Abstract
The reemergence of the New World screwworm (Cochliomyia hominivorax) poses a significant threat to animal and public health with minimal regulatory oversight. This study analyzes the potential distribution and reemergence of this pest, which is endemic to South America but was previously eradicated in North America. We first developed bioclimatic suitability models, and then incorporated these findings along with reemergence records and inspection point data to simulate possible dispersal routes into Mexico and the United States. Our results document the historical distribution of C. hominivorax across the Americas and recent reemergence events in Panama (2023) and Mexico (2024–2025). Findings indicate high invasion potential from Central America, with significant risk along Mexico’s Pacific and Atlantic coasts and the Yucatan Peninsula. In the United States, Texas and Florida face the highest risk. Regions with high livestock density in both countries demonstrate considerable climatic suitability for the pest. Our simulations identify Chiapas, Campeche, Tabasco, and Veracruz as critical northern dispersal points. The invadable areas contain substantial populations of domestic hosts, primarily cattle and horses. These findings will enable governmental authorities to develop comprehensive prevention and control strategies to address this emerging threat.
Similar content being viewed by others
Introduction
Livestock in North America and the New World screwworm
North America is one of the world’s leading livestock-producing regions1. Following the signing of North American Free Trade Agreement (NAFTA; now United States-Mexico-Canada Agreement, USMCA), cattle exports have increased significantly, with an average annual intra-regional trade of 2 million head of cattle2. Mexico plays a key role in beef production, ranking as the world’s seventh-largest producer in 2022 3,4,5. Most of the live cattle exported from Mexico are destined for the United States3,6making Mexico the primary supplier of live cattle of North America2,3,6,7.
In 2023, the live cattle market reached a value of $3 billion, with 965,169 head exported to the U.S.8. A significant increase happened in 2024 to 1,014,386 head8. This trade represents a significant portion of commercial exchange within the USMCA3,7. Exported cattle are primarily sent to feedlots in the U.S., with northern Mexican states as the main suppliers8. However, other regions of the country also contribute to this trade6. Among the certified entities for export, Chihuahua, Sonora, Durango, Coahuila, Tamaulipas, and Nuevo León stand out. Additionally, Campeche, Veracruz, and Yucatán meet sanitary requirements, such as tuberculosis- and tick-free certification, to export their cattle production8.
Throughout history, various pests have impacted livestock production in the Americas, including the eradication of Texas fever in the U.S., caused by the Rhipicephalus (Boophilus) annulatus tick, and the elimination of the New World screwworm fly (Cochliomyia hominivorax)9. The latter species was successfully controlled using the sterile insect technique, a milestone in the application of gamma radiation to produce sterile males as a pest control method10,11. However, this pest has reemerged as one of the greatest concerns regarding animal health, livestock production and cattle commercialization in the Americas in recent months12.
Cochliomyia hominivorax, known as the New World screwworm, is a dipteran native to tropical and subtropical America that causes myiasis in warm-blooded animals and poses a public health concern13,14,15,16. Due to its impact, the FAO and WHO have classified myiasis caused by C. hominivorax as a priority disease for eradication in the Americas15. Its life cycle is usually complete within 18 days at 29 °C or 24 days at 22 °C, although the pupal stage can extend up to 57 days in adverse climates17. The species goes through three larval stages within its host before completing metamorphosis in the soil, emerging as an adult fly after a pupation period of up to 240 h14,17. Females can lay up to 500 eggs per oviposition, typically performing around four ovipositions within 15 min15,16,18 (Fig. 1c-f), with eggs hatching within 12–24 h at 35 °C. The larvae feed on the host tissue, causing severe lesions before completing their development and falling to the ground to pupate15,17 (Fig. 1g-j). Environmental factors such as humidity and temperature influence pupal survival, favoring their development in warm, moist soils but negatively affecting them under extreme conditions like flooded soils or very low or high temperatures19. C. hominivorax has remarkable flight capabilities, traveling 4–20 km in tropical climates with high host density, and, in some cases, up to an estimated 300 km in less than two weeks while searching for favorable environments20,21.

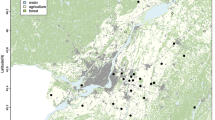
Source of images of panels c-j: Animal and Plant Health Inspection Service, United States Department of Agriculture. Special Collections, National Agricultural Library. Figure created using ArcMap software 10.8 version and edited in Inkscape software 1.2 version.
Geographical distribution and biological generalities of C. hominivorax. (a) Historical chronology of eradication in North and Central America (USDA-National Agricultural Library https://www.nal.usda.gov/exhibits/speccoll/items/show/7335; OIRSA - Regional International Organization for Agricultural Health https://www.iaea.org/sites/default/files/21/03/situacion-del-gbg.pdf), (b) geolocation of the presence records used in this work, (c-j) life stages of C. hominivorax and affected hosts, (c) adult screwworm fly (slide of adult screwworm fly; https://www.nal.usda.gov/exhibits/speccoll/items/show/7324), (d) fly laying eggs in animal wound (slide of fly laying eggs in animal wound; https://www.nal.usda.gov/exhibits/speccoll/exhibits/show/stop-screwworms--selections-fr/item/7330), (e) larvae living in wound (slide of larvae living in wound; https://www.nal.usda.gov/exhibits/speccoll/items/show/7325), (f) pupae burrowing into ground (slide of pupae burrowing into ground; https://www.nal.usda.gov/exhibits/speccoll/exhibits/show/stop-screwworms--selections-fr/item/7326), (g) infested cow (slide of infested cow; https://www.nal.usda.gov/exhibits/speccoll/items/show/7329), (h) infested Lamb (Slide of infested lamb; https://www.nal.usda.gov/exhibits/speccoll/items/show/7327), (i) treating ear of infested pig (slide of treating ear of infested pig; https://www.nal.usda.gov/exhibits/speccoll/items/show/7322), (j) infested back wound horse (slide of infested back wound on horse; https://www.nal.usda.gov/exhibits/speccoll/items/show/7328).
Eradication of Cochliomyia hominivorax in the United States and Mexico
The eradication of the screwworm in the U.S. and Mexico was carried out through the release of sterile flies and binational effort between 1972 and 1991. In the U.S., the campaign began in Florida (1957–1960), producing 50 million sterile flies per week, eradicating the pest with an investment of 11 million dollars and preventing annual losses of 20 million dollars22. The campaign was later expanded across the entire country22,23 (Fig. 1a). In 1972, the Mexico–U.S. Commission for the Eradication of the Screwworm (COMEXA) was established with the goal of eradicating the pest in Mexico and creating a 400 km barrier in the Isthmus of Tehuantepec. Between 1976 and 1977, a plant was opened in Chiapas with the capacity to produce 500 million sterile flies per week22,23. By 1987, the pest had been eliminated in much of Mexico, and between 1988 and 1991, the program expanded to Central America, culminating in Panama in 2001 24 (Fig. 1a). Panama was declared free in 2006 after the inauguration of a plant in Pacora to prevent reinfestations15,16,23. In 2012–2013, COMEXA was dissolved, and the Chiapas plant closed due to absence of funding25. Currently, the only operational facility for the production of sterile flies is in Panama, and was used to contain reinfestations25. From 2018 to 2020, North and Central America were considered free of the pest, while in South America, the pest remains endemic except for Chile16,26.
The cost of eradication in the U.S. and Mexico (1960–1991) was 955 million dollars25. In endemic regions, the pest continues to cause significant economic losses: Uruguay (40–52 million dollars annually)17Brazil (340 million dollars)27and Jamaica (7.7 million dollars)28. Eradication provided annual benefits of 1.350 billion dollars in the U.S., 495 million in Mexico, and 132 million in Central America, adjusted to 2020 inflation17. It is estimated that eradication in South America could generate savings of over 4 billion dollars17.
In Mexico, the reemergence of C. hominivorax would affect the sustainable growth of livestock production, reducing profits by 23% and causing losses in the commercialization and industrialization networks. The Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria from Mexico (National Service for Agri-Food Health, Safety, and Quality; SENASICA) estimates that its reemergence could cause a loss of 8.029 billion of Mexican pesos (470.588 million dollars, adjusted to 2025 inflation )25.
Reemergence and potential impact of Cochliomyia hominivorax
In 2017, a case of C. hominivorax myiasis was recorded in a white-tailed deer (Odocoileus virginianus) on Big Pine Key, Florida, also affecting domestic animals in Miami-Dade County29,30. These cases were controlled through the release of sterile flies30. More recently, a case of screwworm was diagnosed in a 71-year-old adult in Costa Rica31indicating the potential reemergence of C. hominivorax in previously eradicated regions as zoonotic risk.
By December 2024, the Panama–U.S. Commission for the Eradication and Prevention of the Screwworm (COPEG) reported a high incidence of reemergence of C. hominivorax in Panama, Costa Rica, and Nicaragua, with less cases reported in Honduras and Guatemala32. In Mexico, cases were confirmed in November and December of 2024 in cattle detected in Chiapas and Campeche33. As a result, the Animal and Plant Health Inspection Service (APHIS) temporarily suspended the export of Mexican cattle to the U.S. starting on November 25, 2024 34, disrupting the expected export growth compared to 2023 8. The export ban has significantly impacted the Mexican livestock economy. Chihuahua ceased exporting 110,000 calves, with losses of 25–30 million dollars weekly. In Durango, the detention of 5,000 calves represents losses of 7 million dollars per week, while in Tamaulipas, 15,000 cattle destined for export were held back35,36.
Given the sanitary and economic risks posed by the dispersion and establishment of C. hominivorax, as well as its relevance as a zoonotic agent under the One Health approach12this study models its climatic niche and analyzes its potential distribution, providing key information for prevention, control, and surveillance strategies.
Results
The occurrences database compiled for Cochliomyia hominivorax covered a large part of its distribution in the Americas, with a wide latitudinal distribution along the Americas (42º N − 38º S; Fig. 1b) (Supplementary Table S1). Selected ecological niche models (ENM) documented the historical distribution along the continent, also the recent reemergence records (Figs. 1b and 2a and b, and 3) and eradicated places (Fig. 1a). The 82 best models (i.e., those that passed the omission rate and statistical performance criteria) were built with between 2 and 7 bioclimatic variables (Supplementary Table S2); however, the most common number of factors were three. The most recurrent variables were annual mean temperature (bio1), mean diurnal range (mean of monthly (max temp - min temp); bio2), isothermality (bio3), maximum temperature of warmest month (bio5), precipitation of driest month (bio14), and precipitation seasonality (bio15). Moreover, simple models, such as the one formed by variables bio1 and bio14, tended to represent largest potential distributional ranges, representing well historical and reemerging localities (Fig. 2a). These models also aligned with the patterns of preferred and tolerated climates reported by the Centre for Agriculture and Biosciences International (CABI; https://doi.org/10.1079/cabicompendium.11753) (see Supplementary Figs. S1-2 online).

(source: https://dataverse.harvard.edu/dataverse/glw/). Figure created using ArcMap software 10.8 version and edited in Inkscape software 1.2 version.
Predicted suitable areas for C. hominivorax. (a) current climatic suitability in the United States, Mexico and Central America, (b) overlap of suitability in areas with approximately 23 head of livestock/km2, (c) overlap of suitability in areas with approximately 46 head of livestock/km2. The potential distribution of C. hominivorax is indicated as binary suitability in shades of gray with blue outline. The visual representation of the livestock density corresponds to the summarized number of head of livestock including cattle, horses, pigs, sheep, and goats.
Reported cases of C. hominivorax (2021–2025) and predicted suitability. (a) Distribution of reemergence cases of C. hominivorax (2021–2025) and binary suitability model, (b) all reported cases (2021–2025) and the selected climatic suitability model based on mean annual temperature and precipitation of driest month, (c) all reported cases (2021–2025) in suitable areas with approximately 23 head of livestock per km² and binary suitability model, (d) all cases (2021–2025) in suitable areas with approximately 46 head of livestock per km² and binary suitability model. The binary suitability prediction is based on the 10th-percentile threshold of the median of the ten best-performing models. Figure created using ArcMap software version 10.8 and edited with Inkscape software version 1.2.
Suitable areas include the historical distributional range (South America) and the historical invasion range from 1957 to 2001 (North America, Central America and the Antilles; Figs. 1a and b and 2a, and 2b). Reemerging regions were also predicted for points in Panama and Mexico (Figs. 1b and 3). For Mexico, models predicted suitable areas in the south (where reemerging cases have been recorded in 2024–2025) and along both the Pacific and Atlantic slopes. In the U.S., the highest suitable areas were in the southeastern portion of the country (Figs. 1b and 2). The geographical predicted suitable areas are also consistent with recent cases of myiasis caused by C. hominivorax, as reported in the World Animal Health Information System (WAHIS) of the World Organisation for Animal Health (https://wahis.woah.org/#/home). The distribution of cases reported since 2021 aligns with the suitability pattern of our model, particularly in regions with high livestock density (Fig. 3).
We found extensive areas and a high number of suitable connected areas for dispersal in lower cattle abundance patches (~ 23 individuals/km2; Figs. 2b and 3), whereas a higher number of least connected suitable patches increased as the requirement of cattle abundances increased (~ 46 individuals/km2; Figs. 2c and 3; also see Supplementary Fig. S3). High connectivity in suitable patches was located between Central America and Mexico in the south, Pacific, Atlantic, and central regions (Fig. 2b and c; also see Supplementary Fig. S3).
Dispersal simulations showed different rates and suitable extents for C. hominivorax, depending on both the location of the starting points (either reemergence sites or Federal Verification and Inspection Points [PVIF] in Mexico) and the dispersal rates (~ 5 km or ~ 20 km) (see Supplementary Videos online; https://doi.org/10.5281/zenodo.15465751). The highest extent and risk were associated with the largest dispersion rate (see Supplementary Fig. S3).
The dispersal scenario in Central America, starting from Panama, aligned with reports from Panama-United States Commission for the Eradication and Prevention of Screwworm (COPEG; https://www.copeg.org/) up to Nicaragua, the country where C. hominivorax has been homogeneously established in Central America (Fig. 5). Likewise, the simulation indicated high potential for C. hominivorax to spread northwards throughout Central America up to the U.S.-Mexico border (Fig. 5) (see Supplementary Videos online; https://doi.org/10.5281/zenodo.15465751).

(source: https://dataverse.harvard.edu/dataverse/glw/). Figure created using ArcMap software 10.8 version and edited in Inkscape software 1.2 version.
C. hominivorax livestock host density and distribution in the southern U.S., Mexico and Central America. (a) Cattle, (b) horses, (c) pigs, (d) sheep. The visual representation of livestock density reflects the aggregated number of animals, including cattle, horses, pigs, sheep, and goats.
A notable dispersal capacity of C. hominivorax was found from sites located in Chiapas, Campeche, and Veracruz (Fig. 5). These three sites exhibit high suitability for generating a potential geographic expansion pattern for the fly, highlighting that the route through the Gulf of Mexico slope allows for a faster spread toward the U.S. via Texas. Dispersal toward northern Mexico through the Pacific-bordering states also occurs but at a slower rate compared to the Gulf of Mexico route (Fig. 5). This pattern aligns with the current situation in Mexico, as shown by the distribution of reported positive cases in the state of Tabasco (Report FUR_172432)33 and the most recent case, dated May 2025, located in the south of Veracruz (Report IN_174171), as reported in the World Animal Health Information System (WAHIS) of the World Organisation for Animal Health (https://wahis.woah.org/#/home).
Discussion
Invasive species have had a significant impact throughout history upon their introduction to North America, being responsible for economic losses ranging from billions to trillions of dollars on a global scale58,59. In recent years, some of these species, once considered eradicated, have reemerged, causing renewed economic losses in different geographical regions59. Of particular zoonotic interest is the fly Cochliomyia hominivorax, which has historically been considered one of the most important pests affecting livestock in the Americas23. Although its main impact is associated with livestock, it has also been described as a neglected tropical zoonotic myiasis, with notable clinical persistence in both humans and animals, both wild and domestic60. The recent reemergence of C. hominivorax in North America has highlighted the need for mandatory reporting, given the magnitude of the risks it poses to animal production and public health12. The entry of new populations of C. hominivorax into Mexico and the U.S. could have significant economic, biological, and ecological implications61,62.
Climate is a determining factor in the distribution and establishment of invasive species63making its analysis crucial for the design and implementation of control and eradication strategies. In this context, understanding the potential geographic distribution, dispersion routes, and ecological preferences of these species allows for an assessment of their invasive potential59,64,65. Our environmental suitability maps of C. hominivorax suggest that its potential distribution covers nearly all Central American countries, both slopes of Mexico (Pacific and Atlantic), as well as the southern and southeastern states of the country. In the U.S., the southern states of the Atlantic slope and California also present favorable conditions (Figs. 1 and 2). This potential distribution hypothesis is similar to the historical distribution in North America before the species was eradicated in 1970 66 (Fig. 1). Likewise, our predicted environmental suitability matches the current dispersal scenario observed to date in the reemergence of C. hominivorax in Central America and Mexico, according to official WAHIS data (https://wahis.woah.org/#/home).
Cold temperatures limit the distribution of C. hominivorax to tropical and subtropical climates (Figs. 1 and 2), with average winter temperatures below 9 °C preventing its survival due to the absence of a diapause stage67. Laboratory studies have shown that temperatures below 10 °C significantly affect the survival and oviposition capacity of these flies68whereas extremely high temperatures (above 40 °C) in dry environments reduce the biological conditions for reproduction69. In general, C. hominivorax prefers tropical climates with average temperatures of 25 °C, while its populations are significantly lower in high mountainous areas and dry regions69 (https://doi.org/10.1079/cabicompendium.11753) (see Supplementary Figs. S1-2 online).
Previous studies have predicted the distribution of the species70although using different methods and environmental variables that present spatial artifacts45. The most recent case, focusing on South America in Argentina, used data from different sources to estimate the potential distribution of C. hominivorax, showing the usefulness of this approach71. This study highlights the impact of low temperatures as a limiting factor for C. hominivorax populations, restricting its expansion to southern South America72. Our model shows high climatic suitability for North America, highlighting the northern borders with the U.S. and southern Mexico with Central America (Fig. 2), and for South America, it is consistent with field data reported in Brazil73. There have also been contributions to map the climatic favorability of C. hominivorax in Mexico and the southern U.S. during the period 1983–2003 using demographic models based on physiology64,65. The results obtained in these studies are consistent with those presented in our work. According to these models, the highest climatic favorability is predicted in the Yucatán Peninsula and southern tropical Mexico, with lower favorability in the Pacific region. The states with the highest cattle production, such as Chiapas, Veracruz, and San Luis Potosí, are located in areas predicted to be highly suitable for C. hominivorax (Fig. 4a). Likewise, it is evident that equine livestock would play an important role in the spread of the pest, as they are widely distributed and abundant in Central America, Mexico, and the U.S. (Fig. 4b). Additionally, smaller livestock species would have a significant epidemiological role in central Mexico (Fig. 4c-d).
Simulation of potential invasion dynamics of C. hominivorax. Invasion dynamics of C. hominivorax from recent reemergence points in Panama, Chiapas, Campeche and Veracruz, considering two dispersal scenarios (1-neighbor [~ 5 km] and 4-neighbors [~ 20 km]) and a cattle livestock density of approximately 23 heads of livestock/km2. Different time steps in the simulation are shown (Step 20, Step 100, Step 200, and Step 300); each time step corresponds roughly to a reproductive life cycle. Blue areas indicate suitable regions, while green represents occupied areas in each scenario. Figure created using ArcMap software 10.8 version and edited in Inkscape software 1.2 version.
In the tropics, the density of flies is higher in forested habitats74although recent records of their reemergence (see Supplementary Table S3) suggest they have high plasticity across different landscapes17,69. Their potential habitat, where they reach higher densities, includes grasslands, disturbed landscapes with tall vegetation, and the presence of wild or domestic hosts, indicating a wide habitat preference pattern75. Cattle and horses are identified as the most relevant hosts for their distribution as their density is high in geographic areas with the highest suitability according to our models (Fig. 4a and b). This is consistent with recent reemergence cases in Mexico, where reports have occurred primarily in young cattle and horses33. According to the climatic suitability prediction, the greatest risk of dispersion of C. hominivorax would occur in areas with the highest density of hosts (map of binarized cattle, ~ 23–46 livestock/km2) and cattle production systems (see map in76). The finding of this potential dispersal is concerning because it has been reported that the establishment of C. hominivorax populations could lead to mortality rates of up to 20% in affected animals77. Newborn animals are the most vulnerable, as umbilical lesions are a natural attraction for C. hominivorax78. This has been observed in recent reports from Mexico, where the affected animals are neonatal calves33. From an epidemiological standpoint, this scenario is concerning due to the reproductive management of herds in these regions, where the primary calving season occurs during the winter and spring, as previously documented76.
There are natural corridors that facilitate the dispersion of this pest towards Central and North America79. Some regions, such as the Caribbean, have endemic populations that prevail year-round (e.g., 258,689 cases in humans and animals from 1995 to 2015)80. Established populations, along with evidence of environmental suitability, allow the creation of scenarios based on potential dispersion simulations, establishing a precedent for an increased risk of reemergence in North America81. In this context, it has been documented that as little as 1% of a C. hominivorax original population can be sufficient to recover damaging densities in just a few generations82,83.
The recent reemergence of C. hominivorax in southern Mexico has led to modifications in the binational processes for the exportation of Mexican cattle to the United States84. Notably, this includes the introduction of a new certificate verifying the pest-free status of the cattle, the establishment of pre-export inspection centers for New World screwworm, and the training of professionals responsible for their implementation85. As of now, the cattle located at the border have been approved as eligible for export. However, this situation places the rest of the country’s livestock, particularly in the south, at a disadvantage, potentially leading to economic losses. This scenario highlights the need to strengthen control, prevention, and eradication measures for C. hominivorax across all natural corridors in the Americas.
According to our dispersal simulations, the areas with the highest potential risk for the introduction and establishment of C. hominivorax are mainly located in tropical and subtropical regions with high climatic suitability (Fig. 2a). Given the broad climatic suitability for the fly, these simulations indicate that its dispersion could be rapid, spreading from key sites identified in this study—such as the Yucatán Peninsula—throughout the entire Gulf of Mexico slope64,65 and toward central and northern Mexico, posing a significant potential risk of reaching the U.S. Under these hypotheses, the recent reemergence cases reported in Campeche represent the highest risks of dispersion to neighboring states33. Therefore, these areas should be prioritized for control measures, particularly through the release of sterile flies under current conditions78.
Our dispersal simulations are based on maps of overall high suitable climatic conditions for C. hominivorax; however, we must consider the impact of human facilitation in its spread into geographical areas with varying levels of suitability70. Practices related to commercialization and informal mobility could facilitate its expansion into different regions by promoting the transport of livestock infested with screwworm larvae78. Therefore, strengthening epidemiological surveillance measures regarding livestock movement to and within Mexico is crucial to prevent the establishment of C. hominivorax populations. Our methodology for generating dispersal scenarios has been previously used for agricultural pests, thus we anticipate it will have high potential for application in various epidemiological phenomena, such as this one86.
Seasonality of the climate and global warming are key factors in the reemergence and potential expansion of C. hominivorax in North America. A seasonal pattern in the abundance of its populations has been observed, as occurred in Mexico prior to its eradication87. This population dynamic has also been described in South American countries where the species is endemic, showing that warmer seasons coincide with a broader distribution and an increase in C. hominivorax reports88. Climate change could intensify favorable conditions for the species throughout the year and expand its geographic range. This phenomenon has been documented in various diseases of economic and public health concern in the Americas89,90,91,92. Importantly, our current potential distribution projection aligns with the prediction by Gutiérrez et al.65 for the 2045–2055 period, where they noted an increase in climate favorability in Texas and Florida. Since our model incorporates recent presence records of C. hominivorax, our findings further strengthen the evidence of favorable conditions for its establishment in the U.S. Texas is one of the states with the highest livestock production and has a high predicted suitability for C. hominivorax. Therefore, the epidemiological and economic risk for this region is very high93. According to the latest census published by the U.S. Department of Agriculture, National Agricultural Statistics Service93Texas is the leading state with the largest cattle population, totaling 12,543,300, while California is a major producer with 1,574,057 head of cattle. This highlights the imminent risk to cattle health and sustainability in the U.S.
Despite their economic, biological, and ecological relevance, research on flies in the context of health and climate change is limited compared to species such as ticks and mosquitoes89highlighting the need to expand studies in this field. It has been documented that climate change is affecting the biology and ecology of pests relevant to the agri-food sector, emphasizing the importance of using tools for predicting potential geographic distribution62. On the other hand, controlling this pest requires integrated efforts, which have been successful in the past during eradication efforts in North America23as well as in the emergence of this species in the western region of the world during the North African outbreak in 1988, caused by the introduction of infected livestock78,94. An increase in populations has been reported in South America, providing evidence of failures in the use of Doramectin and Ivermectin as preventive methods for the presence of C. hominivorax larvae in cattle61. In this region, the presence of multiparasitic skin conditions in cattle has been documented, including ticks and C. hominivorax myiasis95highlighting the health and economic impact of parasite control in livestock farming.
The utility of Ecological Niche Models (ENM) and Species Distribution Models (SDM) has been demonstrated as an effective tool in designing and implementing vector control strategies in official health campaigns. A notable example is their application in managing Glossina palpalis gambiensis and trypanosomiasis in Africa96. These examples show how these models represent a valuable methodology for predicting the potential distribution of vectors and pests, optimizing control and management efforts in different regions. In this regard, we hope that our results will contribute to the body of evidence currently needed for planning specific strategies for the prevention and control of C. hominivorax in Mexico. Our work highlights the importance of zoosanitary control points, which serve as starting points in the simulations and consistently determine the establishment of quarantine pests.
Conclusion
North America presents potential sites for the establishment and dispersal of C. hominivorax populations, particularly in regions with higher livestock densities. Given the current reemergence of the fly in Central America and southern Mexico, it is anticipated this could lead to an expansion into North America across tropical environments both along the Atlantic and Pacific slopes. Given these scenarios and considering the historical distribution of the fly across these territories, it is essential to strengthen control strategies through: (a) regulation of livestock movement, (b) treatment of infected animals, (c) continuous epidemiological surveillance, and (d) reactivating control campaigns such as the sterile fly liberation. Moreover, greater efforts and collaboration among countries are required to eradicate this pest in the Americas, especially in South and Central America, to prevent significant economic losses and restrictions on the international trade of cattle. As our results suggest, the success of different control strategies would greatly benefit from incorporating species suitability modeling outputs generated through Ecological Niche Modeling (ENM) and Species Distribution Modeling (SDM) methodologies.
Methods
Occurrences
Presence data for C. hominivorax were obtained through an exhaustive literature search and different data providers, including: Global Biodiversity Information Facility (www.gbif.org), Biodiversity Information Serving Our Nation (https://bison.usgs.gov/#home), Berkeley Ecoinformatics Engine (EcoEngine; https://ecoengine.berkeley.edu), Integrated Digitised Biocollections (iDigBio; www.idigbio.org), and iNaturalist (www.inaturalist.org). The search of digital database sources was performed within the R programming environment (R Development Core Team, 2020) using the spocc package37,38. We compiled occurrences, which were organized into a structured database that including latitude, longitude, date, site, collection method, life cycle stage of C. hominivorax, host, and the source from which the information was obtained. This dataset was visually verified in Google Earth Pro 7.3, and records that did not align with actual field data—such as those from cities, museums, or scientific collections—were discarded. Before proceeding with the model selection protocol, we removed duplicates using the ntbox R package39resulting in a dataset of 258 presence records (see Supplementary Table S1). The cleaned and verified dataset was then spatially filtered using a 17 km distance threshold with the spThin package in R40 to prevent model overfitting and reduce spatial autocorrelation.
Calibration area
We formulated two hypotheses regarding the accessible area for C. hominivorax needed for model calibration and evaluation41,42using two different approaches. The first accessible area was modeled with the grinnell package in R43using default settings (see Supplementary Methods S1). For this estimation, we used previously filtered C. hominivorax records and climatic data from WorldClim at a 10-minute arc resolution44. Due to the wide distribution of C. hominivorax in the Americas, bioclimatic variables 8, 9, 18, and 19 were excluded, as they are spatial artifacts43,45. The simulation was run over the geographic extent defined by the window (-128.833, -57, -30.333, 39.166; xmin, ymin – xmax, ymax), according to the distribution and abundance of actual or potential hosts. This methodology is based on cellular automata and has been effectively applied in assessments of species dispersal, colonization, and extinction capacity, as well as for defining the accessible area required for calibrating ecological niche models42,43,46. Our second approach used the terrestrial ecoregions intersecting C. hominivorax occurrences, plus a 100 km buffer. We chose this alternative because it represents a broader accessible area than the first approach, aligning more closely with the species’ high dispersal capacity and historical distribution, which the modeled accessible area did not fully capture.
Ecological niche modelling and suitability distribution
For the calibration of the ecological niche models of C. hominivorax, we used the bioclimatic variables from WorldClim v1.4 (1950–2000) with a 2.5 arc-minute resolution44retaining only those with low correlation (Spearman r < 0.8) at the presence points, while excluding variables 8, 9, 18, and 19, following the criteria outlined in the previous section. This variable selection resulted in the inclusion of bioclimatic variables 1, 2, 3, 5, 12, 14, and 15.
The niche models were constructed using minimum volume ellipsoids (MVEs), implemented in the ntbox package39using 70% of the presence data used for training and the remaining 30% for evaluation of the spatially filtered dataset. We chose to employ MVEs for several key reasons: (a) their convex shape is coherent with biological expectations about fundamental niches47; (b) theoretical and applied works point out that the distance to the center of an ellipsoid is related to some fitness attributes such as population abundance and genetic diversity48,49,50; and (c) fitting MVEs involves significantly fewer decision-making steps compared to other software-based methods51. The selection of the best model was based on significance criteria of the partial-area ROC curve, omission rate (E = 0.05), and the binomial test39. A total of 120 candidate models were generated, of which only 82 met the omission criterion for both training and evaluation data. For representing the suitability of the species, we chose a combination of two sets of models. For the estimate of the binary suitability we selected the model formed by bioclimatic variables 1 (mean annual temperature) and 14 (precipitation of the driest month) which is the model that under visual inspection best coincides with known historical distribution of the species in the 20th century for the extent of study (-123, -59, 3, 37; xmin, xmax, ymin, ymax) under a threshold of 10th percentile from the set of occurrences used for training the model. Subsequently, we obtained the median of the 10 best models according to the omission rate and AUC ratio criterion (see Supplementary Table S2).
Risk assessments of dispersion
We simulated the potential dispersal of C. hominivorax to generate hypotheses about territories that C. hominivorax could reach, as well as to analyze past and recent emergence events from various points of interest and according to different dispersal kernels. The simulations were conducted using cellular automata with the bamm package in R52,53 under two suitability hypotheses: (1) bioclimatic suitability for C. hominivorax based on the binarized climate suitability map described in the previous section, and (2) suitability for C. hominivorax combined with territories containing either \(\:\ge\:\) 500 or 1,000 livestock heads (for the native resolution of the livestock maps, which, in the spatial resolution of our raster layers, translates to 23 or 46 livestock heads) across the study area in North America (see Supplementary Methods S2). The points of interest used to initiate the simulations corresponded to geographical coordinates where positive cases of C. hominivorax have been recorded in Mexico33as well as Federal Verification and Inspection Points (PVIF) from SENASICA, where cattle enter Mexico and are transported within the country (https://www.gob.mx/senasica/documentos/directorio-de-puntos-de-verificacion-e-inspeccion-federales?state=published; https://dj.senasica.gob.mx/sias/Statistics/Inspeccion/PVIF), as well as from data from Panama in 2023 where some of the most recent reemergence events were recorded (see Supplementary Table S4).
Under this approach, the potentially occupied area by the species is determined by the equation:
where Gj(t + 1) represents the distribution of the species at time t + 1, Aj(t) determines whether the accessible cells are climatically suitable (it is derived from a thresholded niche model), Cj is a connectivity matrix that indicates which cells are connected (adjacent) to one another based on a dispersal kernel. In the bamm package, this kernel is based on the “Moore neighborhood”54which defines the dispersal kernel of the species in terms of the number of adjacent cells. Gj(t) denotes the occupied cells at time t53. This model has been used to studying the dispersal dynamics of several taxons including insects and a shark55,56,57. We tested two dispersal scenarios that align with previous observations on the fly’s dispersal capacity20,21: a short-distance dispersal scenario, where C. hominivorax can travel one pixel (~ 5 km) per unit of time t, and a long-distance dispersal scenario, where the species can travel four pixels (~ 20 km).
Data availability
All supplementary information is available in the Zenodo repository (https://doi.org/10.5281/zenodo.15465751).
References
Gilbert, M. et al. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci Data 5, (2018).
United State od Departament of Agriculture (USDA). Livestock and Meat International Trade Data. (2024). https://www.ers.usda.gov/data-products/livestock-and-meat-international-trade-data
Magaña, M. et al. Indicadores de competitividad de La carne Bovina de México En El Mercado mundial. Rev Mex Cienc. Pecu 11, (2020).
Servicio de información agroalimentaria y Pesquera (SIAP). Panorama Agroalimentario 2018–2024. (2024). https://www.gob.mx/siap/acciones-y-programas/panorama-agroalimentario-258035
Gonzales, K., Prado, F. & García, A. Producción y comercio de la carne en el mundo y en México. Sociedades rurales, producción y medio ambiente 23, 136–155 (2023).
Rhonda, S. et al. Exportaciones de Ganado En pie de México Hacia Los Estados unidos: ¿de Dónde viene El Ganado y Hacia Dónde va? Revista Mexicana De Agronegocios. 8, 212–219 (2004).
Rinconada Carbajal, F. & Serna Hinojosa, J. A. Valdez ramírez, R. I. Competitividad de La carne de res Fresca Mexicana En El Mercado estadounidense, 1967–2020. Análisis Económico. 38, 129–148 (2023).
Servicio de información agroalimentaria y Pesquera (SIAP). Exportación de Ganado Bovino. (2024). https://nube.siap.gob.mx/exportacion_ganado/
Bowman, D. D. Successful and currently ongoing parasite eradication programs. Vet Parasitol 139, (2006).
Bushland, R. C. & Hopkins, D. E. Experiments with Screw-Worm flies sterilized by X-Rays1. J Econ. Entomol 44, (1951).
Knipling, E. F. Sterile insect technique as a screwworm control measure: the concept and its development. in Symposium on eradication of the screwworm from the United States and Mexico. ed. Graham in Entomological Society of America, 4–7, (985).
Wainwright, S. H. et al. Reemerging/Notifiable Diseases to Watch. Veterinary Clinics of North America - Food Animal Practice vol. 40 Preprint at (2024). https://doi.org/10.1016/j.cvfa.2024.01.007
Coquerel, C. Note Sur des larves appartenant a Une espece Nouvelle de diptere (Lucilia hominivorax). Ann. Societe Entomologique De France. 27, 171–176 (1858).
Quiroz, H. Gusano Barrenador: biología Del Gusano Barrenador Del Ganado. Imagen Vet. 3, 4–11 (2003).
World Organisation of Animal Health (WOAH). Terrestrial Manual Online Access. Chapter 3.1.14 New World screwworm (Cochliomyia hominivorax) and Old World Screwworm (Chrysomya Bezziana). (2025).
Mastrangelo, T. & Welch, J. B. An overview of the components of AW-IPM campaigns against the New World screwworm. Insects vol. 3 Preprint at (2012). https://doi.org/10.3390/insects3040930
Fresia, P. et al. Historical perspective and new avenues to control the myiasis-causing fly Cochliomyia hominivorax in Uruguay. Agrociencia Uruguay 25, (2021).
Thomas, D. B. & Mangan, R. L. Oviposition and Wound-Visiting behavior of the screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). Ann Entomol. Soc. Am 82, (1989).
Rahn, J. J. & Barger, G. L. Weather conditions and screwworm activity. Agric. Meteorol. 11, 197–211 (1973).
Hightower, B. G., Adams, A. L. & Alley, D. A. Dispersal of released irradiated Laboratory-Reared Screw-Worm Flies1. J. Econ. Entomol. 58, 373–374 (1965).
Mayer, D. G. & Atzeni, M. G. Estimation of dispersal distances for Cochliomyia hominivorax (Diptera: Calliphoridae). Environ Entomol 22, (1993).
Quiroz, H. Gusano Barrenador: historia de La Campaña de erradicación contra El Gusano Barrenador Del Ganado. Imagen Vet. 3, 4–11 (2003).
Krafsur, E. S., Whitten, C. J. & Novy, J. E. Screwworm eradication in North and Central America. Parasitology Today vol. 3 Preprint at (1987). https://doi.org/10.1016/0169-4758(87)90196-7
(Comisión México-Americana para la Erradicación del Gusano Barrenador del Ganado) COMEXA. Gusano Barrenador: XXX aniversario de La planta productora de de Moscas estériles Del Gusano Barrenador Del Ganado. Imagen Vet. 3, 4–11 (2003).
Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). Análisis Del Impacto Potencial Del Gusano Barrenador En México. (2019).
Vargas-Terán, M. The new world screwworm in Mexico and central America. World Anim. Review 28–35 (1991).
Grisi, L. et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Revista Brasileira De Parasitol. Veterinária 23, (2014).
Vo, T. Economic impact of eradicating the New World screwworm (Cochliomyia hominivorax) from Jamaica. in Proceedings: Area-Wide Control of Fruit Flies and Other Insect Pests. International Conference on Area-Wide Control of Insect Pests, and the 5’ International Symposium on Fruit Flies of Economic Importance 113–116Penang, Malaysia, (2000).
Dupuis, J. R. et al. Molecular characterization of the 2016 new world screwworm (Diptera: Calliphoridae) outbreak in the Florida keys. J Med. Entomol 55, (2018).
Skoda, S. R., Phillips, P. L., Welch, J. B. & Screwworm (Diptera: Calliphoridae) in the United States: Response to and elimination of the 2016–2017 outbreak in Florida. Journal of Medical Entomology 55 Preprint at (2018). https://doi.org/10.1093/jme/tjy049
Venegas-Montero, D. P. et al. Case report: Re-emergence of Cochliomyia hominivorax in Costa rica: report of a human myiasis case 23 years after elimination. Am. J. Trop. Med. Hyg. 111, 1020–1023 (2024).
Comisión Panamá – Estados Unidos para la Erradicación y Prevención del Gusano Barrenador del Ganado. Comisión Panamá – Estados Unidos para la Erradicación y Prevención del Gusano Barrenador del Ganado (COPEG). (2025). https://www.copeg.org/
World Animal Health Information System (WAHIS). (2025). https://wahis.woah.org/#/event-management
Animal and Plant Health Inspection Service (APHIS). Importing Live Cattle and Bison from Mexico to the United States. (2024). https://www.aphis.usda.gov/live-animal-import/cattle-bison-germplasm/mexico
Herrera, D. 15 mil cabezas de ganado de Tamaulipas acorraladas por gusano barrenador. Daisy Herrera (2024). https://daisyherrera.com/15-mil-cabezas-de-ganado-de-tamaulipas-acorraladas-por-gusano-barrenador/?fbclid=IwZXh0bgNhZW0CMTEAAR39GAMvaz7XEs8j9s4rrpXCV0xma0e0dTJxgmYYoItiJwS3atzDg4tOs6Q_aem_t_A7pq1qUvDNC8whlT50jA
Barragán, A. El Regreso Del Gusano Barrenador Pone En Jaque al sector Ganadero mexicano: no Podemos Vender Nuestros Becerros. El País (2024). https://elpais.com/mexico/2024-12-16/el-regreso-del-gusano-barrenador-pone-en-jaque-al-sector-ganadero-mexicano-no-podemos-vender-nuestros-becerros.html
Chamberlain, S. Package ‘spocc’ - Interface to Species Occurrence Data Sources. CRAN Repository Preprint at (2018).
Global Biodiversity Information Facility (GBIF). GBIF Occurrence Download. (2024). https://doi.org/10.15468/dl.d4r7tw
Osorio-Olvera, L. et al. ntbox: an R package with graphical user interface for modelling and evaluating multidimensional ecological niches. Methods Ecol. Evol 11, (2020).
Aiello-Lammens, M. E. et al. SpThin: an R package for Spatial thinning of species occurrence records for use in ecological niche models. Ecography 38, (2015).
Soberon, J. & Peterson, A. T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics 2, (2005).
Barve, N. et al. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Modell 222, (2011).
Machado-Stredel, F., Cobos, M. E. & Peterson, A. T. A simulation-based method for selecting calibration areas for ecological niche models and species distribution models. Front Biogeogr 13, (2021).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km Spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Booth, T. H. Checking bioclimatic variables that combine temperature and precipitation data before their use in species distribution models. Austral Ecol. 47, 1506–1514 (2022).
Rojas-Soto, O. et al. Calibration areas in ecological niche and species distribution modelling: unravelling approaches and concepts. J Biogeogr 51, (2024).
Jiménez, L. & Soberón, J. Estimating the fundamental niche: accounting for the uneven availability of existing climates in the calibration area. Ecol Modell 464, (2022).
Osorio-Olvera, L., Soberón, J. & Falconi, M. On population abundance and niche structure. Ecography 42, 1415–1425 (2019).
Osorio-Olvera, L., Yañez-Arenas, C., Martínez-Meyer, E. & Peterson, A. T. Relationships between population densities and niche-centroid distances in North American birds. Ecology Letters vol. 23 Preprint at (2020). https://doi.org/10.1111/ele.13453
Ochoa-Zavala, M. et al. Reduction of genetic variation when Far from the niche centroid: prediction for Mangrove species. Frontiers Conserv. Science 2, (2021).
Nava-Bolaños, A. et al. Critical areas for pollinator conservation in mexico: A cross-border priority. Biol Conserv 283, (2023).
Soberón, J. & Osorio-Olvera, L. A dynamic theory of the area of distribution. J Biogeogr 50, (2023).
Osorio-Olvera, L. & Soberón, J. Species Distribution Models as a Function of Biotic, Abiotic and Movement Factors (BAM). (2024). https://luismurao.github.io/bamm
Gray, L. Mathematician looks at wolfram’ s new kind of science. Notices AMS. 50, 200–211 (2002).
Nuñez-Penichet, C., Soberón, J. & Osorio-Olvera, L. The dispersal patterns of a migratory insect are driven by biotic interactions. J Biogeogr 50, (2023).
Nuñez-Penichet, C. et al. Geographic potential of the world’s largest hornet, Vespa mandarinia Smith (Hymenoptera: Vespidae), worldwide and particularly in North America. PeerJ 9, (2021).
De Wysiecki, A. M., Cortés, F., Jaureguizar, A. J. & Barnett, A. Potential global distribution of a temperate marine coastal predator: the role of barriers and dispersal corridors on subpopulation connectivity. Limnol Oceanogr 67, (2022).
Pimentel, D., Zuniga, R. & Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the united States. Ecological Economics 52, (2005).
Gutierrez, A. P. & Ponti, L. Eradication of invasive species: why the biology matters. Environ Entomol 42, (2013).
McGarry, J. W. Tropical myiases: neglected and well travelled. The Lancet Infect. Diseases 14, (2014).
Muchiut, S., Miró, M. V., Anziani, O., Nava, S. & Lifschitz, A. Failure of doramectin and Ivermectin in preventing Cochliomyia hominivorax myiasis in a subtropical region: A pharmacokinetic-pharmacodynamic study. Vet. Parasitol. 334, 110384 (2025).
Subedi, B., Poudel, A. & Aryal, S. The impact of climate change on insect pest biology and ecology: implications for pest management strategies, crop production, and food security. J Agric. Food Res 14, (2023).
Hulme, P. E. Invasive species challenge the global response to emerging diseases. Trends Parasitology 30, (2014).
Gutierrez, A. P. & Ponti, L. The new world screwworm: prospective distribution and role of weather in eradication. Agric Entomol 16, (2014).
Gutierrez, A. P., Ponti, L. & Arias, P. A. Deconstructing the eradication of new world screwworm in North america: retrospective analysis and climate warming effects. Med Vet. Entomol 33, (2019).
Baumhover, A. H. A Personal Account of programs to eradicate the screwworm, Cochliomyia hominivorax, in the United States and Mexico with special emphasis on the Florida program. in Pioneer Lecture presentation sponsored by the Florida Entomological Society (1997).
Lindquist, A. W. & Barrett, W. L. Overwintering of Cochliomyia americana at uvalde, Texas. J Econ. Entomol 38, (1945).
Adams, T. S. The reproductive physiology of the screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae) II. Effect of constant temperatures on oogenesis. J. Med. Entomol. 15, 484–487 (1979).
Forero Becerra, E., Cortes, J. A. & Villamil, L. C. Ecologia y epidemiologia Del Gusano Barrenador Del Ganado Cochliomyia hominivorax (Coquerel, 1858). Rev Med. Vet. (Bogota) (2007).
Fresia, P., Azeredo-Espin, A. M. L. & Lyra, M. L. The phylogeographic history of the new world screwworm fly, inferred by approximate bayesian computation analysis. PLoS One 8, (2013).
Mulieri, P. R. & Patitucci, L. D. Using ecological niche models to describe the geographical distribution of the myiasis-causing Cochliomyia hominivorax (Diptera: Calliphoridae) in Southern South America. Parasitology Research 118, (2019).
Anziani, O. S. & Volpogni, M. M. Incidence of bovine myiasis (Cochliomyia hominivorax) in the central area of Argentina. in Annals New. York Acad. Sciences 791, (1996).
Costa-Júnior, L. M. et al. A review on the occurrence of Cochliomyia hominivorax (Diptera: Calliphoridae) in Brazil. Revista Brasileira De Parasitol. Veterinaria 28, (2019).
Phillips, P. L., Welch, J. B. & Kramer, M. Seasonal and Spatial distributions of adult screwworms (Diptera: Calliphoridae) in the Panama Canal area, Republic of Panama. J. Med. Entomol. 41, 121–129 (2004).
Brenner, R. J. Distribution of screwworms (Diptera: Calliphoridae) relative to land use and topography in the humid tropics of Southern Mexico. Ann Entomol. Soc. Am 78, (1985).
Lassala, A., Hernández-Cerón, J., Pedernera, M., González-Padilla, E. & Gutiérrez, C. G. Cow-calf management practices in mexico: reproduction and breeding. Veterinaria Mexico 7, (2020).
James, M. T. The Flies That Cause Myiasis in Man /. (U.S. Dept. of Agriculture, Washington, D.C.:, (1947). https://doi.org/10.5962/bhl.title.65688
Lindquist, D. A., Abusowa, M. & Hall, M. J. R. The new world screwworm fly in libya: a review of its introduction and eradication. Med Vet. Entomol 6, (1992).
Fresia, P. et al. Applying Spatial analysis of genetic and environmental data to predict connection corridors to the new world screwworm populations in South America. Acta Trop 138, (2014).
Tietjen, M., Pfeiffer, V. & Poh, K. C. Insights into the genetic landscape and presence of Cochliomyia hominivorax in the Caribbean. Parasitol Res 122, (2023).
Tietjen, M. et al. Geographic population genetic structure of the new world screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae), Using SNPs. J Med. Entomol 59, (2022).
Knipling, E. The Basic Principles of Insect Population Suppression and Management. (1979).
Knipling, E. F. Entomology and the management of man’s environment. Aust J. Entomol 11, (1972).
United States Departament of Agriculture (USDA). Cattle and Bison Imports from Mexico Resume Under New Protocol. (2025). https://www.usda.gov/about-usda/news/press-releases/2025/02/01/cattle-and-bison-imports-mexico-resume-under-new-protocol
Animal and Plant Health Inspection Service- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (APHIS-SENASICA). APHIS-SENASICA New World screwworm Protocol for the Importation of Ruminants from Mexico to the United States. (2025). https://www.aphis.usda.gov/sites/default/files/aphis-senasica-nws-protocol-import-ruminants.pdf?utm_medium=email&utm_source=govdelivery
Fadda, L. A., Osorio-Olvera, L., Ibarra-Juárez, L. A., Soberón, J. & Lira-Noriega, A. Predicting the dispersal and invasion dynamics of ambrosia beetles through demographic reconstruction and process-explicit modeling. Sci. Rep. 14, 7561 (2024).
Hightower, B. G., Davis, R. B., Baumhover, A. H. & Graham, O. H. Seasonal abundance of the Screw-Worm in Northern Mexico1. J Econ. Entomol 59, (1966).
Coronado, A. & Kowalski, A. Current status of the new world screwworm Cochliomyia hominivorax in Venezuela. Med Vet. Entomol 23, (2009).
Van De Vuurst, P. & Escobar, L. E. Climate change and infectious disease: a review of evidence and research trends. Infect Dis. Poverty 12, (2023).
Van de Vuurst, P., Qiao, H., Soler-Tovar, D. & Escobar, L. E. Climate change linked to vampire Bat expansion and rabies virus spillover. Ecography https://doi.org/10.1111/ecog.06714 (2023).
Ogden, N. H. & Lindsay, L. R. Effects of climate and climate change on vectors and Vector-Borne diseases: ticks are different. Trends Parasitology32, (2016).
Altizer, S., Ostfeld, R. S., Johnson, P. T. J., Kutz, S. & Harvell, C. D. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, (2013).
United States Departament of Agriculture (USDA). 2022 Census of Agriculture. (2024). https://www.nass.usda.gov/Publications/AgCensus/2022/index.php
Cunningham, E. P. et al. Le programme d’éradication de La Lucilie bouchère d’afrique du Nord. Rev Elev Med. Vet. Pays Trop 45, (1992).
Reck, J. et al. Does Rhipicephalus microplus tick infestation increase the risk for myiasis caused by Cochliomyia hominivorax in cattle? Prev Vet. Med 113, (2014).
Dicko, A. H. et al. Using species distribution models to optimize vector control in the framework of the Tsetse eradication campaign in Senegal. Proc. Natl. Acad. Sci. U S A. 111, 10149–10154 (2014).
Acknowledgements
Special thanks to the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (Secihti), Mexico, for providing scholarship support to UMV-E (CVU 835901) and LAF (CVU 1013389) for their doctoral studies at the Instituto de Ecología, A.C. (INECOL). LO-O acknowledges financial support from the UNAM Research and Technological Innovation Support Program (PAPIIT IA202824) and the Secihti Frontier Science Project (CF-2023-I-115). LO-O also thanks Nancy Gálvez-Reyes, Graciela García-Guzmán, and Miguel Baltazar Gálvez for their technical support on projects IA202824 and CF-2023-I-115. AL-N acknowledges support from the Alexander von Humboldt Foundation through a Georg Forster Research Fellowship for a research stay at Georg-August Universität Göttingen.
Author information
Authors and Affiliations
Contributions
U.M.V.-E., D.J.-G., and A.L.-N. conceived and designed the study. U.M.V.-E. and L.A.-F. collected the data. U.M.V.-E., R.M., D.J.-G., and A.L.-N. conducted the ecological niche models, while L.O.-O. conducted the dispersal models. U.M.V.-E. and L.A.-F. created the final figures. All authors contributed to the first draft, made substantial revisions to the analysis and interpretation of the results, and worked on the final manuscript. All authors reviewed and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
All supplementary information is available in the Zenodo repository (https://doi.org/10.5281/zenodo.15465751).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Valdez-Espinoza, U.M., Fadda, L.A., Marques, R. et al. The reemergence of the New World screwworm and its potential distribution in North America. Sci Rep 15, 23819 (2025). https://doi.org/10.1038/s41598-025-04804-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04804-9