Abstract
In this study, the effect of Zr elements on Al–10Ce alloys were investigated in terms of the microstructure, mechanical properties at room and high temperatures, and corrosion resistance. Meanwhile, the finite element simulations on tensile fracture was adopted to explain the fracture process of Al–Ce alloys. Results showed that as Zr content increased from 0.05 to 0.15%, the eutectic Al11Ce3 phase were gradually coarsened. However, further increase in Zr content led to the refinement and homogenization. Meanwhile, a significant transformation in the morphology of eutectic Al11Ce3 from layered to rod-like or bar-like structures happened. Correspondingly, mechanical properties at room temperature such as hardness, tensile strength, and yield strength initially decreased but subsequently increased. The tensile strength and yield strength of the alloys could reach the maximum values of 160 MPa and 96 MPa, respectively, and the elongation of the alloy reached the highest of 14.4%. The yield strength and the tensile strength at high temperature of 300 °C exhibited the highest values of 62.5 Mpa and 74 Mpa, respectively, when the Zr content is 0.05%. Moreover, the corrosion current of the alloys was minimized to 1.158 × 10− 7 µA/cm2 when the content of Zr was 0.05%, which attributed to the observed optimal microstructural characteristics of the Al11Ce3 phase at this concentration. Therefore, it is believed that Al–10Ce–Zr alloys are excellent candidate engineering materials in extreme environment fields.
Similar content being viewed by others
Introduction
Rare earth (RE) aluminum alloys have been extensively studied due to their excellent mechanical properties, wear resistance and high temperature performance1,2,3,4. They have wide applications in aerospace, automotive and defense fields where high strength-to-weight ratio and capability to withstand extreme environments are critical5,6. However, the complexity of RE-Al alloy systems presents challenges for further improving their properties through alloy modification.
Recent advancements in aluminum-cerium (Al-Ce) alloys have highlighted cerium’s potential in enhancing the microstructure and properties of aluminum alloys7,8,9,10,11,12,13. The Al-Ce binary phase diagram demonstrates a pivotal transformation around 10 wt% Ce at 640 °C, leading to coexistence of α-Al and Al11Ce3 phases14,15,16. This tailored composition can improve thermal stability and mechanical properties, marking a significant leap in alloy technology. However, the challenge persists in augmenting the strength and high-temperature resilience of Al-Ce alloys. One promising strategy is the incorporation of transition metals, particularly focusing on zirconium (Zr) due to its high melting point and robust Al-Zr interactions, fostering the formation of beneficial intermetallic.
In recent years, the strengthening mechanisms of Zr have been widely studied in Al alloys. The addition of Zr elements to Al alloys can greatly refine the grain to further enhance the strength and ductility. Feng et al. demonstrated that Al3Zr particles observed at or near the centre of the particles are high-strength nuclei of α-Al in aluminum17. Dunand et al. pointed out that during aging, supersaturated Al-Sc, Al-Zr, or Al-Zr-Sc solid solutions could form the nanoscale elastically hard Al3Sc, Al3Zr, or Al3(Sc, Zr) precipitates in the Al matrix18,19,20. In unsaturated aluminum alloys, the Zr element is mainly solid-solved within the alloy and plays a role in solid solution strengthening of the alloy, while the Zr element inhibits the segregation of other elements, which also enhances the strengthening of the solid solution to a certain extent21. In supersaturated alloys, the addition of Zr generates Al3Zr particles with the Al matrix, and Al3Zr particles can play a role in grain refinement, and Al3Zr particles will be pinned at grain boundaries to improve the room temperature mechanical properties of the alloy22,23. It has been found that the addition of 0.06 at% Zr to pure Al increased the microhardness value of the alloy from 200 MPa to 295 MPa24. The study by Mohammed et al. indicated that the addition of Zr significantly reduced the grain size of the Al-9Ce alloy while successfully maintaining the unique shape of the Al11Ce3 phase, which implies that the tensile strength of the alloys with the addition of the Zr element was significantly higher than that of the binary Al-Ce alloys at both room and elevated temperatures25. Meanwhile, in Al-5wt.%Ce hypoeutectic alloys, the average grain size was refined from 576 ± 182 μm (Zr-free) to 452 ± 148 μm (0.5 wt% Zr), and ultimate tensile strength was increased from 126 MPa to 137 MPa, and elongation was improved from 32 to 37%, and these results showed that the addition of Zr effectively addressed the strength-ductility trade-off of alloys26. The research from the addition of Zr in Al-Fe alloys showed that the refined grain structure and enhanced mechanical properties were realized by forming L12-Al3Zr precipitates27. Therefore, it is concluded that the introduction of Zr in Al-Ce alloys can effectively optimize the properties by Zr-induced grain refinement and strengthening.
While significant advancements have been made in understanding the impact of Zirconium (Zr) on Aluminum-Cerium (Al-Ce) alloys, critical aspects remain underexplored. Specifically, comprehensive insights into how Zr addition influences the corrosion resistance, microstructural stability, and overall mechanical properties of Al-Ce alloys are still lacking. However, further investigation is necessary to fully comprehend the holistic understanding of Zr’s role in Al-Ce alloy systems.
Therefore, the present work aims to perform a comprehensive study on the influences of Zr content on the microstructure, mechanical properties and corrosion behavior of Al-Ce alloys. By combining experiments and simulations, this study will provide new insights into the role of Zr in modifying the microstructure and properties of Al-Ce based alloys.
Experimental process
Alloy preparation
High-purity aluminum (99.99 wt%), cerium (99.99 wt%) and zirconium (99.99 wt%) supplied by Youyan Rare-Earth New Materials Co., Ltd. were used to prepare Al-Ce-Zr alloys. The designed compositions were listed in Table 1.
The raw materials were melted in a graphite crucible in a resistive furnace following the process as shown in Fig. 1. After the aluminum was placed in graphite crucible in furnace, the temperature was heated to 720 °C with a heating rate of 10 °C/min and held for 4 h to melt the aluminum completely. Then, the cerium and zirconium wrapped in aluminum foil were placed in melted aluminum and the temperature was increased to 800 °C for 2 h with a heating rate of 10 °C/min to realize the melting. During the heating, the alloy melting was mechanically stirred for 1 h to reach the homogeneity, and then was cast into a preheated graphite mold at 300 °C for 1 h. Cylindrical alloy ingots with 10 mm diameter were obtained after solidification.
Characterization
The microstructures of the alloys were examined by optical microscope (DMI8M, Leica) after etching using Keller solution (95ml H2O, 2.5 ml HNO3, 1.5 ml HCl and 1 ml HF). Additionally, scanning electron microscope (SEM, SU8010, Hitachi) with EDS (EDAX E1506-C2B) was used to analyze the polished surface morphology, tensile fracture surface morphology and elemental distributions of alloys. The phase compositions of alloys were identified by X-ray diffraction (D8 Advance, Bruker) with Cu Kα radiation at a scanning rate of 2°/min within the 2θ range of 20°-80°. Hardness was measured using a hardness tester (MHVD-30IS) by taking ten different points for each samples. Tensile tests were performed on a universal testing machine (CMT5105) to obtain tensile strength, yield strength, and elongation. In addition, the corrosion behavior was evaluated using an electrochemical workstation (CHI660E) using electrochemical methods in 3.5wt% NaCl solution.
Finite element simulation
The Al-Cr-Zr material plastic model parameters were inputted into the ABAQUS software 2020 for finite element simulation. As shown in Fig. 2, the finite element simulation model was established based on the real specimen size for modeling. Figure 2(a) showed the grid size division, where the C3D4 grid was used and the middle region was focused on for mesh refinement, with a total of 23,418 mesh cells. Figure 2(b) showed the setup of boundary conditions for the finite element model, where the lower end was fully fixed and a displacement load was applied to the upper end in the y-axis direction to simulate tensile experiments. The damage model was set for flexibility failure. The simulated strain rate was 10− 3s− 1, obtaining the load-displacement curve of the smooth specimen. The simulated load-displacement curve was compared with the experimental load-displacement curve to determine the feasibility of the model, as shown in Fig. 2(c). In this study, the key to establish the constitutive model and failure model of Al-Ce-Zr alloy was to estimate the material parameters in the model according to the existing experimental data. The results indicated a high degree of correlation between the simulated and experimental data, suggesting the model’s effectiveness in predicting the behavior of Al-Ce-Zr alloys under tensile stress. This validation opened avenues for further simulations to optimize alloy properties and explore new alloy compositions.
Results and discussion
Microstructures of Al–Ce–Zr alloy
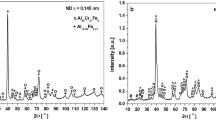
The XRD patterns of cast Al-Ce-Zr alloys with different Zr contents are shown in Fig. 3(a). It can be observed that all alloy samples exhibit the diffraction peaks corresponding to α-Al phase and Al11Ce3 phase. The increasing Zr content has no evident effect on the phase composition of alloys. In addition, Al-Zr or Al-Ce-Zr phases are not detected owing to their amounts are below the detection limit25. Figure 3(b-f) shows the optical morphology images of cast Al-Ce-Zr alloys with different Zr contents. The distribution of the eutectic Al11Ce3 phase in alloys are observed. According to the binary phase diagram of Al-Ce, the eutectic point of Al-Ce alloys is at 10 wt% Ce, so the eutectic Al11Ce3 phase is the main phase in the casting Al-10Ce-xZr (x = 0.05, 0.1, 0.15, 0.2 and 0.25 wt%) alloys. It is observed from the optical images that the eutectic Al11Ce3 phase undergoes partial segregation when the Zr content is 0.05%, and the segregation of the eutectic Al11Ce3 phase is intensified and the spacing between eutectic Al11Ce3 phases increased from 1 μm to 1.67 μm with a continuous increase of the Zr content from 0.1 to 0.15%. When the Zr content is greater than 0.15%, the segregation of eutectic Al11Ce3 phase decreases with the increase of Zr content, and the spacing between eutectic phases decreases from 1.67 μm to 1.1 μm and 0.34 μm, respectively. The detailed scanning of the Al-10Ce-xZr alloys’ microstructure in Fig. 3(b-f) shows that Zr additions affect the eutectic Al11Ce3 phase segregation and their spacing. As Zr content rises to 0.15 wt%, segregation and spacing among the eutectic phases are enlarged, but further addition of Zr beyond this point results in a denser eutectic structure with reduced phase spacing. The refinement in the microstructure is observed when Zr content exceeds 0.15 wt%, indicating a shift towards a more homogeneous and compact eutectic arrangement. This structural change, marked by decreased spacing among the eutectic Al11Ce3 phases, suggests that higher Zr additions contribute to a more uniform microstructural distribution, potentially leading to enhanced mechanical properties of the alloys. As shown in the study by Ye et al., the tensile and yield strengths increased, while the total elongation decreased with increasing Zr content. The strengthening mechanism is attributed to fine-grain, solution, and second-phase strengthening28.
Figure 4(a-e) shows the SEM images of cast Al-Ce-Zr alloys with different Zr contents. It can be seen more clearly that the eutectic Al11Ce3 phase in the alloys shows different morphologies and sizes. With the increase of Zr content, the morphology of eutectic Al11Ce3 phase changes from layered and rod-shaped to spherical and dot-shaped, and the size of eutectic Al11Ce3 phase also varies from several microns to tens of nanometers. These changes indicate that the addition of Zr element has a significant effect on the microstructure of Al-Ce-Zr alloys, which might be related to the dissolution and precipitation of Zr elements. Figure 4(I-V) shows the magnified SEM image of cast Al-Ce-Zr alloy, and it can be seen that a large amount of eutectic Al11Ce3 phase is distributed on the α-Al matrix, and its morphology is granular and rod-shaped. Meanwhile, the eutectic Al11Ce3 phase changes from layered to rod-shaped or bar-shaped with the increase of Zr contents.
Figure 5 shows the EDS point and mapping scan images of cast Al-10Ce-0.1Zr alloy. It is found that a large amount of Ce element, a small amount of Al element and Zr element are distributed in the white second phase, which is speculated to be a Ce-rich intermetallic. In order to further explore the composition, EDS point scan is performed on the second phase with different morphologies, it is found that the black matrix (point 3) is the α-Al phase, and the white second phase with different morphologies (point 1 and point 2) are both the Al11Ce3 phase. A large amount of Zr atoms are uniformly distributed in the white second phase, and a small amount of Zr atoms are also present at the edge of the black matrix and the white second phase, indicating that most of the Zr atoms are dissolved in the Al11Ce3 phase, Zr element replaces some of the Ce elements in the Al11Ce3 phase, and a small part of Zr atoms are dissolved in the α-Al matrix and aggregated at the α-Al/Al11Ce3 interface. Therefore, the refinement of Al11Ce3 phase by Zr element may be due to the change of undercooling. It has been shown that the different morphologies of the Al11Ce3 phase are strongly related to the Zr element, mainly because the Zr element changes the undercooling degree of the melt during the solidification process, which results in the change of the morphology of the Al11Ce3 phase from lamellar to short rod-like29. Meanwhile, the transition of the second phase from a lamellar structure to a short rod-shaped structure has been proven to effectively improve the plasticity of Al-Si alloys30,31. Therefore, the refinement of Al11Ce3 phase by Zr element may be due to the change of undercooling degree32.
Effect of adding Zr element on the mechanical properties of Al-Ce alloys
Room temperature properties
The effect of different Zr contents on the Vickers hardness and room temperature tensile properties of cast Al-10Ce-xZr (x = 0.05, 0.1, 0.15, 0.2 and 0.25 wt%) alloys are investigated. Meanwhile, the finite element simulation is used to analyze the fracture behavior of the alloys. Figure 6(a) shows the variation of Vickers hardness of cast Al-Ce-Zr alloys with different Zr contents. With the increase of Zr content in the alloy, the hardness of the alloy shows a trend of first decreasing and then slightly increasing. The hardness of the alloy reaches the highest value of 47.6 HV when the Zr content is 0.05%, and the hardness of the alloy has the lowest value of 38.35 HV when the Zr content is 0.15%. The addition of a small amount of Zr element intensifies the segregation of α-Al phase inside the alloy, resulting in the decrease of the hardness of the alloy. However, the addition of further Zr element breaks the lamellar Al11Ce3 phase into rod-like or strip-like. After the addition of 0.2% Zr, the average grain size is reduced from 1320 μm to 495 μm. Meanwhile, Zr element also plays a role in refining the grains, leading to an increase in the hardness of the alloy. This increase was attributed to the presence of more grain or subgrain boundaries in the alloy due to smaller grains, thereby enhancing the alloy’s properties33,34,35. However, the strengthening effect of grain refinement on hardness is limited, being less than 5 MPa36. Therefore, the increased hardness of alloy is mainly due to the solid solution strengthening effect of Zr element, while the decrease of alloy hardness is mainly due to the segregation effect of eutectic phase.
Figure 6(b) shows the stress-strain curve of cast Al-Ce-Zr alloys with different Zr contents, and the room temperature tensile properties, including tensile strength, yield strength and elongation, are shown in Table 2. With the increase of Zr content, the tensile strength and yield strength of the alloys show an overall trend of first decreasing and then increasing. When the Zr content is 0.05%, the tensile strength and yield strength of the alloys are 152 MPa and 100 MPa, respectively. As the Zr content increases to 0.15%, the tensile strength and yield strength of the alloys reach the lowest values, which are 109 MPa and 87 MPa, respectively. However, when the Zr content reaches 0.2%, the tensile strength and yield strength of the alloys reach the maximum values, which are 160 MPa and 96 MPa, respectively. When the Zr content increases to 0.25%, the strength of the alloy decreases. The elongation of the alloy shows a trend of first increasing and then decreasing. When the Zr content is 0.1%, the elongation of the alloy reaches the highest of 14.4%. The segregation and coarsening of eutectic phase reduce the strength of the alloy, while the refinement and homogenization of eutectic phase increase the strength of the alloy. In addition, grain refinement and uniform distribution of second phase are beneficial to improve the elongation of the alloy. It was reported that the ultimate tensile strength was 126 MPa26 and 95 MPa36 for as-cast Al-5wt.%Ce alloys, which are lower than the optimal tensile strength of alloys in this work. For instance, Zhu et al. conducted the grain refinement and modification combined in a hypoeutectic Al-Si-Mg-Ti-Be cast alloy, resulting in enhanced ultimate tensile strength and elongation at fracture. Furthermore, it had been found that grain refinement and the uniform distribution of the second phase contributed to the improvement of the alloy’s elongation37. Research has shown that after extending the aging time, the distribution of GP zones in the matrix of 7005 aluminum alloy becomes more uniform, leading to an increase in both strength and elongation. This finding supports the view that a uniform distribution of the second phase can enhance mechanical properties38.
The presence of numerous dimples, either elliptical or circular, on the room temperature tensile fracture surface of the Al-Ce-0.2Zr alloys, as depicted in Fig. 6(c), typically signifies a ductile fracture mechanism. Dimpled rupture is a hallmark of ductile fracture, where the material undergoes considerable plastic deformation before failure. The shapes and sizes of these dimples can provide insights into the stress state and deformation behavior of the material during fracture. Elliptical dimples often suggest uniaxial tensile stress, while circular dimples may indicate a more isotropic stress state. This observation of ductile fracture in the Al-Ce-0.2Zr alloy aligns with the expected behavior of ductile materials under tensile loading, corroborating the fracture mechanism as predominantly ductile in nature. The stress distribution of the alloys (Fig. 6(d)) during the tensile fracture process is further explored by finite element model, and the result is shown in Fig. 6(e). It can be seen that the fracture position is consistent with that of the experiment, and they are in the middle of the specimen. The simulated stress concentration cloud diagram of different stages is shown in Fig. 7, which gives the concept of stress distribution and plastic deformation in the material during the tensile process. It is worth noting that the fracture elongation and fracture position have consistency no matter how the tensile stage is, indicating that the material has uniform fracture at different strain levels. This observation result is very consistent with the experimental results, which emphasizes the reliability of the simulation in capturing the plastic deformation behavior of the material. Wang et al. also used finite element simulation to simulate the tensile behavior of smooth specimens. As the plastic strain increased, the crack propagation stage and ductile fracture stage were displayed. The simulation curves were in good agreement with the experimental results, and the ductile tearing phenomenon of the tensile failure specimen was determined39.
High temperature properties
As shown in Fig. 8(a), both the yield strength and the tensile strength at high temperature of 300 °C exhibits the highest values when the Zr content is 0.05%, which are 62.5 Mpa and 74 Mpa respectively, indicating the potential strengthening effect of Zr at low concentration. However, when the Zr content increases to 0.1% and 0.15%, the yield strength and the tensile strength decrease significantly, which might be due to the increased internal stress caused by the segregation of eutectic Al phase40,41,42. Notably, when the Zr content is 0.2%, the strength of the alloy rebounds and reaches the peak. This phenomenon might be attributed to the further refinement of the grain size and the better control of the dislocation movement by the presence of Zr. However, exceeding 0.2% of Zr content leads to the decrease of strength, and this reduction reflects a complex interaction among factors such as the alloying elements’ distribution, the size and distribution of Zr-containing phases, the presence of impurities, the effects of solidification rate on microstructure, and the possible formation of new phases or precipitates. In addition, the elongation of the alloy shows a trend of first increasing and then decreasing, where the highest elongation is observed when the Zr content is 0.1%, which might be related to the grain refinement and the uniform distribution of second phase. Ye et al. concluded that the Al11Ce3 phase was favorable for improving the mechanical properties of alloys at high temperatures. The microhardness of alloys with different Zr additions increased with exposure time at 300◦ C, indicating that the Zr-rich phase had a strengthening effect43. In summary, these findings highlight the complex influence of Zr content on the mechanical behavior of cast Al-Ce-Zr alloys at high temperature, which has implications for the alloy design and application.
The macro and micro fracture of high-temperature tensile of cast Al-Ce-Zr alloys are shown in Fig. 8(b-c), where relatively rounded dimples are observed in the micro fracture of cast Al-Ce-Zr alloys. The presence of these tough dimples indicates that the micro cracks in the alloy undergoes plastic deformation under stress during the tensile process, eventually leading to fracture. Compared with the room-temperature tensile, the micro fracture of high-temperature tensile shows more uniform distribution of dimples, which might be due to the increased plastic deformation at high temperature, making the internal micro cracks more evenly distributed in the alloy44. The macro fracture of high-temperature tensile shows obvious ductility, and the fracture position occurs in the upper middle part of the alloy. This indicates that the fracture mode of the alloy is ductile fracture under high-temperature conditions. The finite element simulation with the addition of the temperature parameter shows a similar situation (Fig. 8(d)), with significant plastic deformation and then fracture in tension, in agreement with the experimental situation.
In Fig. 8, the stress-strain curves of cast Al-Ce-Zr alloys at various Zr concentrations reveal the mechanical behavior under high-temperature conditions. The fracture morphology of the Al-10Ce-0.2Zr alloy, captured by scanning electron microscopy, displays characteristic features that are integral to understanding the material’s response to stress. These include the presence of shallow dimples and pores which are indicative of the ductile nature of the fracture at high temperatures, as well as the stress distribution and localization at the microstructural level which ultimately lead to the formation of microcracks and voids, thereby contributing to the overall fracture process45.
Comparatively, in Fig. 9, the ABAQUS simulation of tensile stress at elevated temperatures shows the distribution of stress concentrations throughout the material, with areas of high stress indicating potential sites of plastic deformation or failure. The simulation reveals that under high-temperature conditions, the material exhibits significant plastic behavior, as evidenced by the extensive spread of stress concentration areas, which suggests a more uniform stress distribution across the material. To ensure a comprehensive understanding and verification of the finite element model’s accuracy, the simulation results were meticulously compared with the experimental findings. This comparison is pivotal, as it confirms the reliability of the constitutive and damage parameters used in the simulation, providing a concrete foundation for the interpretation of the fracture morphology observed experimentally.
The experimental tensile data, showing the tensile strength and the percentage elongation after fracture, resonate with the simulated behavior of the alloy. The simulation, which effectively reproduces the entire sequence from crack initiation to complete fracture. In essence, the integration of experimental data with finite element analysis elucidates the mechanical response under high-temperature tensile loads. The simulation provides a virtual yet realistic representation of the material’s fracture behavior, which, when aligned with experimental observations, offers a robust framework for predicting the performance of cast Al-Ce-Zr alloys in high-temperature applications.
Corrosion resistance of Al–Ce–Zr alloy
Figure 10(a) shows the Tafel polarization curves of cast Al-Ce-Zr alloys in 3.5 wt% NaCl solution. The aluminum alloys underwent cathodic polarization firstly. When the potential is higher than the self-corrosion potential, the aluminum alloys undergo anodic polarization, and the matrix of the aluminum alloys dissolves at this time. With the electrode potential moving positively, the corrosion current density increases. Table 3 shows the self-corrosion potential (Ecorr) and corrosion current density (Icorr) of cast Al-Ce-Zr alloys with different Zr contents in 3.5 wt% NaCl solution. With the increase of Zr content, the self-corrosion potential shifts negatively significantly, and the corrosion tendency increases. The corrosion current density shows a trend of increasing first and then decreasing. When the Zr content is 0.05%, the corrosion current of the alloy is the smallest value of 1.158 × 10− 7 µA/cm2, indicating that the working electrode with 0.05% Zr has the greatest ability to hinder the charge transfer, and the corrosion resistance of the alloy is the excellent at this time. When the Zr content is 0.1%, the corrosion current of the alloy is the largest value of 2.502 × 10 − 7 µA/cm2, indicating that the charge transfer resistance of the alloy is small, and the corrosion resistance of the alloy is the worst. When the Zr content exceeds 0.1%, the corrosion resistance of the alloy is gradually improved with the increase of Zr content. By observing the corrosion current density, the relationship between the Zr content and the corrosion resistance of the alloy could be found. With the increase of Zr content, the corrosion resistance of the alloy shows a phenomenon of weakening first and then strengthening and then weakening again, which is mainly related to the morphology, size and distribution of the internal Al11Ce3 phase.
The arc resistance represents the double layer behavior between the metal matrix and the solution, and the larger the radius, the better the surface corrosion resistance. Figure 10(b) shows the Nyquist plots of cast Al-Ce-Zr alloys with different Zr contents. It can be seen that the electrochemical impedance of the alloy is composed of medium to high frequency capacitive reactance arc and low frequency induction arc. The medium to high frequency capacitive arc is generated by the oxide film on the surface of aluminum alloy. The generation of arc resistance is caused by an irreversible reaction, such as the adsorption of Cl− during alloy corrosion46. It can be seen that only one arc resistance appeared in the high frequency region in the Nyquist plots of the alloys with different Zr contents in the 3.5% NaCl corrosion medium, and no inductive arc appeared in the low frequency region, indicating that the passivation film of the alloy has dissolved at this time, and the surface matrix of the alloy is corroded, corresponding to the matrix dissolution of the Al-Ce-Zr alloys. When the Zr content is 0.05%, its radius is the largest, indicating that the corrosion resistance of the alloy is highest, which is consistent with the analysis result of the Tafel curve.
Figure 10(c) shows the bode plot of the frequency and phase angle relationship. It can be seen that all the curves show one characteristic peak, corresponding to the one arc resistance that appeared in the high frequency region in the Nyquist plot. Therefore, the test result of the nyquist plot is also well verified by the bode plot. Figure 10(d) shows the equivalent circuit diagram of the impedance spectrum of cast Al-Ce-Zr alloy fitted by zview software. After the alloy is immersed in 3.5% NaCl solution, the smaller the pitting pit of the alloy is in the initial stage, the larger the value of the pitting pit resistance R2 is. It can be found from the fitting data that when the Zr content is 0.05%, the solution resistance R1 is the smallest and the pitting pit resistance R2 is the largest, indicating that the corrosion resistance of the alloy is the excellent when the Zr content is 0.05%.
Conclusion
This study aimed to study their effects of Zr element on the microstructure, mechanical properties and corrosion resistance of the Al-10Ce alloys by comparing the room-temperature and high-temperature tensile tests and electrochemical experiments. The main conclusions were as follows:
-
(1)
In the as-cast Al-Ce-Zr system, the eutectic Al11Ce3 phase segregated and coarsened with the increase of Zr content from 0.05 to 0.15%, and then refined and homogenized with the further increase of Zr content. The morphology of eutectic Al11Ce3 phase changed from layered to rod-shaped or bar-shaped with the increase of Zr content.
-
(2)
In the as-cast Al-Ce-Zr system, the hardness, tensile strength and yield strength showed a trend of decreasing first and then increasing with the increase of Zr content. The addition of appropriate amount of Zr improved the microstructure and mechanical properties of the alloy.
-
(3)
In the cast Al-Ce-Zr alloy system, the addition of Zr had a significant effect on increasing the tensile strength and plasticity, and the optimum Zr addition (0.05-0.2%) was found to increase the hardness and tensile strength, with the strength peaking at 0.2% Zr. Room temperature tests showed a ductile fracture pattern, with strength and ductility initially increasing and then decreasing with increasing zirconium content at elevated temperatures. This study emphasized the important role of Zr in balancing the strength and ductility of Al-Ce alloys, which was critical for high temperature applications. Finite element modeling also confirmed accurately prediction of the tensile stress distribution at room and high temperature conditions.
-
(4)
By observing the Tafel curves and impedance spectra of cast Al-Ce-Zr alloys, it was found that for cast Al-Ce-Zr alloys with different Zr contents, the corrosion current of the alloys was minimized to 1.158 × 10− 7 µA/cm2 when the content of Zr is 0.05%, and at this time, the morphology and distribution of the Al11Ce3 phase were optimal in the alloys, which improved the corrosion resistance of the alloys.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Ding, W. et al. Effect of rare Earth Y and Al–Ti–B master alloy on the microstructure and mechanical properties of 6063 aluminum alloy. J. Alloys Compd. 830 (2020).
He, Y. et al. Microstructure evolution and mechanical properties of Al-La alloys with varying La contents. Mater. Sci. Eng. A. 701, 134–142 (2017).
Lu, Z., Li, X. & Zhang, L. Thermodynamic description of Al-Si-Mg-Ce quaternary system in Al-Rich corner and its experimental validation. J. Phase Equilib. Diff. 39 (1), 57–67 (2017).
Huang, X. & Yan, H. Effect of trace La addition on the microstructure and mechanical property of as-cast ADC12 Al-Alloy. J. Wuhan Univ. Technology-Mater Sci. Ed. 28 (1), 202–205 (2013).
Ng, D. S. & Dunand, D. C. Aging- and creep-resistance of a cast hypoeutectic Al-6.9Ce-9.3Mg (wt%) alloy. Mater. Sci. Eng. A 786 (2020).
Liu, Y., Michi, R. A. & Dunand, D. C. Cast near-eutectic Al-12.5 wt% Ce alloy with high coarsening and creep resistance. Mater. Sci. Eng. A 767 (2019).
Habashi, F. Extractive metallurgy of rare earths. Can. Metall. Q. 52 (3), 224–233 (2013).
Sims, Z. C. et al. High performance aluminum–cerium alloys for high-temperature applications. Mater. Horiz. 4 (6), 1070–1078 (2017).
Liao, H. C., Liu, Y., Lü, C. L. & Wang, Q. G. Effect of Ce addition on castability, mechanical properties and electric conductivity of Al–0.3Si–0.2Mg alloy. Int. J. Cast. Met. Res. 28 (4), 213–220 (2015).
Czerwinski, F. Cerium in aluminum alloys. J. Mater. Sci. 55 (1), 24–72 (2019).
Sims, Z. C. et al. Cerium-Based, Intermetallic-Strengthened aluminum casting alloy: High-Volume Co-product development. JOM 68 (7), 1940–1947 (2016).
Okamoto, H. Al-Ce (Aluminum-Cerium). J. Phase Equilib. Diff. 32 (4), 392–393 (2011).
Zhang, Z., Wang, Y. & Bian, X. Microstructure selection map for rapidly solidified Al-rich Al–Ce alloys. J. Cryst. Growth. 260 (3–4), 557–565 (2004).
Okamoto, H. Al-Ce (aluminum-cerium). J. Phase Equilib. 19 (4), 395–396 (1998).
Guo, Y. et al. Microstructure diversity dominated by the interplay between primary intermetallics and eutectics for Al–Ce heat-resistant alloys. J. Alloys Compd. 899 (2022).
Zhou, L. et al. Laser powder bed fusion of Al–10 wt% Ce alloys: microstructure and tensile property. J. Mater. Sci. 55 (29), 14611–14625 (2020).
Wang, F. et al. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 61 (15), 5636–5645 (2013).
Knipling, K. E., Karnesky, R. A., Lee, C. P., Dunand, D. C. & Seidman, D. N. Precipitation evolution in Al–0.1Sc, Al–0.1Zr and Al–0.1Sc–0.1Zr (at.%) alloys during isochronal aging. Acta Mater. 58 (15), 5184–5195 (2010).
Dn, F. C. D. Creep propreties of coarse-grained Al(Sc) alloys at 300 °C. Scr. Mater. 40 (6) (1999).
Xu, P. et al. Precipitation characteristics and morphological transitions of Al3Sc precipitates. J. Alloys Compd. 790, 509–516 (2019).
Miao, D. et al. Improvement in the mechanical properties of as-rapidly solidified Al–Zn–Mg–Cu alloys with Zr addition before and after aging. Mater. Lett. 337 (2023).
Suwanpreecha, C., Pandee, P., Patakham, U. & Limmaneevichitr, C. New generation of eutectic Al-Ni casting alloys for elevated temperature services. Mater. Sci. Eng. A 709, 46–54 (2018).
Pandey, P., Makineni, S. K., Gault, B. & Chattopadhyay, K. On the origin of a remarkable increase in the strength and stability of an al rich al-Ni eutectic alloy by Zr addition. Acta Mater. 170, 205–217 (2019).
Knipling, K. E., Seidman, D. N. & Dunand, D. C. Ambient- and high-temperature mechanical properties of isochronally aged Al–0.06Sc, Al–0.06Zr and Al–0.06Sc–0.06Zr (at.%) alloys. Acta Mater. 59 (3), 943–954 (2011).
Mohammed, A. A. et al. Enhancing ambient and elevated temperature performance of hypoeutectic Al–Ce cast alloys by Al3(Sc,Zr) precipitate. J. Mater. Res. Technol. 28, 1188–1197 (2024).
Wu, J. J. et al. Revealing the grain refinement and strengthening mechanisms of Al-Ce-Zr alloy via in-situ synchrotron X-ray imaging and diffraction. Mater. Charact. 223, 114965 (2025).
Chankitmunkong, S., Wang, F., Pandee, P. & Eskin, D. G. Precipitation Hardening and Structure Evolution in Hypereutectic Al-6% Fe-Zr Alloys Subjected to Ultrasonic Melt Processing (2024).
Ye, J., Guan, R., Zhao, H., He, C. & Xiong, K. Effect of Zr microalloying on the microstructures and strengthening mechanism of As-Cast Al-Fe-Zr alloys. Materials 13 (21) (2020).
Zhang, L. J. et al. Effects of rare-earth element, Y, additions on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. Mater. Sci. Eng. A. 725, 437–446 (2018).
Ye, J., Dai, K., Chen, J., Guan, R. & Gao, M. Primary phase transformation mechanism in a hypereutectic Al-Ce alloy during rapid solidification. Mater. Lett. 340 (2023).
Akopyan, T. K., Belov, N. A., Lukyanchuk, A. A., Letyagin, N. V. & Musin, A. F. Effect of high pressure torsion on the precipitation hardening in Al–Ca–La based eutectic alloy. Mater. Sci. Eng. A 802 (2020).
Wang, L. et al. Improved tensile strength of Al-5Ce alloy by permanent magnet stirring. Metall. Mater. Trans. A. 51 (5), 1972–1977 (2020).
Wang, W. et al. Internal friction and heat resistance of al, Al–Ce, Al–Ce–Zr and Al–Ce–(Sc)–(Y) aluminum alloys with high strength and high electrical conductivity. J. Mater. Res. Technol. 14, 1255–1274 (2021).
Zhao, Y. H., Liao, X. Z., Jin, Z., Valiev, R. Z. & Zhu, Y. T. Microstructures and mechanical properties of ultrafine grained 7075 al alloy processed by ECAP and their evolutions during annealing. Acta Mater. 52 (15), 4589–4599 (2004).
Sabirov, I., Murashkin, M. Y. & Valiev, R. Z. Nanostructured aluminium alloys produced by severe plastic deformation: new horizons in development. Mater. Sci. Eng. A. 560, 1–24 (2013).
Wang, L., Ye, B., Bai, Y., Zhao, B. & Ding, W. Effect of Zr and Sc micro-additions on the microstructure and mechanical properties of as-cast Al-5Ce alloy. Mater. Sci. Eng. A 822 (2021).
Zhu Xiaoping, W. et al. Grain refinement and modification of an Al–Si–Mg–Ti–Be cast alloy: microstructure and tensile property. Ferroelectrics 601, 194–204 (2022).
Tian, N. et al. Effect of precipitates on the mechanical performance of 7005 aluminum alloy plates. Materials 15 (17) (2022).
Zhao, Z., Ji, H., Zhong, Y., Han, C. & Tang, X. Mechanical properties and fracture behavior of a TC4 titanium alloy sheet. Materials 15 (23) (2022).
Cao, H. & WESSÉN, M. Effect of microstructure on mechanical properties of as-cast Mg-Al alloys. Metall. Mater. Trans. A. 35, 309–319 (2004).
Dahle, A., Sannes, S., John, D. S. & Westengen, H. Formation of defect bands in high pressure die cast magnesium alloys. J. Light Met. 1, 99–103 (2001).
Kainer, K. Magnesium alloys and their applications, Magnesium Alloys and Their Applications (1999).
Hu, G., Zhu, C., Xu, D., Dong, P. & Chen, K. Effect of cerium on microstructure, mechanical properties and corrosion properties of Al-Zn-Mg alloy. J. Rare Earths. 39 (2), 208–216 (2021).
Fang, X., Jing, W., Xiufeng, H. & Jianfeng, Z. Tensile properties and fracture behaviors of electron-beam welded Ti6242 titanium alloy joints. Eng. Res. Express 6 (1) (2023).
Chai, Y., Yan, J., Wang, C., Zhang, L. & Liang, M. Effect of temperature and thermal stress on micropores and cracks in aluminum alloy film during micro-arc oxidation. J. Electrochem. Soc. 170 (8) (2023).
Chen, K., Chen, S., Peng, G., Fang, H. & Xiao, D. Effects of deformation rate on recrystallization and properties of 7150 aluminum alloy. Special Cast. Nonferrous Alloys. 30, 103–107 (2010).
Author information
Authors and Affiliations
Contributions
Guoqiang Qin wrote the main manuscript, research process; Xinran Lv and Kaili Li conducted the part research, Zhigang Yang wrote and revised the manuscript, Tianqing Sheng and Shuqin Zhang conducted some data analysis, Xuguang Wei and Gang Yu conducted part images, Jianbo Yu revised the manuscript and provided funding, Ruili Song revised the manuscript and draw some figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, G., Lv, X., Li, K. et al. Excellent mechanical properties and corrosion resistance of Al–Ce–Zr alloys. Sci Rep 15, 21965 (2025). https://doi.org/10.1038/s41598-025-04817-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04817-4
Keywords
This article is cited by
-
The Microstructure and Mechanical Properties of the Ball-Milled and Hot Press-Sintered Al–Mn–Ce–Zr Alloy
Metallurgical and Materials Transactions A (2025)