, Cary, North Carolina, USA). The primary outcome was defined as the total BCSS score at 12 weeks. All other outcomes, including individual BCSS components (breathlessness, cough, sputum) and VAS measures, were considered exploratory. For changes in BCSS, VAS, hematological tests, blood chemistry, vital signs, and anthropometry were evaluated before and after consumption. To account for multiple comparisons in the analyses of individual BCSS symptoms and subgroup analysis, we applied the Bonferroni correction. Paired t-test was used for within-group comparison and two-sample t-test or Wilcoxon rank-sum test was used for between-groups comparison. Demographic and lifestyle factors were analyzed using a generalized linear model (GLM, using ANCOVA) with the baseline characteristics as covariates. Moreover, AEs were analyzed using chi-square test or Fisher’s exact test, whereas UA was performed using McNemar test (NCT05528705).
Results
Study subjects
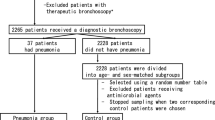
A total of 126 volunteers were screened for eligibility, of which, 123 were selected and randomized. Thirty-three participants (27.6%) were excluded either for violating the inclusion/exclusion criteria or withdrawing their consent, and the dropout rated did not differ between the experimental group and placebo groups (24.6% vs. 27.4%, p > 0.05). Finally, the experimental group included 46 participants, whereas the control group included 44 participants who successfully completed the trial (Fig. 1). The safety analysis included all the enrolled subjects. Analysis of BCSS were conducted for the subjects who completed the study (per protocol).
Primary outcomes
As per protocol, the primary outcome analysis was conducted among participants who completed the study. Changes in BCSS are summarized in Fig. 2. Total BCSS score changes after six weeks were −1.98 ± 1.37 and −1.64 ± 1.42 points in the experimental and control groups, respectively, showing no significant difference between the two groups. Similarly, the change in the total BCSS scores after 12 weeks was −2.48 ± 1.11 and −2.27 ± 1.34 points in the experimental and control groups, respectively, showing no significant difference in changes between the groups. The dropout rate of 27.6% in this study may introduce bias in interpreting these results, which we acknowledge as a limitation.
BCSS score changes over 6, 12 weeks of GCWB1001 consumption in patients with mild respiratory conditions. The figure represents changes in the BCSS scores as mean ± standard deviation. Each panel represents changes in (a) total; (b) breathlessness; (c) cough; (d) sputum; and (e) breathlessness and sputum BCSS scores.
Secondary outcomes
Since these analyses were not pre-specified as primary endpoints, all findings should be considered exploratory. Overall, numerical improvements were observed in certain BCSS sub-scores (e.g., breathlessness, sputum) and VAS measures in the experimental group compared to placebo, yet none of these changes reached statistical significance after correcting for multiple comparisons.
For instance, the breathlessness sub-score (BCSS) showed a greater reduction in the experimental group at Week 6 (e.g., −0.33 ± 0.47 vs. −0.14 ± 0.51), and the combined breathlessness-and-sputum sub-score displayed a nominal improvement at Week 12 (e.g., −1.39 ± 0.74 vs. −1.00 ± 0.84). Subgroup analyses further suggested that GCWB1001 might exert more pronounced effects in men and individuals over 40 years old. In the male subgroup, the total BCSS showed a greater reduction at Week 6, whereas in participants older than 40, the breathlessness sub-score and the combined breathlessness-and-sputum sub-score exhibited numeric improvements at Week 6 and Week 12, respectively (data not shown). However, these subgroup findings were post hoc and should be interpreted solely as hypothesis-generating.
Changes in VAS scores are summarized in Fig. 3. While VAS cough scores did not differ meaningfully between groups at either Week 6 or Week 12, there were nominal improvements in sputum and breathlessness scores in the experimental group. Specifically, the sputum VAS decreased more in the GCWB1001 group at Week 12 (e.g., −16.95 ± 13.82 vs. −11.22 ± 12.79), and the breathlessness VAS decreased more at Week 6 (e.g., −5.36 ± 11.8 vs. −0.68 ± 11.15). Combined VAS scores for breathlessness and sputum also appeared to improve; however, like the BCSS analyses, these findings did not reach significance following multiple-comparison adjustment and should be viewed as exploratory.
Changes in the VAS score after 6, 12 weeks of GCWB1001 consumption in patients with mild respiratory conditions. This figure represents the changes in VAS scores as mean ± standard deviation. Each panel represents changes in (a) VAS sputum; and (b) VAS breathlessness scores. The data were analyzed using two-paired t-test or Wilcoxon rank-sum test. * Indicates that the p-value between the two groups is < 0.05. (a) 12 weeks VAS sputum, p-value (p = 0.0231, Wilcoxon rank-sum test) (b) 6 weeks VAS Breathlessness, p-value (p = 0.0479, Wilcoxon rank-sum test).
Safety of GCWB1001
The safety of GCWB1001 was assessed in participants who had consumed the investigational product at least once (61 in the experimental group and 62 in the control group). During the study period, a total of five participants (8.2%) in the experimental group and one participant (1.6%) in the control group discontinued the study due to adverse events, as indicated in Fig. 1. The adverse events leading to dropout were mild to moderate in severity and were not considered related to the study product by the investigators. There were no serious adverse events in either group.
In addition to adverse events, some participants withdrew consent or were lost to follow-up, but there was no statistically significant difference in dropout rates between the groups. Furthermore, no significant differences were detected between the experimental and control groups in hematological tests, blood chemistry (Supplement 1,2), urinalysis, vital signs, or anthropometric parameters.
Discussion
In this randomized, double-blind, placebo-controlled trial, GCWB1001 supplementation did not yield a statistically significant improvement in the primary outcome—i.e., the total BCSS (Breathlessness, Cough, and Sputum Scale) score—after 12 weeks. This finding remained consistent despite the per-protocol analysis, which may have been biased by a dropout rate of 27.6%. While numerical or nominal improvements were noted in certain exploratory endpoints (e.g., sputum production and breathlessness), these should be interpreted with caution. They were secondary measures that did not undergo a priori multiple comparison adjustment in the initial statistical plan and thus serve merely as hypotheses for future investigation. Moreover, post hoc subgroup analyses suggested potential benefits for men and adults over 40, but these findings were purely exploratory and cannot be generalized without further study.
The prevalence of respiratory diseases continues to increase annually. Despite the development of various therapeutic interventions—such as biologics, long-acting anticholinergics, macrolides, antileukotrienes, and low-dose systemic steroids—these options carry risks of adverse events (AEs), including diarrhea, hearing loss, liver damage, arrhythmia, cataracts, and exacerbation of respiratory conditions. Against this backdrop, we investigated GCWB1001 as a potentially safer option that could help maintain respiratory health. Our study findings indicate that GCWB1001 intake appears safe and may be beneficial for respiratory symptoms, although larger trials are needed to confirm its efficacy.
Our aim was to develop a probiotic product intended not as a direct therapy but as a functional ingredient to support respiratory health. To focus on mild or subclinical respiratory discomfort, we enrolled semi-healthy individuals aged 19–70, with FEV1/FVC ≥ 70% and a BCSS score of 4–8 points, excluding those with clinically diagnosed asthma or COPD. Given that sputum production is a common marker of airway inflammation in conditions such as COPD, chronic bronchitis, and bronchiectasis10,11,12, we also considered sputum-related endpoints10,13. Improvements noted in sputum indices—though exploratory—suggest that GCWB1001 could be more helpful for individuals whose primary symptom is sputum production rather than overall symptom severity.
Although the total BCSS score did not significantly improve, nominal or numerical improvements in breathlessness, sputum, and their combined scores were observed in the GCWB1001 group. Chronic respiratory symptoms often vary among individuals, with some experiencing more pronounced breathlessness or sputum production. The potential demographic variability—particularly in males or older adults—also raises the possibility of personalized approaches. Nonetheless, these subgroup observations are post hoc and require confirmation in larger, well-powered trials that incorporate multiple-comparison corrections and intention-to-treat (ITT) analyses.
In terms of cough-related outcomes, we found no clear difference from placebo, potentially reflecting the subjective nature of cough assessments, which often makes severity and frequency difficult to quantify14,15,16,17. Additionally, cough is deemed chronic only after eight consecutive weeks, complicating the evaluation of shorter-term interventions in a relatively mild population.
Preclinical evidence for GCWB1001 efficacy was previously demonstrated in a murine model, where it mitigated diesel exhaust particulate matter (DEPM)-exacerbated allergic inflammation, reduced sputum production, and improved alveolar structure and smooth muscle function. These findings suggest that GCWB1001 may act on the respiratory system by inhibiting allergy-induced cytokine production and preventing inflammation-related damage. However, our current study also has limitations. First, we did not employ an ITT analysis; doing so might have provided more reliable, generalizable conclusions. Second, both BCSS and VAS rely on subjective symptom reporting, which can introduce bias or variability. Objective measures of respiratory function (e.g., detailed spirometry or inflammatory biomarkers) were not incorporated, further limiting the scope of our conclusions.
Given these limitations, we advocate for larger-scale trials employing an ITT design, a prespecified plan for multiple-comparison corrections, and objective measures of respiratory function. Such studies would help clarify whether GCWB1001 confers meaningful clinical benefits for individuals with mild respiratory symptoms and could elucidate any potential role in personalized or targeted respiratory health interventions.
Conclusions
Although GCWB1001 did not significantly improve the primary endpoint of total BCSS scores in this study, exploratory analyses suggest that certain respiratory symptoms, such as breathlessness and sputum, may show numerical improvement. These findings, while not definitive, highlight the potential need for further research with larger sample sizes, ITT analyses, and more rigorous statistical controls to determine whether GCWB1001 can provide meaningful respiratory health benefits.
Data availability
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Bloom, C. I. Covid-19 pandemic and asthma: What did we learn?. Respirology 28, 603–614. https://doi.org/10.1111/resp.14515 (2023).
Guarnieri, M. & Balmes, J. R. Outdoor air pollution and asthma. Lancet 383, 1581–1592. https://doi.org/10.1016/S0140-6736(14)60617-6 (2014).
Song, W. J. et al. Confronting COVID-19-associated cough and the post-COVID syndrome: Role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir. Med. 9, 533–544. https://doi.org/10.1016/S2213-2600(21)00125-9 (2021).
Baig, A. M. Chronic long-COVID syndrome: A protracted COVID-19 illness with neurological dysfunctions. CNS Neurosci. Ther. 27, 1433–1436. https://doi.org/10.1111/cns.13737 (2021).
Garcia-Vicente, P. et al. Chronic cough in post-COVID syndrome: Laryngeal electromyography findings in vagus nerve neuropathy. PLoS ONE 18, e0283758. https://doi.org/10.1371/journal.pone.0283758 (2023).
Jha, N. K. et al. Evidence of coronavirus (CoV) pathogenesis and emerging pathogen SARS-CoV-2 in the nervous system: A review on neurological impairments and manifestations. J. Mol. Neurosci. 71, 2192–2209. https://doi.org/10.1007/s12031-020-01767-6 (2021).
Jin, S. W. et al. Lactic acid bacteria ameliorate diesel exhaust particulate matter-exacerbated allergic inflammation in a murine model of asthma. Life 10, 260. https://doi.org/10.3390/life10110260 (2020).
Leidy, N. K. et al. Evaluating symptoms in chronic obstructive pulmonary disease: Validation of the Breathlessness, Cough and Sputum Scale©. Respir. Med. 97, S59–S70 (2003).
Kirichenko, T. V. et al. Clinical effectiveness of a combination of black elder berries, violet herb, and calendula flowers in chronic obstructive pulmonary disease: The results of a double-blinded placebo-controlled study. Biology 9(4), 83 (2020).
Beeh, K. et al. A single nasal allergen challenge increases induced sputum inflammatory markers in non-asthmatic subjects with seasonal allergic rhinitis: Correlation with plasma interleukin-5. Clin. Exp. Allergy 33, 475–482 (2003).
Paone, G. et al. Blood and sputum biomarkers in COPD and asthma: A review. Eur. Rev. Med. Pharmacol. Sci. 20, 698–708 (2016).
Fahy, J. V. et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am. J. Respir. Crit. Care Med. 163, 1470–1475 (2001).
Bacci, E. et al. Induced sputum is a reproducible method to assess airway inflammation in asthma. Mediat. Inflamm. 11, 293–298. https://doi.org/10.1080/09629350210000015692 (2002).
The Korean Academy of Asthma, Allergy and Clinical Immunology. Korean Guidelines for Chronic cough 2018 (2018). www.allergy.or.kr.
Ministry of food and drug safety, Health functional food functionality evaluation guideline: Respiratory(trachea·bronchi) can 370 help health. www.mfds.go.kr. Accessed 03 Sep 2020.
Oh, M.-H. et al. Research on outcome indicators in clinical trials of asthma, cough, and sputum by reviewing papers searched from pubmed. J. Internal Korean Med. 28, 519–530 (2007).
Irwin, R. S. Assessing cough severity and efficacy of therapy in clinical research: ACCP evidence-based clinical practice guidelines. Chest 129, 232S-237S (2006).
Acknowledgements
We express our heartfelt appreciation to the participants of the human trial, the Clinical Research Organization (CRO) NeoNeutra, the trial institutions (Chungbuk National University Hospital, Hallym University Dongtan Sacred Heart Hospital, Hallym University Chuncheon Sacred Heart Hospital, and Hanyang University Hospital), and Dr. Sang-Heon Kim for his meticulous review of the manuscript
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry(IPET) through -High Value-added Food Technology Development Program (or Project), funded by Ministry of Agriculture, Food and Rural Affairs(MAFRA) (321035053HD060).
Author information
Authors and Affiliations
Contributions
J.-H.C., S.-H.K., and C.-R.L contributed study conceptualization; J.-H.C., M.-G. K and C.-R.L. contributed methodology; J.-H.C., J.Y.-K. and S.-H.K. performed validation; M.-G.K. T.-B-J., J.Y.-K and S.-H.K. conducted data analysis; M.-G.K., T.-B.J and C.-R.L. wrote and prepared the original draft; J.-H.C., S.-H. K. contributed writing, reviewing, and editing; J.-H.C., and C.-R. L. provided supervision; J.W.-K and C.-R. L. oversaw funding acquisition; M.-G.K., J.-H.C. S.-H. L. and C.-R.L. enrolled subjects. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
T.-B. J, J.-Y. K and J.-W. K are full-time employees of GREEN CROSS Wellbeing Co., Ltd., the company that manufactures and markets GCWB1001 used in this study. J.-H. C, M.-G. K, S.-H. K, C.-R. L and S.-H. L declare no competing interests.
Institutional review board statement
All subjects provided written informed consent. This study was conducted in accordance with the International Conference on Harmonization—Good Clinical Practice (ICH-GCP) guidelines. The study protocol was reviewed and approved by the independent Institutional Review Boards (IRB) of participating institutions: Chungbuk National University Hospital (IRB No: CBNUH 2022-05-011-003), Hallym University Dongtan Sacred Heart Hospital (IRB No: HDT 2022-05-007), Hallym University Chuncheon Sacred Heart Hospital (IRB No: CHUNCHEON 2022-05-006), and Hanyang University Hospital (IRB No: HYUH 2022-05-035). All methods were performed in accordance with the relevant guidelines and regulations. The study was registered at ClinicalTrials.gov under the identifier [NCT05544942].
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kang, MG., Choi, JH., Kim, SH. et al. Efficacy and safety of Lactobacillus plantarum GCWB1001 for respiratory health in a double blind randomized placebo controlled trial. Sci Rep 15, 22700 (2025). https://doi.org/10.1038/s41598-025-04612-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04612-1