Abstract
Oil-based drill cuttings (OBDC) are a typical hazardous waste generated during the development of shale gas fields, and pyrolysis can recover harmful components for resource recycling. This study evaluates the pyrolysis behavior through the study of pyrolysis kinetics and analyzes the impact of pyrolysis temperature on the yield of gaseous products, the quality of liquid products, and the migration of heavy metals in the solid phase. The results indicate that the pyrolysis of OBDC is divided into three stages, with the activation energy increasing as the reaction progresses. Moreover, the temperature at which the maximum weight loss rate occurs under high heating rates is higher. The four typical methods selected (FM, OFW, KAS, and Starink) all provide excellent fits to the weight loss curves. The pyrolysis temperature significantly affects the distribution of products; as the temperature rises, the total gas yield increases to varying degrees, the liquid phase undergoes degradation, resulting in the formation of new complex substances, and the solid phase content gradually increases. Additionally, after thermal treatment at corresponding times, the content of coke and the recovery rate of mineral oil in OBDC change differently. The heavy metals Pb, Zn, Mn, and Cr in the coke show the same trend in content and residual rate after pyrolysis treatment, with Cr, Cu, and Zn being enriched and fixed in the coke, indicating that pyrolysis has a certain effect on the stabilization of heavy metals in oil-based drill cuttings. This information on the solid products generated from the pyrolysis of industrial OBDC alerts future researchers to pay attention to the final disposal of the entire technical output.
Similar content being viewed by others
Introduction
As an efficient unconventional energy, shale gas has been considered by many countries as one of the most promising alternative energy sources in the 21st century due to its large storage capacity, high energy utilization rate and clean combustion1,2. However, with the burgeoning development of shale gas exploration and extraction, the environmental issues it brings cannot be overlooked, among which the most significant is the massive generation of oil-based drill cuttings (OBDC).
OBDC are solid-phase materials composed of heteroatoms, heavy metals, separated cuttings, and drilling fluids3. They contain a variety of harmful pollutants, including high concentrations of soluble salts, organic matter, and heavy metals4, which can have a severe impact on local ecosystem. Estimates suggest that the annual production of OBDC in China currently exceeds 3 million tons5. On the other hand, OBDC, with its complex composition and high oil content, holds significant recyclable value6. Technologies for the treatment and utilization of OBDC include landfilling, solidification, and incineration7,8. Traditional landfilling techniques pose potential pollution risks to the subsurface environment and lack economic benefits. Solidification methods do not effectively recycle petroleum hydrocarbon resources and have high investment and operational costs. Pyrolysis technology is considered an efficient and feasible treatment method for industrial scale due to its thorough pollutant disposal, high resource utilization rate, and stable operation9,10. Additionally, thermogravimetric analysis (TGA) is commonly used to study the pyrolysis process of various oil sludges, and the assessment of pyrolysis kinetic parameters for OBDC is also deemed most suitable11. Existing research has predominantly focused on the pyrolysis of petroleum refinery sludge and coal tar residues12,13. However, studies on the pyrolysis patterns and product characteristics of shale-gas oil-based drill cuttings are scarce. This scarcity limits the precise control of the pyrolysis process. Therefore, thoroughly investigating the pyrolysis-conversion patterns and behaviors of oil-based drill cuttings is of great significance. It can effectively remove harmful substances and fully utilize the decomposition products.
OBDC pose significant environmental hazards due to their composition, yet they also hold considerable value for recycling. Pyrolysis technology stands out as one of the most reliable and widely applied methods under integrated conditions. By accurately calculating thermodynamic and kinetic parameters through various methods, the quality and key properties of the pyrolysis products have been examined, and the migration characteristics of heavy metals in the residual char have been analyzed and evaluated. The examination of thermodynamic and kinetic parameters is instrumental in elucidating the pyrolysis behavior of industrial OBDC. This analysis not only deepens the comprehension of the pyrolytic process but also furnishes essential knowledge for environmental impact assessments. Additionally, it serves as a basis for delineating strategies aimed at maximizing the industrial value and ensuring the safety and eco-friendliness of both OBDC and its pyrolyzed products.
Materials and methods
Materials and experiments
The oil-based drilling cuttings samples were collected from the Fuling shale area of Chongqing, SINOPEC. The OBDC was dark viscous solid waste.
Material characterization
The pH of OBDCs was determined in accordance with the standard procedure for assessing corrosivity in solid wastes using a glass electrode, as outlined in GB/T 15555.12-199514. The slag content was quantified adhering to the “Industrial Analysis of Coal” protocols. Moisture content was evaluated using distillation methods, while petroleum substance concentration was ascertained through Soxhlet extraction followed by analysis with infrared spectrophotometry. Elemental carbon and hydrogen were quantified using the Vario EL III organic elemental analyzer (absolute error < 0.1%) from Elemeraor, Germany. Chlorine concentration in the OBDCs was measured via the Eschka mixture fusion-sample-potassium thiocyanate titration method, following the “Standard Test Method for Chlorine in Coal”. Sulfur content was evaluated using a similar Eschka method, as per the “Standard Test Method for Total Sulfur in Coal” (China)15. The inorganic mineral composition was elucidated through X-ray diffraction (XRD) and X-ray fluorescence spectroscopy (XRF) analyses. Proximate analysis were determined following the ASTM D3172 standard. The properties of the experimental samples are detailed in Table 1.
Experimental equipment and process
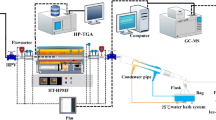
Pyrolysis trials were executed within the confines of a fixed-bed apparatus under ambient pressure, as delineated in Fig. 1. The pyrolysis experimental system is composed of key components including a gas supply module, a fixed-bed reactor, a distillation condensation system, and a product collection system. The OBDC samples were mixed uniformly and filtered. Upon reaching the experimental temperature, 20 g of the filtered sample was placed into a porcelain crucible that had been cleaned with chloroform, then pushed into the center of a corundum tube and maintained for 50 min, with a nitrogen flow rate of 0.5 L/min maintained throughout the pyrolysis process. Gases produced during the pyrolysis were collected using gas bags for further characterization. Liquid products were obtained through an oil collection apparatus. After each experiment, the residual material was meticulously gathered and its mass determined, categorizing it as the solid phase.
Utilizing the American TA-SDTQ600 synchronous thermal analyzer (temperature accuracy of 0.2 °C with a calorimetric error of ± 2%), thermogravimetric analysis (TGA) was conducted to delineate the pyrolytic behavior of OBDC. The experimental protocol involved the precise weighing of 15 mg of OBDC for each run, within an environment controlled by a nitrogen flow of 50 mL/min. The temperature profile spanned from room temperature to 900 °C, establishing a baseline for thermal decomposition analysis. Post-data acquisition, the recorded TGA traces were subjected to further analytical procedures to elucidate the thermal characteristics of the OBDC samples. After obtaining the experimental recording data, further analysis was carried out.
Product analysis
The analysis of gaseous products, including methane CH4, carbon dioxide CO2, hydrogen H2, and ethylene C2H4, was conducted employing a gas chromatography-mass spectrometry system (GC-MS). The quantification of oil content in the coke matrix adhered to the guidelines set forth by the Chinese standard (HJ 1051–2019). For the characterization of pyrolytic mineral oils, the C6 ~ C9 hydrocarbons were evaluated following US EPA 8260 C, while the C10 ~ C14, C15 ~ C28, and C29 ~ C36 hydrocarbons were assessed according to US EPA 8015 C. Heavy metal concentrations within the OBDC, including chromium (Cr), copper (Cu), lead (Pb), zinc (Zn), manganese (Mn), and nickel (Ni), were determined utilizing atomic absorption spectrophotometry with a Shimadzu AA-6300 C instrument. Concurrently, mercury levels were quantified by cold vapor atomic absorption analysis using the SHIMADZU MVU-1 A instrument.
Pyrolysis kinetic equation
Basic dynamic equations
The thermal decomposition of OBDC entails intricate thermochemical transformations. In essence, the pyrolytic reactions of solid waste, such as OBDC, can be broadly represented by a generalized formula that encapsulates the collective behavior of the system during pyrolysis. This formula can be expressed as:
The pyrolysis or combustion kinetics of the same conversion rate \(\alpha\) at different heating rates can be expressed as Eqs. (2)–(6) :
Where w0 represents the initial mass of the reactant in grams, g; wt indicates the mass at a specific time of the process, also in grams, g; \({w_\infty }\)is final mass after reaction, g; k is the reaction rate constant determined by Arrhenius law; A is the pre-exponential factor, min− 1; \(f(\alpha )\) is the correlation function of the reaction mechanism; \({E_\alpha }\) represents the activation energy of the reaction in joules per mole (J/mol), R denotes the ideal gas constant in joules per mole-kelvin (J/(mol K)), and T signifies the temperature in kelvins (K).
Selection of mechanism function
Equation 6 represents the fundamental integral form of kinetic analysis in thermoanalysis, which does not have an exact analytical solution in mathematics. Different methods can yield different model functions. Typically, thermokinetic analysis can be divided into model fitting methods and iso-conversional methods. The model fitting method, due to its inherent limitations, cannot reflect the complex nature of solid-state reactions16. Iso-conversional methods refer to the approach of conducting thermokinetic analysis on multiple thermogravimetric curves obtained at different heating rates. By employing repeated temperature scanning techniques, they reveal the complex nature of multi-step reactions behind the seemingly simple process, allowing for the acquisition of a more reliable activation energy \({E_\alpha }\). The principle of iso-conversionality lays the foundation for iso-conversional methods, which are commonly divided into two forms: integral and differential.
In this study, four iso-conversional methods were selected for analysis: Ozawa–Flynn–Wall (OFW) and the Kissinger–Akahira–Sunose (KAS) are typical integral methods17,18. They do not rely on specific reaction mechanism models and can be applied to various complex reaction systems. The Friedman (FR) and Starink methods, due to their differential nature, offer flexibility in data processing and also have better noise resistance capabilities. Combining these four iso-conversional methods allows for a more comprehensive and in-depth understanding of the pyrolysis behavior of OBDC. Their mathematical representations are delineated by the Eqs. (7)–(10):
Ozawa–Flynn–Wall (OFW):
Kissinger–Akahira–Sunose (KAS):
Friedman (FR):
Starink:
By incorporating the reaction kinetics from the pyrolysis mechanism into the thermogravimetric (TG) data, various models enable curve fitting and subsequent calculations. The activation energy \({E_\alpha }\), can be compared and obtained from the linear plot through the linear equation Y = aX + b. where a is the slope and b is the intercept of the line.
Results and discussion
Pyrolysis kinetic analysis
Based on the four iso-conversion methods established above, this section first analyzes the pyrolysis stages of OBDC through TG-DTG curves, and then estimates the pyrolysis activation energy of OBDC using the four iso-conversion methods, so as to deeply analyze the pyrolysis kinetics characteristics of OBDC.
TG-DTG analysis
Thermogravimetric analysis is a technique employed to investigate the thermal stability of materials, ascertain the thermodynamic parameters of various stages of pyrolysis, and thereby analyze a series of complex reactions in OBDCs. Throughout the process of thermogravimetric analysis, we adhered to the same experimental methodologies and conditions as those employed in prior research, ensuring the comparability of the data. The TG-DTG curves presented in Fig. 2 not only corroborate the findings of our previous studies but also, through detailed analysis under different heating rates, further elucidate the weight loss behavior of OBDC during the pyrolysis process. The curves distinctly illustrate the progression of weight loss in relation to temperature as the sample is heated from room temperature to 900 °C, with heating rates of 10, 20, and 50 °C per minute. From the figure, it is evident that elevating the heating rate displaces the TG and DTG curves to higher temperature zones. This displacement is likely due to thermal inertia stemming from constraints in heat transfer and medium diffusion—a finding consistent with observations in earlier research19. The weight loss trends of OBDC at different heating rates are generally consistent, with the maximum weight loss rate increasing as the heating rate is elevated. With the increase in temperature, the thermal weight loss of OBDC can be divided into three stages based on the TG-DTG curve trends: below 350 °C (Stage I), between 350 and 550 °C (Stage II), and between 550 and 900 °C (Stage III).
The stage I is characterized by rapid weight loss, predominantly attributed to the rapid evaporation of moisture and volatile hydrocarbons. At heating rates of 10 °C/min and 20 °C/min, there are less pronounced fluctuations, which may be attributed to the slow depolymerization of molecules with non-covalent bonds in large molecular substances20. The stage II is characterized by a gradual weight loss, with a deceleration in the rate of mass reduction relative to the initial phase. This is mainly due to the pyrolysis of mineral oil alkanes in OBDC, with principal components including alkanes, aromatic hydrocarbons, resins, asphaltenes, and a small amount of carboxylic acids, ketones, or aldehydes formed through maturation processes21. The dynamic analysis was selected in the stage II. The stage III is characterized by a gradual weight loss, ranging from approximately 10.6–12.9%. This phase is predominantly defined by the pyrolytic breakdown and carbonization of complex hydrocarbons, along with the decomposition of inorganic minerals. These hydrocarbons, typically viscous and solid at room temperature, contribute to the waxy nature of OBDC. Interestingly, the TG curve exhibits a peak during the third stage, which can be reasonably explained by the initiation of secondary cracking of complex organic and inorganic substances as the temperature rises22, with higher heating rates intensifying this process. The residual mass loss above 900 °C is due to the decomposition of stable inorganic minerals and the gasification of residual carbon. These processes generate a small amount of gas, causing the curve to show a gradual downward trend.
Assessment of activation energy
Building on the foundation of thermogravimetric analysis, this study employs four isoconversional methods (Friedman, OFW, Starink and KAS) to estimate the Eα of the reaction. The study selected nine distinct conversion rates, α, ranging from 0.1 to 0.9, and, in conjunction with the three stages of the pyrolysis process described in the preceding text, conducted an in-depth analysis of the entire pyrolysis process. The linear fitting results of the different isoconversional methods at each conversion rate are presented in Fig. 3.
Activation energy represents the minimum energy threshold that must be reached for a chemical reaction to initiate. Generally, the higher the activation energy, the slower the reaction rate and the more difficult it is to start the reaction. In this study, after a meticulous analysis of the thermogravimetric analysis data, we divided the pyrolysis process into three stages and performed calculations using predictive functions. Subsequently, by estimating the slope of the fitting curves, we calculated the Eα for different stages, as shown in Table 2. Observations reveal that as the pyrolysis stage progresses, the Eα values obtained by the four iso-conversional methods (FR, OFW, KAS, and Starink) all exhibit an increasing trend. The pyrolysis process of OBDC is a complex one that includes parallel reactions, competitive reactions, and consecutive reactions, with the Eα values being closely related to the pyrolysis reaction mechanism. The specific ranges of Eα values are: for the FR method, 161.0 kJ/mol to 203.5 kJ/mol; for the OFW method, 157.6 kJ/mol to 193.3 kJ/mol; for the KAS method, 142.3 kJ/mol to 203.5 kJ/mol; and for the Starink method, 142.9 kJ/mol to 184.2 kJ/mol. Under the three stages, the correlation coefficient R2 values obtained by the four methods range from 0.98 to 0.99, demonstrating a high degree of fit with the experimental data, thereby validating the reliability of the methods employed.
Activation energy (Eα) and correlation coefficients (R2) for OBDC pyrolysis stages. Eα increases with stage progression (Stage I < III), with FR yielding the highest and Starink the lowest values. The variation in the average Eα results obtained from different models is not significantly pronounced. The Eα estimated using the Friedman method is slightly higher than that from other methods, likely due to its relationship with the simple differential form of the kinetic rate law. The average activation energies estimated by the Kissinger–Akahira–Sunose (KAS) and Starink methods are remarkably close, with values of 166.2 kJ/mol and 166.0 kJ/mol, respectively. These methods exhibit insensitivity to data noise, demonstrate high precision and reliability in activation energy estimation, and are particularly suitable for complex reaction systems [0]. The results suggest that they may be more applicable to the pyrolysis reaction of OBDC23. The results derived from these estimates may be more applicable to the pyrolysis reactions of OBDC. The variation in OBDC’s Eα with temperature reveals the unique thermal response mechanism of organic–inorganic composite pollutants in shale gas drill cuttings. This finding offers a theoretical basis for developing a staged temperature–controlled pyrolysis process.
Analysis of pyrolysis OBDC production
Based on the energy barrier characteristics revealed by kinetic analysis, this paper further conducts a multi-dimensional analysis of the gaseous products’ composition, oil component distribution, and solid residue characteristics. It systematically assesses the environmental risks and resource value of pyrolysis products. Figure 4 is the distribution of products corresponding to different treatment temperatures of thermal desorption.
Figure 4 illustrates the distribution of pyrolysis products, highlighting that the solid phase consistently predominates, with char as the principal component. This phase’s proportion declines as the target temperature rises, with a marked decrease from 88.2 at 250 to 73.6% at 500 °C. This trend matches the weight loss in TGA curves, indicating the coke yield drop is mainly due to the phased release of volatile components. This trend suggests that at elevated temperatures, the core temperature of the material aligns closely with the set point, enhancing heat transfer and the volatilization of oil into gas24. Additionally, the increased temperature promotes the decomposition of heavier, non-volatile components, contributing to a more thorough removal of organic pollutants. Beyond 400 °C, the gas phase yield plateaus at approximately 4.4%, with a subsequent increase due to secondary cracking reactions that generate smaller molecular weight gases, elevating the overall gas yield. Meanwhile, the weight loss zones of this–stage TGA curve’s weight loss peak largely overlap, further confirming that oil–phase generation mainly comes from the pyrolysis of large–molecule substances. It can be observed that at a temperature of 500 °C, there is a diminution in the proportion of the oil phase and a concomitant augmentation in the proportion of the solid phase. This may be due to the polymerization of pyrolyzed oil into larger molecules. These high molecular weight compounds may be adsorbed into the pores of the solid phase25, thereby increasing the solid phase content.
Gas phase analysis
Figure 5 shows the effect of pyrolysis temperature on gas production. The main gaseous products including CH4, CO2, H2, and C2H4 in the pyrolysis process of oil-based drilling cuttings were recorded.
In general, when the temperature increases from 400 to 550 °C, the total gas yield shows different degrees of improvement; this may be due to the cracking, dehydrogenation, decarboxylation, decarbonylation, and other reactions at high temperatures, which lead to the cracking of long-chain hydrocarbons into short-chain hydrocarbons. Specifically, with the increase of temperature, the C–H cracking process of organic matter in oil-based drilling cuttings produces H2, and its yield increases with the increase of temperature, and the content is very low at lower temperatures. This may be due to the aromatization and secondary cracking of volatiles26. The effect is enhanced with increasing temperature27. The yield of light hydrocarbons (C1–C3) escalates as the temperature rises, predominantly attributed to the cleavage of C–C bonds28. With the increase in temperature, methoxyl ( AOCH3), methyl (ACH3), and methylene (ACH2A) in OBDC decompose to produce CH429. The formation of CO2 is mainly due to the cleavage of the carbonyl and carboxyl groups inherent in aliphatic and aromatic hydrocarbons30,31, while the carbonyl and carboxyl groups are unstable at high temperatures.
Oil phase analysis
The mineral oil of the second-stage experimental group in the product was analyzed, and the results are shown in Fig. 6.
In the pyrolytic decomposition of OBDC, observable alterations within the hydrocarbon spectrum were noted. A significant reduction of 3.26% within the C15 to C28 hydrocarbons was juxtaposed with a 2.89% increase in the lighter C10 to C14 hydrocarbons, indicative of the pyrolytic cleavage of heavier species and the volatilization of lighter ones. Concurrently, an observed increase in the concentration of heavier hydrocarbons, C29 to C36, suggests a thermal maturation process, where higher temperatures induce the formation of more complex hydrocarbons32. The PAHs underwent a compositional transformation, shifting from lower to higher ring structures, particularly enriching 4-ring configurations post-pyrolysis. This is attributed to the selective volatilization of lower molecular weight PAHs, enhancing the medium to high-ring PAHs in the residue33,34. The PAH distribution is intricately linked to the thermodynamic conditions of pyrolysis, with temperature playing a pivotal role in determining the molecular architecture of the pyrolyzed OBDC. This highlights the necessity for precise temperature management to control pyrolytic outcomes and hydrocarbon distribution.
The GC-MS analysis was performed on pre- and post-pyrolysis mineral oils. Comparative analysis of the Fig. 7 indicates that the compositional differences between the mineral oils in OBDCs and the recovered mineral oils are minimal, suggesting that, to a certain extent, recovered mineral oils can serve as substitutes for diesel fuel35. Nonetheless, within the context of the high-temperature treatment regime, there is a progressive elevation in the concentration of halogenated hydrocarbons. Concurrently, there is a diminution in the quantity of monocyclic aromatic hydrocarbons, and an augmentation in the diversity of polycyclic aromatic hydrocarbons (PAHs), albeit with a relatively marginal increment in their proportion15. This is mainly due to the high-temperature reaction process facilitating reactions that lead to cyclization and polymerization, resulting in the formation of new complex aromatic compounds.
Solid phase analysis
The residual oil content in drill cuttings exhibits a marked reduction, and post-attaining a temperature of 350 °C, the decrement rate of the oil content stabilizes, aligning with the outcomes observed in thermogravimetric analysis. Additionally, as the liquid phase of OBDC continuously volatilizes, the thermal conductivity of the oil-containing solid phase also decreases, leading to a stabilization in the oil content of the residue36. The oil content in the char is crucial for the treatment and disposal of the char. As depicted in Fig. 8, the graphical data delineate the fluctuating ash oil content and oil recovery rates for OBDC over a range of temperatures and heating periods. Upon subjecting the material to thermal processing for the designated intervals, the residual oil content within the char is found to be below the threshold of 0.3%, which can be considered as solidified37.
The order of heavy metal content in OBDC is: Mn > Zn > Pb > Cr > Ni > Cu > Hg (Table 3). Owing to the inherent instability of mercury, it was undetected in the char. Moreover, the levels of all heavy metals in both OBDC and coal char were found to be below the regulatory limits set by the national standard (CJ/T 362–2011, China).
The bio-safety and mobility of heavy metals were assessed through the analysis of their detection levels and residual rates (RR). Where RR is defined as the equation:
where the variables c1 and c2 correspond to the quantities of heavy metals present in OBDC and the resulting char, respectively, mg/kg; refers to the percentage yield of char obtained from the pyrolysis process, %. The recovery rate (RR), which is a measure of the concentration of heavy metals in the char relative to their initial content in OBDC, varies between 0 and 100%. It is noteworthy that an increased RR is indicative of a higher degree of heavy metal enrichment within the char.
As shown in Fig. 9, in general, the content of most heavy metal elements after pyrolysis has increased to varying degrees due to enrichment, with a systematic upward trend as temperature rose, and the content of most metal elements has increased with the increase in temperature. This result is consistent with the conclusion before38,39.This is because metal oxides form dense microparticles to encapsulate heavy metals during pyrolysis, which promotes the solidification of heavy metals40. Specifically, pyrolysis has the greatest impact on Cu retention capacity, with RR increasing from 76.98 to 90.81%. Followed by Cr, from 80.17 to 92.34%. In contrast, Mn content and residual rate stayed stable across temperatures, likely because Mn forms oxide-bound complexes with other metal ions or precipitates as hydroxides, exhibiting stable chemical properties due to strong ionic bonds and high specific surface area41. Because of its strong ionic bond and high specific surface area, the chemical properties are relatively stable. Cu and Mn residual rates displayed non-monotonic behavior with temperature changes: Cu’s residual rate dipped briefly at 300–400 °C before rebounding, while Mn’s fluctuations stayed within the error margin. This is because, as temperature climbs, corresponding heavy metal ion compounds crack and react with original components, reducing content42,43. Moreover, Ni content and residual rate declined with temperature, with the downward slope matching the volatility of Ni in the literature44. The sequestration and immobilization of chromium Cr, copper Cu, and Zn within the char suggest that pyrolysis serves as a mechanism for the stabilization of heavy metals present in OBDC. This process is indicative of the pyrolytic treatment’s efficacy in mitigating the mobility and environmental impact of these potentially harmful elements45. However, the enrichment rates of Pb and Ni are lower and are susceptible to leaching due to temperature effects, which warrants attention as they may pose certain environmental risks.
Conclusion
This work systematically investigated the pyrolysis kinetics and product characteristics of OBDC. The pyrolysis process exhibited three distinct stages: rapid, moderate, and slow weight loss, where increased heating rates reduced total weight loss due to shortened reaction duration inhibiting complete conversion. Four kinetic methods (FR, OFW, KAS and Starink) demonstrated excellent linear fitting of thermogravimetric data, with correlation coefficients R2 exceeding 0.98 within the conversion range of 0.1–0.9. The average activation energies estimated by the KAS and Starink methods are very close, at 166.2 kJ/mol and 166.0 kJ/mol respectively, with a significant increase in the activation energy gradient as conversion rises. Pyrolysis temperature greatly affects product distribution: the main product is solid (over 70%). Gas yield rises with temperature, peaking at 550 °C. The liquid product yield also changes qualitatively with temperature, generating new complex aromatics. Coke analysis shows residual oil below 0.3%, with heavy metals stabilizing differently. Cr, Cu, and Zn are fixed in coke, while Pb and Ni have lower enrichment rates due to temperature effects, posing potential leaching and environmental risks.
The universal framework based on iso-conversation kinetics can be extended to thermo-chemical conversion systems of oily solid wastes like oily sludge and waste lubricating oil residue. Applying this method in multi-phase reactors enables quantitative assessment of energy and mass transfer, offering a technical path to synergistically optimize energy recovery efficiency and product risk control in solid waste thermo-chemical processes. Information from solid product analysis highlights the importance of focusing on TPHs and heavy metal residues and migration in future engineering. This should be considered in harmless treatment and utilization to achieve clean-processing of OBDC pyrolysis.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Hu, Y., Mu, S., Zhang, J. & Li, Q. Regional distribution, properties, treatment technologies, and resource utilization of oil-based drilling cuttings: a review. Chemosphere https://doi.org/10.1016/j.chemosphere.2022.136145 (2022).
Zhou, Y. et al. Engineering design and application of large-scale oil-based drilling cuttings treatment project. Waste Manag. Res. J. Sustain. Circ. Econ. 43(2), 282–292. https://doi.org/10.1177/0734242X241231393 (2025).
Xiong, D. & Wang, C. Risk assessment of human exposure to heavy metals, polycyclic aromatic hydrocarbons, and radionuclides in oil-based drilling cutting residues used for roadbed materials in Chongqing, China. Environ. Sci. Pollut. Res. Int. https://doi.org/10.1007/s11356-021-13871-0 (2021).
Ytrehus, J. D., Lund, B., Taghipour, A. & Saasen, A. Cuttings transport with oil- and water-based drilling fluids1. J. Energy Res. Technol. https://doi.org/10.1115/1.4063838 (2024).
Xia, Z. et al. Co-pyrolysis of waste Polyvinyl chloride and oil-based drilling cuttings: Pyrolysis process and product characteristics analysis. J. Clean. Prod. 318, 128521. https://doi.org/10.1016/j.jclepro.2021.128521 (2021).
Adhami, S., Jamshidi-Zanjani, A. & Darban, A. K. Remediation of oil-based drilling waste using the electrokinetic-Fenton method. Process. Saf. Environ. Prot. 149, 432–441. https://doi.org/10.1016/j.psep.2020.11.018 (2021).
Chen, D. et al. Co-pyrolysis of light bio-oil leached bamboo and heavy bio-oil: Effects of mass ratio, pyrolysis temperature, and residence time on the Biochar. Chem. Eng. J. 437, 135253. https://doi.org/10.1016/j.cej.2022.135253 (2022).
Feng, Y. et al. Microwave-combined advanced oxidation for organic pollutants in the environmental remediation: An overview of influence, mechanism, and prospective. Chem. Eng. J. 441, 135924. https://doi.org/10.1016/j.cej.2022.135924 (2022).
Hu, G. et al. Life cycle assessment of low-temperature thermal desorption-based technologies for drill cuttings treatment. J. Hazard. Mater. 401, 123865. https://doi.org/10.1016/j.jhazmat.2020.123865 (2021).
Hu, G. et al. Low-temperature thermal desorption and secure landfill for oil-based drill cuttings management: Pollution control, human health risk, and probabilistic cost assessment. J. Hazard. Mater. https://doi.org/10.1016/j.jhazmat.2020.124570 (2020).
Qu, Y., Li, A., Wang, D., Zhang, L. & Ji, G. Kinetic study of the effect of in-situ mineral solids on pyrolysis process of oil sludge. Chem. Eng. J. 374, 338–346. https://doi.org/10.1016/j.cej.2019.05.183 (2019).
Wang, A. et al. Catalytic hydroconversion of extraction portion from coal Tar residue to clean liquid fuel over a Ni based ZSM-5/PC derived from coal gasification fine slag. J. Anal. Appl. Pyrol. 180, 106542. https://doi.org/10.1016/j.jaap.2024.106542 (2024).
Hasan, A. M. A., Kamal, R. S., Farag, R. K. & Abdel-Raouf, M. E. Petroleum sludge formation and its treatment methodologies: A review. Environ. Sci. Pollut. Res. Int. 31 (6), 8369–8386. https://doi.org/10.1007/s11356-023-31674-3 (2024).
Li, J. et al. Investigation on the co-pyrolysis of agricultural waste and high-density polyethylene using TG-FTIR and artificial neural network modelling. Process. Saf. Environ. Prot. 160, 341–353. https://doi.org/10.1016/j.psep.2022.02.033 (2022).
Liu, P., Xiao, Q., Dai, N., Liu, Z. & Wang, C. Study on pyrolysis of shale gas oil-based drilling cuttings: Kinetics, process parameters, and product yield. ACS Omega. https://doi.org/10.1021/acsomega.2c07379 (2023).
Vengosh, A., Jackson, R. B., Warner, N., Darrah, T. H. & Kondash, A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ. Sci. Technol. 48(15), 8334–8348. https://doi.org/10.1021/es405118y (2014).
Chen, F. et al. Investigation of pyrolysis kinetics, thermodynamics, product characteristics and reaction mechanism of rubber seed oil. Energy Convers. Manag. 244, 114535. https://doi.org/10.1016/j.enconman.2021.114535 (2021).
Mong, G. R. et al. Pyrolysis of waste activated sludge from food manufacturing industry: Thermal degradation, kinetics and thermodynamics analysis. Energy (Oxf). 235, 121264. https://doi.org/10.1016/j.energy.2021.121264 (2021).
Mishra, R. K. & Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 251, 63–74. https://doi.org/10.1016/j.biortech.2017.12.029 (2018).
Yu, H. et al. Pyrolysis/combustion potential and heavy metal risk of oily sludge and derived products in industrial scale. Fuel (Lond). 344, 128044. https://doi.org/10.1016/j.fuel.2023.128044 (2023).
Singh, B. & Kumar, P. Physicochemical characteristics of hazardous sludge from effluent treatment plant of petroleum refinery as feedstock for thermochemical processes. J. Environ. Chem. Eng. 8(4), 103817. https://doi.org/10.1016/j.jece.2020.103817 (2020).
Leng, L. et al. Insights into glycine pyrolysis mechanisms: Integrated experimental and molecular dynamics/DFT simulation studies. Fuel (Lond). 351, 128949. https://doi.org/10.1016/j.fuel.2023.128949 (2023).
Tagade, A., Kandpal, S. & Sawarkar, A. N. Insights into pyrolysis of Pearl millet (Pennisetum glaucum) straw through thermogravimetric analysis: Physico-chemical characterization, kinetics, and reaction mechanism. Bioresour.Technol. 391, 129930. https://doi.org/10.1016/j.biortech.2023.129930 (2024).
Liu, W. et al. Promoting the resource utilization of oil-based drilling cuttings pyrolysis residues in cement products. Constr. Build. Mater. 373, 130886. https://doi.org/10.1016/j.conbuildmat.2023.130886 (2023).
Zalazar-Garcia, D. et al. Exergo-ecological analysis and life cycle assessment of agro-wastes using a combined simulation approach based on Cape-Open to Cape-Open (COCO) and SimaPro free-software. Renew. Energy 201, 60–71. https://doi.org/10.1016/j.renene.2022.10.084. (2022).
Song, Q. et al. Characterization of the products obtained by pyrolysis of oil sludge with steel slag in a continuous pyrolysis-magnetic separation reactor. Fuel (Lond). 255, 115711. https://doi.org/10.1016/j.fuel.2019.115711 (2019).
Kazmi, B. et al. Techno-economic assessment of sunflower husk pellets treated with waste glycerol for the bio-hydrogen production–a simulation-based case study. Fuel (Lond). 348, 128635. https://doi.org/10.1016/j.fuel.2023.128635 (2023).
Lv, Q. et al. Pyrolysis of oil-based drill cuttings from shale gas field: Kinetic, thermodynamic, and product properties. Fuel (Lond). 323, 124332. https://doi.org/10.1016/j.fuel.2022.124332 (2022).
Kazemi, S., Alavi, S. M., Rezaei, M. & Akbari, E. Preparation of the NiO/FeAl2O4 nanopowders and their application in CH4 thermocatalytic decomposition to produce high purity of hydrogen: Optimization of the synthesis parameters using Taguchi design of experiment. Int. J. Hydrogen Energy. 51, 1429–1449. https://doi.org/10.1016/j.ijhydene.2023.07.332 (2024).
Deng, S. et al. Investigation on the fast co-pyrolysis of sewage sludge with biomass and the combustion reactivity of residual char. Bioresour. Technol. 239, 302–310. https://doi.org/10.1016/j.biortech.2017.04.067 (2017).
Zhuofan, Z., Junhu, Z., Jinlong, W., Jianzhong, L. & Weijuan, Y. Pyrolytic gas analysis and evaluation from thermal plasma pyrolysis of simulated oil-based drill cuttings. Waste Dispos. Sustain. Energy. https://doi.org/10.1007/s42768-023-00153-3 (2023).
Liu, H., Li, J., Zhao, M., Li, Y. & Chen, Y. Remediation of oil-based drill cuttings using low-temperature thermal desorption: Performance and kinetics modeling. Chemosphere 235, 1081–1088. https://doi.org/10.1016/j.chemosphere.2019.07.047 (2019).
Guida, P. et al. Chemical kinetics of asphaltene pyrolysis. Energy Fuels 35(10), 8672–8684. https://doi.org/10.1021/acs.energyfuels.0c04358 (2021).
Guo, W., Zhang, X., Sun, Y., Li, Q. & Liu, Z. Migration mechanism of pyrolysis oil during oil shale in situ pyrolysis exploitation. Energy (Oxf). 285, 128769. https://doi.org/10.1016/j.energy.2023.128769 (2023).
Chen, G. et al. Nitrogen, sulfur, Chlorine containing pollutants releasing characteristics during pyrolysis and combustion of oily sludge. Fuel (Lond). 273, 117772. https://doi.org/10.1016/j.fuel.2020.117772 (2020).
Zhao, T. et al. Comparative analysis of seven machine learning algorithms and five empirical models to estimate soil thermal conductivity. Agric. Meteorol. 323, 109080. https://doi.org/10.1016/j.agrformet.2022.109080 (2022).
Robinson, J. P. et al. Scale-up and design of a continuous microwave treatment system for the processing of oil-contaminated drill cuttings. Chem. Eng. Res. Des. 88(2), 146–154. https://doi.org/10.1016/j.cherd.2009.07.011 (2010).
Adnan, M. et al. Heavy metals pollution from smelting activities: A threat to soil and groundwater. Ecotoxicol. Environ. Saf. 274, 116189. https://doi.org/10.1016/j.ecoenv.2024.116189 (2024).
Fulke, A. B., Ratanpal, S. & Sonker, S. Understanding heavy metal toxicity: Implications on human health, marine ecosystems and bioremediation strategies. Mar. Pollut. Bull. 206, 116707. https://doi.org/10.1016/j.marpolbul.2024.116707 (2024).
Koyama, H., Kamogashira, T. & Yamasoba, T. Heavy metal exposure: Molecular pathways, clinical implications, and protective strategies. Antioxidants (Basel). 13(1), 76. https://doi.org/10.3390/antiox13010076 (2024).
Wang, T. et al. Co-combustion behavior of dyeing sludge and rice husk by using TG-MS: Thermal conversion, gas evolution, and kinetic analyses. Bioresour. Technol. 311, 123527. https://doi.org/10.1016/j.biortech.2020.123527 (2020).
Swain, C. K. Environmental pollution indices: A review on concentration of heavy metals in air, water, and soil near industrialization and urbanisation. Discover Environ. https://doi.org/10.1007/s44274-024-00030-8 (2024).
Ali, H., Khan, E. & Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 1–14. https://doi.org/10.1155/2019/6730305 (2019).
Zhang, H. et al. Microwave-assisted pyrolysis of textile dyeing sludge, and migration and distribution of heavy metals. J. Hazard. Mater. 355, 128–135. https://doi.org/10.1016/j.jhazmat.2018.04.080 (2018).
Soria, J., Gauthier, D., Flamant, G., Rodriguez, R. & Mazza, G. Coupling scales for modelling heavy metal vaporization from municipal solid waste incineration in a fluid bed by CFD. Waste Manag. 43, 176–187. https://doi.org/10.1016/j.wasman.2015.05.021 (2015).
Author information
Authors and Affiliations
Contributions
P. L. conceptualization, methodology writing; The first draft of the manuscript was written by W.L. Software, analysis and funding acquisition formed by T. R.F.; Conceptualization, review formed by Z.B. L. B. Z., supervision, funding acquisition; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, P., Li, W., Tan, R. et al. Investigation of pyrolysis behavior shale gas oil-based drilling cuttings kinetics and product characteristics. Sci Rep 15, 19775 (2025). https://doi.org/10.1038/s41598-025-04640-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04640-x