Abstract
Peripheral amyloid-beta (Aβ), including those in bloodstream and intestine, is implicated in the pathogenesis of Alzheimer’s disease (AD). Additionally, diabetes and insulin resistance are associated with the impairment of Aβ metabolism and an increased risk of developing cognitive decline and AD. Previous research has demonstrated the neuroprotective effects of cannabidiol (CBD) and S-allyl-cysteine (SAC); however, their impact on Aβ levels in the context of diabetes remains unexplored. 5-week-old diabetic db/db mice underwent 23 weeks of dietary interventions with SAC, CBD, or a combination of SAC and CBD. Immunofluorescent imaging quantified enterocytic Aβ abundance and plasma concentrations of Aβ42, Aβ40, and Aβ oligomers were measured using ELISA. Our results reveal a progressive increase in enterocytic Aβ expression in db/db mice, which was mitigated by SAC administration. In contrast, CBD treatment alone and in combination with SAC, increased enterocytic Aβ abundance and elevated plasma Aβ42 concentrations. The combination therapy reduced Aβ oligomers. These findings suggest that the neuroprotective effects of SAC are partially mediated by its impact on peripheral Aβ levels. CBD could potentially exacerbate Aβ accumulation in diabetes.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is characterised by the accumulation of amyloid-beta (Aβ) peptides in the brain, leading to neurodegeneration and cognitive decline1,2. While cerebral Aβ deposition is a hallmark of AD, emerging evidence suggests that peripheral factors, particularly related to metabolic diseases such as diabetes, may play a critical role in accelerating AD pathology3,4,5. Type 2 diabetes has been strongly associated with an increased risk of cognitive decline and AD6, with multiple longitudinal studies linking mid-life insulin resistance and diabetes to increased cerebral amyloid load in cognitively normal individuals7,8,9.
The mechanisms by which diabetes enhances cerebral Aβ deposition are not fully understood. However, prior work, including our recent study in db/db mice, a model of type 2 diabetes, has demonstrated that diabetes is associated with elevated levels of Aβ in both the intestine and plasma10. These peripheral changes were accompanied by blood–brain barrier breakdown, suggesting that diabetes may promote the transport of Aβ from the periphery to the brain, contributing to the early stages of AD pathology. Importantly, these alterations occurred prior to detectable cognitive dysfunction, highlighting a potential window for intervention.
Given the link between diabetes and peripheral Aβ dynamics, targeting pathways that regulate Aβ synthesis and clearance in peripheral tissues, such as the gut and plasma, may offer novel strategies for delaying the progression to AD in diabetic populations. S-allyl cysteine (SAC), a bioactive component of garlic, and cannabidiol (CBD), a non-psychoactive compound derived from cannabis, have both demonstrated antioxidative, anti-inflammatory, and neuroprotective properties in preclinical models of AD11,12. Despite these promising effects on brain pathology, their ability to modulate peripheral Aβ homeostasis, particularly in the context of diabetes, remains largely unexplored.
This study aims to investigate the potential of SAC and CBD, individually or in combination, to regulate intestinal Aβ synthesis and plasma Aβ levels in db/db diabetic mice. By examining how these compounds influence peripheral Aβ dynamics, this work seeks to establish their therapeutic relevance in mitigating diabetes-related risk factors for AD progression.
Methods
Animals and experimental design
All experimental procedures in this study were approved by the Curtin Animal Ethics Committee (approval no. ARE 2018-19) and conducted in accordance with the ARRIVE guidelines13 and the Australian Code for the Care and Use of Animals for Scientific Purposes.
4-week-old male db/db and db/ + C57BLK/6 J mice were obtained from the Animal Resource Centre (Perth, WA) and housed at the Curtin Animal Facility under controlled temperature and a 12-h light/dark cycle. After one week of acclimation, 8–13 mice were randomly assigned to either a 6- or 14-week control group or a 28-week study group. The control group included both db/db and db/ + mice, with animals in the 6-week group euthanised for baseline tissue samples. The 28-week groups comprised control db/db and db/ + mice, as well as db/db mice fed diets containing SAC, CBD, or SAC + CBD starting at five weeks of age for 23 weeks.
The control mice were provided with AIN93M-based chow, while the intervention groups received AIN93M-based chow supplemented with one of the following: 0.04% w/w SAC (ChemScene, New Jersey, USA), 0.06% w/w CBD (Zelira Therapeutics, Perth, Western Australia), or a combination of 0.04% w/w SAC and 0.06% w/w CBD. CBD (> 99% purity) was solubilised in Miglyol 812N at a concentration of 14.5% prior to incorporation into the chow.
Based on the average daily food intake of db/db mice (5–6 g) and an average body weight of 48 g, the dietary concentrations corresponded to approximate dosages of 50 mg/kg SAC and 75 mg/kg CBD.
Sample collection and tissue preparation:
Mice were anaesthetised with isoflurane and blood samples were collected by cardiac puncture into ethylene-diamine-tetracetic acid-coated tubes. Plasma was separated by centrifugation for 10 min at 3000 rpm and 4 °C. The mice were then euthanised via cervical dislocation. A 2 cm segment of the proximal end of small intestine was carefully removed and fixed in 4% paraformaldehyde for a minimum of 24 h. Subsequently, the sample were processed and embedded in paraffin wax blocks, from which 5-μm thick sections were cut and mounted on silanised-coated slides for subsequent immunostaining.
Immunofluorescent detection of enterocytic Aβ and ApoB
The enterocytic abundance of Aβ was measured using immunofluorescent microscopy as previously described14. Briefly, tissue sections of 5-μm thickness were deparaffinised, rehydrated, and placed in boiling deionised water for 15 min to retrieve the antigen-binding sites. The sections were then permeabilised in a PBS-Tween-20 solution for 10 min and subsequently blocked with 20% goat serum for 1 h. The sections were then incubated overnight with rabbit anti-mouse Aβ (1–40/42) antiserum (1:200, Millipore) at 4 °C. Immunofluorescence was detected using Alexa546-labeled anti-rabbit IgG (1:100), and cell nuclei was stained with HOECHST (1:2000, ThermoFisher). Fluorescent images were captured using a Zeiss Axioscan Z1 slide scanner, and pixel intensity was quantified to assess Aβ abundance using Zeiss Zen Blue software.
Plasma Aβ analysis
Plasma concentrations of Aβ40, Aβ42, and Aβ oligomers were measured using commercially available ELISA kit (Wako, Japan) according to the manufacturer’s instructions. The measurements were calibrated against standards of Aβ40 (0–100 pmol/L), Aβ42 (0–20 pmol/L), and Aβ oligomers (0–100 pmol/L).
Measurement of plasma glucose, insulin, and triglyceride levels
Plasma glucose and insulin concentrations were measured using a Colorimetric Assay Kit (Cayman Chemical) and a Mouse Insulin ELISA Kit (Mercodia), respectively, according to the instructions provided by each manufacturer. Optical density measurements were taken using an EnSight Multimode Plate Reader, set at wavelengths of 500 nm for glucose and 450 nm for insulin.
Plasma triglyceride levels were evaluated using the Randox Colorimetric Kit (Randox Laboratories, UK) following the manufacturer’s guidelines. Briefly, 2 µL of either plasma samples or standard solutions were combined with 200 µL of reaction solution in a 96-well plate and incubated at 37 °C for 5 min. Absorbance was subsequently measured at 500 nm.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 9 (U.S.A). All data are presented as mean ± SEM. For normally distributed data, analysis was performed using Brown–Forsythe and Welch ANOVA with unpaired t with Welch’s correction. Non-normally distributed data were analysed using the Kruskal–Wallis test, followed by Dunn’s multiple comparisons test. Statistical significance was established at p < 0.05, with significance levels of p < 0.01 and p < 0.001 also reported where applicable.
Results
The expression of enterocytic Aβ increased with age in diabetic db/db mice
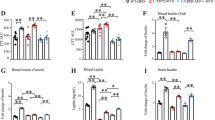
Immunohistochemical quantification of intestinal Aβ revealed no significant age-related differences in Aβ levels among non-diabetic db/ + mice at 6, 14, and 28 weeks (Fig. 1). In contrast, diabetic db/db mice showed statistically significant age-dependent increases in enterocytic Aβ. In particular, Aβ levels were higher in 14-week-old db/db mice compared to those in 6-week-old db/db mice, with 28-week-old db/db mice displaying further increases, exceeding levels in both younger diabetic db/db mice and age-matched non-diabetic db/ + controls.
Semi-quantitative immunomicroscopy for enterocytic abundance of Aβ in control mice. The intensity sums of enterocytic Aβ expressed per nuclei area (A) and representative immunomicrographs showing Aβ (orange) and nuclei (blue) in the intestinal villi (B). The white arrow indicates lamina propria (Lp) and Lu labels the lumen. Data are expressed as mean ± SEM. Statistical significance was estimated by Brown–Forsythe and Welch ANOVA (n = 8–12,*p < 0.05, **p < 0.01, ***p < 0.001).
SAC fully normalised the enterocytic abundance of Aβ in db/db mice
SAC supplementation significantly lowered enterocytic Aβ levels in db/db mice (Fig. 2A,B). Conversely, CBD alone and combined with SAC elevated Aβ levels, with the co-administration nearing statistical significance (p = 0.054).
Differential effect of SAC, CBD and SAC+CBD on enterocytic abundance of Aβ. The intensity sum of enterocytic Aβ expressed per nuclei area (A) and representative immunomicrographs showing Aβ (orange) and nuclei (blue) in the intestinal villi (B). The white arrow indicates lamina propria (Lp) and Lu labels the lumen. Data are expressed as mean ± SEM. Statistical significance was estimated by Brown–Forsythe and Welch ANOVA (n = 7–14, *p < 0.05).
CBD increases the levels of Aβ oligomer and Aβ42 in plasma
Treatment with SAC did not significantly affect Aβ42 concentrations, however minor reductions were noted in Aβ40 and Aβ oligomers levels (Fig. 3A–C). In contrast, CBD treatment, both alone or combined with SAC, significantly enhanced Aβ42 levels and led to a non-significant increase in Aβ40 levels. Moreover, Aβ oligomer levels were lowered in both treatment groups, although the difference was only significant in the combination therapy.
Differential effect of SAC, CBD and SAC+CBD on the concentrations of plasma Aβ isoforms. Pairwise comparisons of plasma Ab40 (A), Aβ42 (B), Aβ42/Aβ40 (C) and Aβ oligomer (D). Values are expressed as ± SEM (5–12 mice per group). Statistical significance was estimated by Brown–Forsythe and Welch ANOVA for the data sets of Aβ42 and Kruskal–Wallis for the data set of Aβ40 and Aβ oligomer (n = 6–9, *p < 0.05, **p < 0.01).
CBD and SAC ameliorate hyperinsulinemia and moderately reduce triglycerides
Treatment with SAC and CBD, both individually and in combination, increased plasma glucose levels, despite reducing circulating insulin concentrations (Fig. 4A,B). All treatment groups showed a decrease in plasma triglycerides to levels comparable to non-diabetic controls (db/ +), though this reduction did not reach statistical significance (Fig. 4C). Furthermore, only the CBD-treated group exhibited a significant decrease in body weight (Fig. 4D).
Differential effect of SAC, CBD and SAC+CBD on metabolic parameters. Pairwise comparisons of plasma glucose (A), Insulin (B), triglycerides (C) and body weight (D). Values are expressed as ± SEM (3–12 mice per group). Statistical significance was estimated by Brown–Forsythe and Welch ANOVA (n = 6–9, *p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
Consistent with our previous study, we observed a significant increase in Aβ expression within the absorptive cells of the small intestine in diabetic db/db mice10. Our results further extend these observations by demonstrating a progressive increase in enterocytic Aβ levels in db/db mice with age, a pattern not observed in db/ + mice (Fig. 1). Elevated plasma levels of Aβ40 and Aβ oligomers in db/db mice were also noted, while Aβ42 levels remained unchanged. Whilst the precise mechanisms by which diabetes elevates enterocytic Aβ are not fully understood, factors such as hyperphagia and intestinal hypertrophy, common in db/db mice, may contribute to increased Aβ levels through enhanced dietary fat intake. Previous studies suggest that dietary fats influence Aβ abundance in wild-type mice, which may be relevant here5,15.
The present study also examined the effects of SAC and CBD on enterocytic and plasma Aβ levels. SAC administration significantly reduced enterocytic Aβ levels (Fig. 2), consistent with its reported ability to suppress Aβ synthesis in the brain and modulate amyloid precursor protein processing16. The observed reduction in enterocytic Aβ by SAC may also stem from its ability to mitigate hyperinsulinemia and oxidative stress17, both pathways implicated in amyloidogenic processes18. However, the absence of a significant reduction in plasma Aβ suggests that systemic clearance mechanisms, predominantly hepatic, remained unaffected. The paradoxical rise in plasma glucose despite lowered insulin levels further supports the possibility of impaired hepatic insulin sensitivity.
In contrast, while CBD exhibited metabolic alterations similar to SAC, its impact on Aβ homeostasis differed markedly. CBD monotherapy increased enterocytic Aβ expression and elevated plasma Aβ42 levels (Figs. 2 and 3). Interestingly, CBD co-administration with SAC countered the inhibitory effect of SAC on enterocytic Aβ and increased the levels of plasma Aβ42 whereas the levels of Aβ oligomers were reduced. The effect of CBD on plasma membrane fluidity19,20 and intracellular cholesterol handling21,22,23, may partially account for these findings, although the exact mechanisms remain unclear. Previous research has demonstrated inconsistent findings regarding the effects of CBD on Aβ in the brain12,24,25. This study, however, is the first to indicate an upregulation of peripheral Aβ. Further research is necessary to determine the pathways through which CBD influences Aβ metabolism, particularly in the context of diabetes.
Conclusion
In conclusion, our study demonstrated a progressive increase in enterocytic Aβ levels in diabetic db/db mice, with SAC effectively reducing these levels. In contrast, CBD increased Aβ expression in the intestines and plasma. These findings suggest that SAC may have therapeutic potential in regulating Aβ metabolism, whereas caution may be warranted when using CBD, particularly in diabetic models. Further research is required to fully understand the mechanisms behind these effects and to clarify the therapeutic implications for managing Aβ-related pathologies in diabetes.
Data availability
The datasets used in the current study are available from the corresponding author on reasonable request.
Abbreviations
- Aβ:
-
Amyloid-beta
- AD:
-
Alzheimer’s disease
- CBD:
-
Cannabidiol
- SAC:
-
S-allyl-cysteine
References
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 12, 383–388. https://doi.org/10.1016/0165-6147(91)90609-V (1991).
Selkoe, D. J. The molecular pathology of Alzheimer’s disease. Neuron 6, 487–498. https://doi.org/10.1016/0896-6273(91)90052-2 (1991).
Takechi, R. et al. Differential effects of dietary fatty acids on the cerebral distribution of plasma-derived apo B lipoproteins with amyloid-beta. Br. J. Nutr. 103, 652–662. https://doi.org/10.1017/S0007114509992194 (2010).
Takechi, R., Pallebage-Gamarallage, M. M., Lam, V., Giles, C. & Mamo, J. C. Aging-related changes in blood-brain barrier integrity and the effect of dietary fat. Neurodegener. Dis. 12, 125–135. https://doi.org/10.1159/000343211 (2013).
Galloway, S., Jian, L., Johnsen, R., Chew, S. & Mamo, J. C. L. beta-amyloid or its precursor protein is found in epithelial cells of the small intestine and is stimulated by high-fat feeding. J. Nutr. Biochem. 18, 279–284. https://doi.org/10.1016/J.JNUTBIO.2006.07.003 (2007).
Gudala, K., Bansal, D., Schifano, F. & Bhansali, A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 4, 640–650. https://doi.org/10.1111/JDI.12087 (2013).
van Arendonk, J. et al. Diabetes and hypertension are related to amyloid-beta burden in the population-based Rotterdam study. Brain 146, 337–348. https://doi.org/10.1093/BRAIN/AWAC354 (2023).
Pietilä, E. et al. Midlife insulin resistance, APOE genotype, and change in late-life brain beta-amyloid accumulation—A 5-year follow-up [11C]PIB-PET study. Neurobiol. Dis. 190, 106385. https://doi.org/10.1016/J.NBD.2023.106385 (2024).
Matsuzaki, T. et al. Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama study. Neurology 75, 764–770. https://doi.org/10.1212/WNL.0B013E3181EEE25F/ASSET/E0178E4E-73B8-47C0-B64B-490934116B16/ASSETS/GRAPHIC/PAP.GIF (2010).
Sharif, A., Mamo, J., Lam, V., Watts, G. F., Luna, G., Nesbit, M. et al. Effects of HA1 (a Probucol Analogue) and ApoC3 siRNA on lipoprotein-amyloid metabolism, neurovascular integrity and cognitive function in db/db Mice. BioRxiv 2024:2024.11.05.622070. https://doi.org/10.1101/2024.11.05.622070.
Javed, H. et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 1389, 133–142. https://doi.org/10.1016/J.BRAINRES.2011.02.072 (2011).
Watt, G. et al. Chronic treatment with 50 mg/kg cannabidiol improves cognition and moderately reduces Aβ 40 levels in 12-month-old male AβPP swe/PS1ΔE9 transgenic mice. J. Alzheimer’s Dis. 74, 937–950. https://doi.org/10.3233/JAD-191242 (2020).
Percie du Sert, N. et al. The ARRIVE guidelines 20: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 40, 1769–1777. https://doi.org/10.1177/0271678X20943823 (2020).
Galloway, S., Takechi, R., Pallebage-Gamarallage, M. M. S., Dhaliwal, S. S. & Mamo, J. C. L. Amyloid-Β colocalizes with apolipoprotein B in absorptive cells of the small intestine. Lipids Health Dis. 8, 1–7. https://doi.org/10.1186/1476-511X-8-46/FIGURES/3 (2009).
Galloway, S. et al. The differential effects of fatty acids on enterocytic abundance of amyloid-beta. Lipids Health Dis. 18, 1–6. https://doi.org/10.1186/S12944-019-1162-9/TABLES/2 (2019).
Liu, X. J., Wei, J., Shang, Y. H., Huang, H. C. & Lao, F. X. Modulation of AβPP and GSK3β by endoplasmic reticulum stress and involvement in Alzheimer’s disease. J. Alzheimer’s Dis. 57, 1157–1170. https://doi.org/10.3233/JAD-161111 (2017).
Colín-González, A. L. et al. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid. Med. Cell Longev. 2012, 907162. https://doi.org/10.1155/2012/907162 (2012).
Cheignon, C. et al. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 14, 450–464. https://doi.org/10.1016/J.REDOX.2017.10.014 (2018).
Watkins, A. R., Phaterpekar, T., Ruben, P. C. & Thewalt, J. L. Cannabidiol affects chain packing in lipid membranes. Biophys. J. 118, 389a. https://doi.org/10.1016/j.bpj.2019.11.2217 (2020).
Mahadeo, M. N., Lee, E., Bains, J., Ruben, P. C. & Thewalt, J. L. Cannabidiol and cannabinol have distinct effects on membrane structure. Biophys. J. 122, 83a. https://doi.org/10.1016/j.bpj.2022.11.653 (2023).
Perez, E. et al. Differential interaction of cannabidiol with biomembranes dependent on cholesterol concentration. ACS Chem. Neurosci. 13, 1046–1054. https://doi.org/10.1021/ACSCHEMNEURO.2C00040/ASSET/IMAGES/LARGE/CN2C00040_0006.JPEG (2022).
Guard, S. E. et al. Multiomic analysis reveals disruption of cholesterol homeostasis by Cannabidiol in human cell lines. Mol. Cell. Proteomics 21, 100262. https://doi.org/10.1016/J.MCPRO.2022.100262/ATTACHMENT/B79B2E18-6ECD-4DEC-BFDB-1221BE958D9A/MMC10.PDF (2022).
Brown, C., Kariuki, W., Zhong, H. A., Kippes, A. & Sui, Y. Cannabidiol promotes intestinal cholesterol uptake mediated by Pregnane X receptor. Front. Endocrinol. (Lausanne) 15, 1398462. https://doi.org/10.3389/FENDO.2024.1398462/BIBTEX (2024).
Arnanz, M. A. et al. Effects of chronic, low-dose cannabinoids, cannabidiol, Delta-9-tetrahydrocannabinol and a combination of both, on amyloid pathology in the 5xFAD mouse model of Alzheimer’s disease. Cannabis Cannabinoid Res. https://doi.org/10.1089/CAN.2023.0101/ASSET/IMAGES/CAN.2023.0101_FIGURE8.JPG (2023).
Cheng, D., Spiro, A. S., Jenner, A. M., Garner, B. & Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimer’s Dis. 42, 1383–1396. https://doi.org/10.3233/JAD-140921 (2014).
Acknowledgement
The authors acknowledge the Curtin Medical Research Institute for provision of laboratory space and technology platforms utilised in this study.
Funding
This research was supported by an Australian Government Research Training Program (RTP) Scholarship awarded through Curtin University and NHMRC. The Zeiss Axioscan Z.1 digital slide scanner was funded by the Australian Research Council under grant LE200100122.
Author information
Authors and Affiliations
Contributions
Arazu Sharif: Methodology, Formal analysis, Data curation, Conceptualisation, Writing—original draft. Maimuna Majimbi: Methodology, Data curation, Conceptualisation. John Mamo: Funding acquisition, Conceptualisation, Writing—review & editing, Supervision. Virginie Lam: Funding acquisition, Writing—review & editing, Supervision. Michael Nesbit: Methodology, Formal analysis. Ryu Takechi: Methodology, Funding acquisition, Data curation, Conceptualisation, Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experimental procedures in this study were approved by the Curtin Animal Ethics Committee (Approval No. ARE 2018-19) and conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sharif, A., Majimbi, M., Mamo, J. et al. Differential effects of S-allyl cysteine and cannabidiol on enterocytic and plasma amyloid-β in db/db diabetic mice. Sci Rep 15, 20448 (2025). https://doi.org/10.1038/s41598-025-04658-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04658-1