Abstract
In this study, we retrospectively analyzed whether serum lactate levels were elevated in patients with schizophrenia (SCZ) and explored cognitive deficits, abnormalities in lactate metabolism, and neuroinflammation in an dizocilpine (MK-801)-induced N-methyl-d-aspartate (NMDA) receptor (NMDAR) inhibition model using the Morris water maze (MWM) test, biochemical assays, immunofluorescence (IF), Western blot (WB), and enzyme-linked immunosorbent assay (ELISA). We found that serum lactate levels were significantly higher than the normal range in patients with schizophrenia, and they were significantly and positively correlated with both length of hospitalization and serum triglyceride levels. In addition, we found that MK-801 induced cognitive deficits in Sprague-Dawley (SD) rats, accompanied by markedly elevated levels of lactate, pyruvate, glutamate and lactate dehydrogenase (LDH) activity in serum and frontal cortex (FCX). MK-801 caused a significant increase in the expression of NOD-, LRR-, and pyrin-containing protein 3 (NLRP3) and Caspase-1 proteins in FCX of rats; and elevated the levels of interleukin (IL)-1β and IL-18 in serum and FCX (P<0.05). We also found that serum lactate was significantly and positively correlated with serum pyruvate and glutamate levels, LDH activity, and IL-1β and IL-18 levels in SD rats. These data suggest that serum lactate is abnormally elevated in both SCZ patients and NMDA receptor inhibition models. Furthermore, there may be a link between MK-801-induced cognitive impairment, elevated serum lactate, and aberrant activation of the NLRP3/Caspase-1/IL-1βinflammatory pathway in rats. Modulation of serum/brain lactate levels and the NLRP3/Caspase-1/IL-1β pathway in SCZ patients may serve as potential targets for improving cognitive impairment in SCZ.
Clinical trial registration number: ChiCTR2400091186.
Similar content being viewed by others
Introduction
Schizophrenia (SCZ) is considered to be one of the most common psychiatric disorders, with mortality rates in patients ranging from two to four times higher than in the healthy population1. And both environmental and genetic factors can increase the risk of developing schizophrenia. Excessive glutamate during the early stages of life may cause excitotoxicity and structural brain defects2. This disorder is clinically characterized by the presence of positive and negative symptoms and cognitive dysfunction. And cognitive deficits mainly include impaired memory, attention deficits and executive dysfunction3. Cognitive dysfunction is considered to be a core feature of SCZ4, and one of the main causes of poor functional outcomes in patients. However, the etiology and pathogenesis of SCZ remain complex and not fully understood. With the rise and development of animal models in the field of mental disorders, breakthroughs have been made in the exploration of the pathogenesis of schizophrenia and the mechanism of action of antipsychotic drugs. Currently, animal models used to simulate schizophrenia mainly include pharmacological models, neurodevelopmental models and genetic models5. A pharmacological model of schizophrenia with N-methyl-D-aspartate (NMDA) receptor (NMDAR) hypofunction can summarize the range of positive, negative, and cognitive symptoms of schizophrenia. The pharmacological approaches to induce NMDAR hypofunction include phencyclidine (PCP), ketamine (KET), and dizocilpine (MK- 801)6. The NMDAR, an ionotropic glutamate receptor, plays a critical role in glutamatergic neurotransmission, synaptic plasticity, and local rhythmic activity7. And, it is also involved in a variety of brain physiological processes, such as learning, memory, and long-term neuronal enhancement. Faure A et al. found that after acute intervention with ketamine and MK-801 in C57BL/6 J mice, these mice generally exhibited communication and socialization deficits8.
Studies have shown that the pathological mechanisms of schizophrenia are related to a variety of factors such as impaired energy metabolism, mitochondrial dysfunction, neuroinflammation, redox dysregulation, and NMDAR hypofunction. These pathological processes create a positive feedback loop in the brain centered on oxidative stress. They interact and amplify each other, which is the basis for the dysfunctions that occur in patients’ sensory, cognitive, emotional and social function9,10,11. Glausier JR et al. found significant changes in the expression of genes related to mitochondrial function in the prefrontal gray matter of patients with schizophrenia. A total of 871 mitochondria-related genes were detected, of which 83% (296 genes) were downregulated and 17% (60 genes) were upregulated. The authors concluded that the down-regulated genes, which accounted for 83%, were associated with reduced brain energy demand and decreased neuronal activity12. Interestingly, Interestingly, lactate is the most important source of energy for the brain, L-lactate is the major form of lactate in living organisms. Brain lactate is mainly produced by astrocyte glycolysis, and it can subsequently be shuttled from astrocytes to neurons via the astrocyte-neuron lactate shuttle (ANLS) to energize neurons13,14. In addition, lactate in peripheral blood can also cross the blood-brain barrier (BBB) to enter the brain. Importantly, under physiological conditions, the BBB exerts a limited regulatory effect on this process15. Proper levels of L-lactate in the brain are essential for normal learning, memory, and behavior16. Previous studies have confirmed that abnormal lactate levels are closely linked to various psychiatric disorders. An animal study showed that lactate levels were significantly elevated in the brain homogenates of mouse models of autism, bipolar disorder, and schizophrenia17, and that there was a significant negative correlation between elevated lactate levels and decreased PH values. In our published article18, we also extensively discussed lactate metabolism abnormalities in SCZ patients and animal models. In addition, Rahman T et al. also found that increased neuroinflammation in the cerebral cortex of SCZ patients was associated with lower levels of the GluN1 subunit of the NMDA receptor19. During the neuroinflammatory response, hyperactivation of microglia can lead to the production of large amounts of proinflammatory cytokines, increase astrocyte activity, and promote the release of glutamate, which can have neurotoxic effects on the central nervous system20. Neuroinflammation is closely linked to the activation of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome21. The NLRP3 inflammasome is made up of the NLRP3 protein, apoptosis-associated speck-like protein (ASC), and pro-Caspase-122. When NLRP3 inflammasome is activated, it promotes the release of cytokines such as interleukin (IL) −1β, IL-18, IL-2, IL-6, tumour necrosis factor α (TNF-α), high mobility group box-1 (HMGB1), inducible nitric oxide synthase, cyclooxygenase-2, and type I interferon -γ23,24. We have thoroughly discussed the aberrant activation of the NLRP3/Caspase-1/IL-1β pathway in both SCZ patients and animal models in our published article25. However, it remains uncertain whether there is a link between lactate metabolism dysfunction and the aberrant activation of the NLRP3 inflammasome pathway in schizophrenia (see Fig. 1). An in-depth study of the potential link between the two and their possible mechanisms of action has important clinical implications for the diagnosis and treatment of schizophrenia.
Astrocyte-neuron lactate shuttle disorders and abnormal activation of microglia in schizophrenia. Astrocyte-neuron lactate shuttle disorders in schizophrenia can lead to abnormal accumulation of lactate in the brain, while abnormal activation of microglia can further lead to abnormal activation of the NLRP3/ASC/Caspase-1/IL-1β inflammatory pathway18,25. This figure was created by Figdraw2.0, https://www.figdraw.com.
Methods
Clinical research
A retrospective study method was used to find and select all schizophrenia patients hospitalized from April 2022 to December 2022 in the electronic medical record system of Yunnan Provincial Psychiatric Hospital, and 142 patients were finally included according to strict inclusion and exclusion criteria (see Fig. 2.).The diagnosis of the patients strictly followed the diagnostic criteria for schizophrenia in the Diagnostic and statistical manual of mental disorders, Fourth edition (DSM-V) in the U.S. To ensure diagnostic accuracy and consistency, the patient’s medical record data was reviewed, double-checked, and entered by another master’s student with extensive clinical experience. The information involved in the study included the patient’s basic condition, number of hospitalizations, length of stay, severity of clinical symptoms, and biochemical test results. The severity of psychiatric symptoms was assessed using the positive and negative syndrome scale (PANSS), which is commonly used in clinical practice. The biochemical tests included serum lactate, lactic dehydrogenase (LDH), fasting blood glucose, triglyceride (TG), and low-density lipoprotein (LDL), and the results of the included biochemical tests were obtained within one week of the patient’s admission to the hospital. The patients were 18–80 years old, of any gender, and of Han Chinese ethnicity (There was no significant correlation between cerebrospinal fluid lactate levels and age, gender, and whether or not they smoked26, but there was a significant correlation with ethnicity27.). No history of mental retardation or other psychiatric disorders. No history of infection or alcohol and/or psychoactive substance abuse prior to one week. No viral encephalitis, diabetes mellitus, thyroid dysfunction, hypertension, fatty liver disease, gout, cardiac failure, renal failure, or other medical disorders. Exclude patients who are wheelchair or bedridden or have eating disorders. Exclude female patients who are pregnant or breastfeeding. Exclude patients who have used or are using hypoglycemic, lipid-lowering or antihypertensive drugs.
Ethics approval and China clinical trial registration
The clinical retrospective study was conducted in compliance with China’s Administrative Measures for Clinical Trials. This study protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Medical Ethics Committee of Yunnan Provincial Psychiatric Hospital (Ethics Approval No. YNJS-20241015-001). According to Chinese law and the Medical Ethics Committee of Yunnan Provincial Psychiatric Hospital, there was no necessity to retroactively obtain informed consent due to the importance of this study subject and the retrospective study design [we only analyzed the available medical record data for correlations and did not directly intervene in the patient’s course of treatment. In addition, this researcher can ensure that patients’ personal information is not disclosed. Taken together, this study meets the second condition of the waiver of informed consent for Yunnan Provincial Psychiatric Hospital “research that utilizes medical records and biospecimens obtained in previous studies (secondary use of research medical records/biospecimens)”]. In addition, this study was registered with the China Clinical Trial Registry (ChiCTR, https://www.chictr.org.cn/, registration number: ChiCTR2400091186, PID: 247916).
Animal experiments
Animals and groups
The study protocols were approved by the Ethical Review Committee for Animal Experiments of Kunming Medical University (Approval No.: kmmu20230164). We can confirm that all methods were performed in accordance with the China’s Regulations on the Administration of Laboratory Animals and Measures for the Administration of Ethical Review of Laboratory Animals, as well as the International Council for Laboratory Animal Science (ICLAS), and in compliance with the ARRIVE guidelines. Thirty-two male SD rats were purchased from Kunming Medical University (KMMU) and housed in a pathogen-free animal house at KMMU with a 12/12 h light/dark cycle, and medication was administered after 1 week of acclimatization. All rats were randomly and equally divided into four groups (8 rats in each group), i.e., MK (MK-801) group, MK + CLO (MK-801 + clozapine) group, healthy control (HC) group, and CLO (clozapine) group [Chronic intraperitoneal injection (i.p.) of CLO significantly increased the postmortem lactic acid level in adult male SD rats28. The MK group was induced with MK-801 (Sigma, 0.2 mg/kg/d, 14 days, i.p.) in the SCZ animal model29. The MK + CLO group was injected with clozapine (10 mg/kg/d, 7 days, i.p., Shanghai Jingfeng Biotechnology Co., Ltd.) on the 8 th day of MK-801 injection (0.2 mg/kg/d, 14 days), and the clozapine was injected 15 min after MK-801 injection. The HC group received an intraperitoneal injection of saline for 14 days and the CLO group received an intraperitoneal injection of clozapine (10 mg/kg/d) for 14 days. Behavioral tests were performed on days 8–14 of drug administration. The Flowchart of the experiment is shown in Fig. 2.
Morris water maze (MWM) test
MWM can be used to assess spatial learning and memory in rats under different experimental conditions30. The water maze was 120 cm in diameter and 45 cm in height, filled with 25 cm of water (25 ± 1 °C) and divided into four quadrants. Finding platform training was performed on the 7 th day of drug administration, the platform was fixed in one quadrant (the fourth quadrant), the platform was 1 cm above the water surface, rats were randomly placed from the four quadrants tightly attached to the wall of the water tank (the fourth quadrant), and the time taken by the rats to climb up the platform was recorded for a total of 4 times/day, and the average value was taken. The localization navigation experiment was conducted on days 8–14 of drug administration by placing the platform 1 cm below the water surface in the four quadrants, placing rats randomly from the four quadrants immediately adjacent to the wall of the aquarium (fourth quadrant), and recording the time required for the rats to climb onto the platform for 4 times/day for 7 d, and taking the average value. The spatial exploration experiment was conducted on the 14 th day, the platform in the pool was withdrawn 1 h after the end of the localization navigation test, and the SD rats were put into the tank from the original entry point so that the rats were facing the tank wall, and the number of times the rats traversed the platform and the swimming time in the target quadrant (the fourth quadrant) inside the platform were recorded in 60 s. The average time was taken 4 times/day for 7 d consecutively.
Serum and brain tissue collection
After the behavioral test, rats were gas-anesthetized with isoflurane, and blood was collected from the abdominal aorta using blood collection tubes without anticoagulant or procoagulant, centrifuged, and the upper serum and lower precipitate were separately preserved and stored at −80 ℃ for use in serum biochemical assays, including assays for serum lactate, pyruvate, and glutamate levels and lactate dehydrogenase activity. Four of each group were randomly selected for brain tissue perfusion sampling, 4% paraformaldehyde fixation for immunofluorescence detection. The remaining 4 portions of each group were subjected to fresh sampling of brain tissue, quick-frozen in liquid nitrogen and stored at −80℃ for biochemical detection (lactate, pyruvate, and glutamate levels and lactate dehydrogenase activity), WB and ELISA. The rats were euthanized immediately after sampling by cervical dislocation to ensure rapid loss of life and minimize pain and discomfort. The kits used for biochemical testing include lactate test kits (Nanjing Jiancheng Bioengineering Institute A019-2-1), glutamate test kits (Nanjing Jiancheng Bioengineering Institute A074-1-1), pyruvate assay kits (Nanjing Jiancheng Bioengineering Institute A081-1-1), and lactate dehydrogenase (LDH) kits (Nanjing Jiancheng Bioengineering Institute 020-1-2).
Immunofluorescence
Brain sections were placed in a constant temperature oven at 60℃ for at least 30 min.Sections were routinely dewaxed to water. Rinsing in 0.01 mol/L Tris-Buffer saline (TBS) solution, 5 min×3 times; Antigen repair, citrate at pH 6.0, microwave treatment on high for 20 min. Next, 0.01 mol/L PBS solution rinsing, 5 min×3 times; After blotting the water around the sections with filter paper, a circle was drawn around the sections with a histochemical pen to prevent liquid leakage during subsequent staining; 5% sheep serum containment, 37 °C, 1 h; Shake off the excess sealing liquid, add 2% sheep serum diluted with the appropriate concentration of primary antibody including anti-NLRP3 (1:100; bioss), anti-Caspase-1 (1:100; bioss), and refrigerate at 4℃ overnight. Then, rinse with 0.01 mol/L PBST solution for 5 min×5 times. Add secondary antibody including CY3-labeled goat anti-rabbit IgG (1:800; Servicebio) and incubate at 37℃ for 60 min. Finally, rinse with 0.01 mol/L PBST for 5 min×3 times. Seal the film with fluorescent sealer containing DAPI (ZSGB-BIO) and scan holographically (nuclei were blue and antibody positive expression was red) and analyzed by Image J software.
Western blot (WB)
The rats were put to death in compliance with animal ethics, and four randomly selected from each group were subjected to fresh sampling of brain tissue, which was preserved in liquid nitrogen until further processing. Protein blotting analysis was performed using 50 mg of prefrontal cortex tissue. GAPDH was chosen as an internal control to ensure equal amounts of protein. Densitometric quantification of the bands was performed using Image J software. The primary antibodies used in the experiment and their dilution ratios: Caspase-1 (proteintech 22915-1-AP, 1:2000), NLRP3 (proteintech, 68102-1-Ig, 1:2000), GAPDH (ZSGB-BIO TA-08, 1:2000). The secondary antibodies used in the experiment and their dilution ratios: generalized secondary antibody (Servicebio GB23303, 1: 4000), generalized secondary antibody (abmart M21001S, 1: 4000).
Enzyme linked immunosorbent assay (ELISA)
The quantitatively analyzed of IL-1β, IL-18 in the serum and prefrontal cortex was carried out using ELISA kit according to the manufacturer’s instructions, including Rat Interleukin 1β (IL-1β) ELISA Kit (Keqiao Biotechnology KQ112342) and Rat Interleukin 18 (IL-18) ELISA Kit (Keqiao Biotechnology KQ112340). The concentration was calculated according to the corresponding standard curve. Duplication was set for each sample.
Statistical analysis
All statistical analyses were performed by SPSS 19.0 (IBM, USA). Measures that conformed to normal distribution were expressed as mean ± standard deviation \((\bar{x}\pm s)\), and those that did not conform to normal distribution were expressed as M (P25, P75). For measures that conformed to normal distribution with homogeneous variance, independent samples t-test was used for between-group comparisons; for measures that conformed to normal distribution with heterogeneous variance, independent samples t’ test was used for between-group comparisons; and for data that did not conform to normal distribution, Mann-Whitney U-test was used for between-group comparisons in the nonparametric test for independent samples. Count data were expressed as rates or component ratios, and the Pearson chi-square test was used for comparison between groups. For correlation analysis, Pearson correlation analysis was used for information that satisfied normal distribution and met the test of chi-square, and Spearman correlation analysis was used for severely skewed data or data with noncontinuous variables. The level of all tests was set at 0.05, with P < 0.05 being considered a statistically significant difference. Graph Pad Prism 5.0 biostatistics software was used for graphing.
Results
Analysis of serum lactate test results in patients with schizophrenia
142 patients with schizophrenia were included in the study and their mean age was 46.42 ± 16.36 years. The mean serum lactate value of the patients when they were first admitted to the hospital was 2.71 ± 0.65 mmol/L, and the normal value range of lactate from the lactate testing instrument was 1.33–1.78 mmol/L. The serum lactate level of the patients when they were first admitted to the hospital was analyzed by using a one-sample t-test, and the result showed that serum lactate level of the patients with schizophrenia was significantly higher than the normal range (T = 17.043, P < 0.000). In addition, we found significant positive correlations between serum lactate levels and patients’ age (r = 0.211, P < 0.05), LDL (r = 0.196, P < 0.05), glucose (r = 0.157, P >0.05), triglycerides (r = 0.352, P < 0.001), and hospital stays (r = 0.243, P < 0.01) (Tables 1 and 2).
In all patients with schizophrenia included in this study, there was a significant positive correlation between the age of the patients and the hospital stays (r = 0.496, P < 0.01), triglycerides and low-density lipoprotein (r = 0.426, P < 0.01) were also significantly positively correlated (Table 1). Partial correlation analysis revealed that after controlling for the covariate of age, there was a significant positive correlation between patients’ serum lactate concentration and hospital stays (pr = 0.180, P < 0.033) (Table 3; Fig. 3A); and after controlling for serum LDL, there was a significant positive correlation between patients’ serum lactate concentration and serum triglyceride level (pr = 0.313, P < 0.001) (Table 3; Fig. 3B).
Analysis of the detection results of MWM localization and navigation stages in each group of rats
Escape latency was tested in four groups of rats during the 7-day orientation navigation phase using the Morris water maze. The results showed that the main effect of testing time was significant (Waldχ2 = 58.801, P time < 0.001), i.e., the difference of the time factor (day) was statistically significant; the main effect between the treatment groups was significant (Waldχ2 = 15.127, P group < 0.01), i.e., the factor of the subgroup (group) was statistically significant, and there was an interaction between group and time (Waldχ2 = 159.781, P interaction < 0.001). There was a significant difference in avoidance latency between groups on day two (P < 0.01) and day five (P < 0.05) (Fig. 4A). However, the escape latency was significantly longer in the MK group than in the HC group only on the fifth day (P < 0.05). The difference in the latency to escape on the fifth day between the MK and CLO groups was not statistically significant (P > 0.05). Day 5 escape latency was significantly longer in the MK + CLO group than in the HC group (P < 0.05). However, we did not find statistical differences (P > 0.05) between the MK + CLO and CLO groups and between the MK + CLO and MK groups during the Day 5 escape latency (Fig. 4C).
Four groups of rats were tested for distance traveled during the 7-day orientation voyage phase. The results showed that the main effect of testing time was significant (Waldχ2 = 73.481, P time<0.001), i.e., the difference of the time factor (day) was statistically significant; the main effect between treatment groups was significant (Waldχ2 = 20.029, P group<0.001), i.e., the factor of subgroup (group) was statistically significant; and there was an interaction between group and time (Waldχ2 = 112.876, P interaction < 0.001). There was a significant difference in the distance traveled between the groups on the second, fifth and seventh days at P < 0.01, P < 0.01 and P < 0.05, respectively (Fig. 4B). On days 5 and 7, the distance traveled was significantly longer in the MK group than in the HC and CLO groups (P < 0.05). The difference in distance traveled between the MK + CLO group and the MK group on days 5 and 7 was not statistically significant (P>0.05). The distance traveled on day 5 was significantly longer in the MK + CLO group than in the HC group (P < 0.05); however, we did not find a significant difference in the distance traveled on day 7 between these two groups (P > 0.05). The distance traveled on days 5 and 7 was significantly longer in the MK + CLO group than in the CLO group (P < 0.05) (Fig. 4D and E). In addition, the four groups of rats were tested for locomotor speed during the 7-day localization voyage phase. The results showed that the grouping factor (group) was not statistically significant (Waldχ2 = 1.685, P group > 0.05), so no further analysis was done.
Morris water maze (MWM) test. (A) The escape latency during the 7 days of training period. (B) The distance traveled during the 7 days of training period. (C) The escape latency on the day 5 of training period. (D) The distance traveled on the day 5 of training period. (E) The distance traveled on the day 7 of training period. Note: *P < 0.05, ** P < 0.01, *** P < 0.001, ns P>0.05. Abbreviations: healthy control group, HC; clozapine group, CLO; MK-801 group, MK; MK-801 + clozapine group, MK + CLO.
Analysis of the results of serum and frontal cortex (FCX) biochemical tests in each group of rats
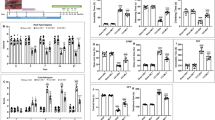
We found that lactate levels in serum and FCX were significantly higher in the MK group than in the HC group (P < 0.001) (Fig. 5A and E). Lactate levels were significantly higher in serum (P < 0.01) and FCX (P < 0.001) in the MK group compared with the CLO group (Fig. 5A and E). Lactate levels in FCX were significantly lower in the MK + CLO group than in the MK group (P < 0.001); however, we did not find a significant difference in serum lactate levels between these two groups (P > 0.05) (Fig. 5A and E). In addition, lactate levels in FCX were significantly higher in the MK + CLO group than in the CLO group (P < 0.001); however, there was no significant difference in serum lactate between these two groups (P > 0.05) (Fig. 5A and E). LDH activity was significantly higher in serum and FCX in the MK group compared with the HC and CLO groups (P < 0.001) (Fig. 5B and F). LDH activity in serum (P < 0.01) and FCX (P < 0.001) was significantly lower in the MK + CLO group than in the MK group (Fig. 5B and F). In addition, LDH activity in serum and FCX was significantly higher in the MK + CLO group than in the CLO group (P < 0.001) (Fig. 5B and F). Pyruvate levels in serum and FCX were significantly higher in the MK group compared with the HC and CLO groups (P < 0.001) (Fig. 5C and G). Pyruvate levels in serum (P < 0.01) and FCX (P < 0.001) were significantly lower in the MK + CLO group than in the MK group (Fig. 5C and G). In addition, the level of pyruvate in serum was significantly higher in the MK + CLO group than in the CLO group (P < 0.01) (Fig. 5C and G). However, the level of pyruvate in FCX of the MK + CLO group was significantly lower than that of the CLO group (P < 0.05) (Fig. 5C and G). Serum and FCX glutamate levels were significantly higher in the MK group than in the HC group (P < 0.001) (Fig. 5D and H). Serum (P < 0.05) and FCX (P < 0.001) glutamate levels were significantly higher in the MK group compared to the CLO group (Fig. 5D and H). FCX glutamate levels were significantly lower in the MK + CLO group compared to the MK group (P < 0.001); however, we did not find a significant difference in serum glutamate levels between these two groups (P > 0.05) (Fig. 5D and H). However, we did not find significant differences in glutamate levels in serum and FCX between the MK + CLO and CLO groups (P > 0.05) (Fig. 5D and H). These results indicate that MK-801-induced schizophrenia model has abnormally elevated levels of lactate, pyruvate, glutamate and LDH activity in serum/FCX. Clozapine ameliorated the abnormally elevated levels of lactate and glutamate in FCX, as well as the abnormally elevated pyruvate levels and LDH activity in serum and FCX.
Analysis of biochemical indices. (A) Statistical diagram of Lac in the serum. (B) Statistical diagram of LDH in the serum. (C) Statistical diagram of Pyruvate in the serum. (D) Statistical diagram of Glutamate in the serum. (E) Statistical diagram of Lac in the FCX. (F) Statistical diagram of LDH in the FCX. (G) Statistical diagram of Pyruvate in the FCX. (H) Statistical diagram of Glutamate in the FCX. Note: *P < 0.05, ** P < 0.01, *** P < 0.001, ns P>0.05. Abbreviations: healthy control group, HC; clozapine group, CLO; MK-801 group, MK; MK-801 + clozapine group, MK + CLO. lactate, Lac; lactate dehydrogenase, LDH.
Analysis of NLRP3 and Caspase-1 protein levels in the frontal cortex of rats in each group
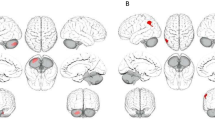
Immunofluorescence results showed that the relative fluorescence intensities of NLRP3 and Caspase-1 were significantly higher (P < 0.001) in FCX of the MK group compared with those of the HC group and CLO group (Fig. 6A, B, C and D). The relative fluorescence intensities of NLRP3 and Caspase-1 in FCX of the MK + CLO group were significantly lower than those of the MK group (P < 0.001) (Fig. 6A, B, C and D). The relative fluorescence intensities of NLRP3 and Caspase-1 in FCX were significantly higher (P < 0.001) in the MK + CLO group compared to the HC and CLO groups (Fig. 6A, B, C and D).
In addition, the protein expression levels of NLRP3 (P < 0.01) and Caspase-1 (P < 0.001) were significantly elevated in FCX from the MK group compared with the HC group (Fig. 6E, F and G). The protein expression level of Caspase-1 in FCX of the MK group was significantly higher than that of the CLO group (P < 0.05); however, we did not find a significant difference in the protein expression level of NLRP3 in FCX of these two groups (P > 0.05) (Fig. 6E, F and G). The protein expression level of Caspase-1 in FCX of the MK + CLO group was significantly lower than that of the MK group (P < 0.01); however, we did not find a significant difference in the protein expression level of NLRP3 in FCX of these two groups (P > 0.05) (Fig. 6E, F and G). The protein expression level of Caspase-1 in FCX of the MK + CLO group was significantly higher than that of the HC group (P < 0.05); however, we did not find a significant difference in the protein expression level of NLRP3 in FCX of these two groups (P > 0.05) (Fig. 6E, F and G). We did not find significant differences in protein expression of NLRP3 and Caspase-1 in FCX between the MK + CLO and CLO groups (P > 0.05) (Fig. 6E, F and G). These results support that the protein levels of NLRP3 and Caspase-1 were abnormally elevated in the FCX of the SCZ model induced by MK-801, and that clozapine could ameliorate the abnormally elevated protein levels of Caspase-1.
Levels of IL-1β and IL-18 in serum and frontal cortex of rats in each group
IL-1β levels in serum (P < 0.001) and FCX (P < 0.05) were significantly higher in the MK group compared with the HC group (Fig. 6H and I). IL-1β levels in serum and FCX were significantly higher in the MK group than in the CLO group (P < 0.001) (Fig. 6H and I). Serum IL-1β levels in the MK + CLO group were significantly lower than those in the MK group (P < 0.001); however, we did not find a significant difference in IL-1β levels in FCX between these two groups (P > 0.05) (Fig. 6H and I). In addition, we found significantly higher IL-1β levels in serum (P < 0.01) and FCX (P < 0.05) in the MK + CLO group than in the CLO group (Fig. 6H and I). IL-18 levels in serum and FCX were significantly higher in the MK group compared to the HC group (P < 0.001) (Fig. 6J and K). IL-18 levels in serum (P < 0.01) and FCX (P < 0.001) were significantly higher in the MK group than in the CLO group (Fig. 6J and K). The level of IL-18 in FCX of the MK + CLO group was significantly lower than that of the MK group (P < 0.05); however, we did not find a significant difference in the serum levels of IL-18 between these two groups (P > 0.05) (Fig. 6J and K). In addition, we found that IL-18 levels in FCX of the MK + CLO group were significantly higher than those of the CLO group (P < 0.001); however, we did not find a significant difference in IL-18 levels in serum of these two groups (P > 0.05) (Fig. 6J and K). These results indicate that IL-1β and IL-18 levels were abnormally elevated in serum/FCX in the MK-801-induced schizophrenia model. Clozapine treatment improved the abnormally elevated IL-1β levels in serum and abnormally elevated IL-18 levels in FCX.
(A) Representative immunofluorescent (IF) staining of NLRP3 (red) with DAPI (blue) in the FCX, Scale bar = 50 μm. (B) IF statistical diagram of NLRP3 in the FCX. (C) Representative immunofluorescent staining of Caspase-1 (red) with DAPI (blue) in the FCX. Scale bar = 50 μm. (D) IF statistical diagram of Caspase-1 in the FCX. (E) Representative western blot images of NLRP3 and Caspase-1 in the FCX, GAPDH was used as a loading control. WB quantitative analysis of NLRP3 (F) and Caspase-1 (G) in the FCX. Quantitative statistics of IL-1β levels in the serum (H) and FCX (I) detected by ELISA. Quantitative statistics of IL-18 levels in the serum (J) and prefrontal cortex (K) detected by ELISA. Note: *P < 0.05, ** P < 0.01, *** P < 0.001, ns P>0.05. Abbreviations: healthy control group, HC; clozapine group, CLO; MK-801 group, MK; MK-801 + clozapine group, MK + CLO.
Analysis of the correlation between serum lactic acid and other assays in various groups of rats
For the correlation analysis between serum lactate and MWM assays, we did not find significant correlations between serum lactate and escape latency on day five (r = 0.118, P = 0.519) and distance traveled (r = 0.307, P = 0.087) (Fig. 7B and D); however, serum lactate was not significantly correlated with escape latency on day two (r = 0.447, P = 0.010), distance traveled on the second day (r = 0.497, P = 0.004), and distance traveled on the seventh day (r = 0.382, P = 0.031) were significantly and positively correlated with each other (Fig. 7A, C and E). Correlation analysis of serum lactate with other serum biochemical indices revealed significant positive correlation between serum lactate and serum levels of LDH activity (r = 0.744, P = 0.000), pyruvate (r = 0.765, P = 0.000) and glutamate (r = 0.694, P = 0.000) (Fig. 7F, G and H). In addition, correlation analysis between serum lactate and serum levels of IL-1β and IL-18 revealed a significant positive correlation between serum lactate levels and serum IL-1β (r = 0.760, P = 0.000) and IL-18 (r = 0.781, P = 0.000) levels (Fig. 7I and J).
Correlation analysis of serum lactate with the escape latency on the day 2 (A) and day 5 (B), the movement distance on the day 2 (C) and day 5 (D) and day 7 (E), serum LDH activity (F), serum Pyruvate (G), serum Glutamate (H), serum IL-1β (I) and serum IL-18 (J). Abbreviations: healthy control group, HC; clozapine group, CLO; MK-801 group, MK; MK-801 + clozapine group, MK + CLO.
Discussion
Disorders of serum lactate metabolism and abnormalities of glucolipid metabolism in SCZ patients
Our findings showed that serum lactate levels were significantly elevated in patients with schizophrenia (T = 13.287, P < 0.001). Kim S et al. found higher levels of lactate, glutamate, alanine, serine, and ornithine in patients with chronic schizophrenia compared to the healthy control group31. Ravanfar P et al. observed increased lactate and phosphocreatine concentrations in the caudal anterior cingulate cortex of 12 chronic SCZ patients32. Interestingly, lactate levels were also significantly higher in the dorsolateral prefrontal cortex (DLPFC) of postmortem schizophrenic patients (P < 0.05)33. In addition, Halim ND et al. found that lactate levels in the postmortem cerebellum of schizophrenic patients increased by 28% (P < 0.01) compared to healthy controls28. They suggested that this disturbance in lactate metabolism may be related to antipsychotic medication rather than primary metabolic disturbances in the prefrontal cortex of the patients. A study by Elmorsy E et al. found no significant difference in arterial blood lactate levels between the schizophrenia patient group and the healthy control group (P > 0.05). However, after three months of treatment with antipsychotics (APs), all patients with schizophrenia showed significantly higher arterial blood lactate levels compared to their baseline levels at the time of the first visit34. Furthermore, patients who developed extrapyramidal reactions had significantly higher lactate levels than those treated with the same antipsychotics. Elmorsy E et al. concluded that abnormally elevated blood lactate levels could serve as biomarkers for antipsychotic side effects. In contrast to Halim ND and Elmorsy E et al., Regenold WT et al. found that the mean lactate concentration in the cerebrospinal fluid of patients with schizophrenia (1.61 ± 0.31 mmol/L) was significantly higher by 23% compared to the healthy control group (1.31 ± 0.21 mmol/L). Interestingly, the use of typical/atypical antipsychotic drugs was significantly associated with lower lactate concentrations in the cerebrospinal fluid27. Among the 30 patients (15 with schizophrenia and 15 with bipolar disorder), 5 had a family history of psychiatric disorders. Their mean cerebrospinal fluid lactate was 2.10 ± 0.34 mmol/L. In contrast, patients without a family history had lactate concentrations of 1.59 ± 0.28 mmol/L. This suggests that elevated lactate levels in patients with schizophrenia may be linked to a genetic predisposition rather than being caused by antipsychotic drugs. It has also been shown that patients with schizophrenia exhibit significant changes in mitochondrial number, morphology, and function. These changes promote glycolysis and lead to abnormally high lactate levels35. Furthermore, the theory of tissue hypoxia and acid retention also explains the decreased pH and increased lactate in the brain tissue of schizophrenic patients36.
In contrast to the above findings, Huang JT et al. observed a decrease in lactate levels in the cerebrospinal fluid of patients with first-episode schizophrenia. They attributed this decrease to the depletion of lactate produced by astrocytes in the brain37. Beasley CL et al. found low levels of both lactate and alanine in the ventral internal capsule forelimb of schizophrenia patients, and importantly, only two samples contained detectable APs38. They suggested that this may indicate altered ANLS energy metabolism coupling in the brain of schizophrenia patients. Additionally, the high metabolic activity in the cerebral white matter of these patients may lead to the depletion of lactate produced by astrocytes. However, there are differences in energy metabolism in different brain regions of schizophrenic patients. Importantly, the energy metabolism of patient’s brain changes dynamically as the disease progresses. Wijtenburg SA et al. compared schizophrenia patients of shorter illness duration (< 5 years) and longer illness duration (> 5 years). And they found significant differences in lactate, glutamatergic and GABAergic in the DLPFC, hippocampus (HPC), anterior cingulate, and centrum semiovale between the two groups39. They also found significant differences in glutamatergic and GABAergic metabolites between SCZ patients, first-degree relatives, and the HC group in different brain regions. Interestingly, our study also found a significant positive correlation between patients’ serum lactate levels and the length of hospitalization (pr = 0.180, P < 0.033).
The current trend of abnormal lactate metabolism in schizophrenia remains controversial26,27,31,32,33,36,38,39,40. Abnormally elevated blood/brain lactate in schizophrenia are recognized by most investigators26,27,31,32,33,36,39,40, and our study also revealed that serum lactate levels were significantly higher than normal in patients with schizophrenia. Physiologically, blood lactate can cross the BBB to enter and energize the brain, and the BBB has a rate-limiting effect on this process15,41. However, studies have confirmed that damage as well as dysfunction can occur in the BBB in schizophrenia42,43. In addition, disturbances in brain lactate metabolism in schizophrenia may be closely associated with ANLS dysfunction. Raquel Pinacho et al. proposed that abnormal NMDA-dependent glycogenolysis in schizophrenia can lead to a temporary localized energy deficit at the ANLS junction44. Sullivan CR et al. suggested that neuronal developmental disorders, hippocampal neuronal apoptosis, reduced expression of mitochondria-related genes in pyramidal neurons, and defects in neuron-specific energy metabolism in the SCZ brain can lead to impaired neuronal lactate utilization, when abnormally activated astrocytes compensatorily produce excess lactate, which can lead to lactate accumulation33. However, Beasley CL et al. supported that cerebral white matter hypermetabolic activity in patients with schizophrenia can alter ANLS and lead to lactate being depleted38. The discrepancy between the views of Sullivan CR et al. and Beasley CL et al. suggests that ANLS and impaired lactate metabolism in the SCZ brain may be progressive. Huang X et al. Interpreted elevated lactate levels in venous blood of schizophrenic patients as gastric acid retention and tissue-derived hypoxia36. Interestingly, astrocyte survival in hypoxia decreased in a time-dependent manner45. In addition, study by Srivastava R et al. has demonstrated that mitochondrial defects in schizophrenic neurons are dynamically changing46. Importantly, we have adequately addressed in a published article that disturbances in lactate metabolism in SCZ may perhaps change dynamically with disease progression: Schizophrenic neurons have impaired utilization of lactate, and activated astrocytes compensatorily produce higher levels of lactate. However, It’s a dynamic process, and as the disease progresses the body may enter a state of decompensation, ultimately leading to severe destruction of brain energy and further neuronal damage, which in turn exacerbates impaired brain energy utilization18.
Furthermore, Yesilkaya UH et al. found that patients with schizophrenia had significantly higher fasting blood glucose levels compared to the HC group47. Roosterman D et al. discovered that glucose metabolism was disturbed in the cerebrospinal fluid (CSF) of schizophrenia patients48. Regenold WT et al. clarified that glucose in CSF is significantly and positively correlated with lactate levels (r = 0.435, P = 0.003)27. Interestingly, our study found no significant positive correlation between serum lactate and blood glucose in patients with schizophrenia (r = 0.157, P > 0.05). However, a study by Bryll A et al. confirmed that nitrosative, oxidative, or sulfuric damage to enzymes of tricarboxylic acid cycle and glycolysis, as well as calcium transport and adenosine triphosphate (ATP) biosynthesis might lead to impaired bioenergetics function in the brain49. This could explain the initial symptoms of schizophrenia, such as mild cognitive impairment. In addition, TianY et al. found that the prevalence of comorbid obesity was 16.4% among 633 Chinese hospitalized schizophrenic patients. Plasma levels of glucose, LDL, triglycerides, cholesterol, and apolipoprotein B were higher in obese patients. Meanwhile, HDL levels were significantly lower in obese patients compared to non-obese patients50. We found a significant positive correlation between serum lactate and triglycerides in SCZ patients (pr = 0.313, P < 0.001). Interestingly, the G-protein-coupled receptor 81 (GPR81) has been shown to promote fat storage in adipocytes, and this process is active in the neocortex and hippocampus of the mammalian brain51. Lactate, as an agonist of the GPR81, can exert its effects through both autocrine and paracrine mechanisms52. In the autocrine pathway, lactate produced by cancer cells activates GPR81 on the surface of the cancer cells. In the paracrine pathway, lactate secreted by cancer cells activates GPR81 on endothelial cells, immune cells, and adipocytes within the tumor stroma.
MK-801 induces cognitive dysfunction in animal models of SCZ
It is now generally confirmed that MK-801 causes cognitive and social deficits in model rats. Previous studies have shown that acute and subchronic MK-801 administration have different effects. Lee J et al. found that acute injection of MK-801 impaired working memory, but did not affect attentional function in rats53. Wang X et al. found that acute MK-801 treatment (0.4 mg/kg) caused cognitive deficits and anxiety in SD rats54. Unal G et al. showed that male rats injected with MK-801 subchronically experienced cognitive deficits in both the novel object recognition experiment and the MWM test55. Maleninska K et al. found that MK-801-induced cognitive deficits related to space and time were dose-dependent. Both MK-801 doses (0.1 mg/kg and 0.12 mg/kg) significantly impaired timing strategies and increased locomotor activity in the dark56. However, only the 0.1 mg/kg dose, not the 0.12 mg/kg dose, impaired spatial avoidance strategies in the light. Our results showed that after chronic injection of MK-801 (0.2 mg/kg/d for 14 days) in SD rats, the escape latency was significantly longer than HC group in the MWM test on day 5 (P < 0.01). In addition, on days 5 and 7, the distance traveled was significantly longer in the MK group than in the HC and CLO groups (P < 0.05). This suggests that MK-801 significantly weakened spatial learning ability in these rats compared to those in the HC and CLO groups. However, the pathological mechanism of MK-801-induced cognitive impairment in rats remains unclear. In the MK-801-induced SCZ rat model, NMDA receptor dysfunction led to severely impaired metabolism of neurotransmitters. This affected the glutamatergic, dopaminergic, and GABAergic systems in the cortico-striato-thalamo-cortical loop57. Our study found that serum and FCX glutamate levels were significantly higher in the MK group than in the HC group (P < 0.001). Glutamate, an important excitatory neurotransmitter, plays a key regulatory role in the pathogenesis of schizophrenia58. Dysfunction of glutamate-gated ion channels (NMDAR) has been demonstrated in schizophrenia. NMDAR antagonists significantly increase prohibitin 2 (PHB2) levels in the prefrontal cortex. Interestingly, PHB2 was significantly elevated in SCZ patients and was significantly negatively correlated with cognitive impairment59,60.
In our experiments, rats in the MK + CLO group had a shorter escape latency on day 5 compared to the MK group; however, we found that this difference was not statistically significant (P > 0.05). In addition, there was no statistically significant difference between the distance traveled of rats in the MK + CLO group and the MK group on day 5 and day 7 (P > 0.05). Interestingly, we found that CLO treatment significantly reduced the abnormally high glutamate levels in FCX of rats induced by MK-801 (P < 0.001). Zhou X et al. showed that clozapine mildly activates NMDA receptors and is effective in treating negative and cognitive symptoms of schizophrenia. They found that clozapine combined with the neuroprotectant pyrroloquinoline quinone improved MK-801-induced schizophrenia-like behaviors61. Regulation of NMDA receptors can reduce the expression levels of NMDAR1 and GluR3, leading to a decrease in tau protein hyperphosphorylation in the hippocampus of rats, and inhibit cell apoptosis through modulation of the Akt/GSK-3β signaling pathway. In addition, two weeks of treatment with 10 mg/kg quetiapine significantly improved cognitive dysfunction and cerebrovascular white matter damage in a mouse model induced by MK-801 (1 mg/kg/day, 1 week, i.p.), and the mechanism may be related to the PI3 K/Akt signaling pathway62. Studies have shown that modulation of the Akt and GSK-3βsignaling pathways partially alleviates MK-801-induced impairments in prepulse inhibition, social interaction, and cognitive function63.
In the MK-801-induced SCZ model, lactate metabolism and glycolytic processes were disrupted
Disturbances in lactate metabolism may contribute to the development of core symptoms of schizophrenia, such as cognitive deficits and negative symptoms64. A study by Eyjolfsson EM et al. found reduced lactate concentrations in the FCX, parietal cortex, thalamus, hippocampus, striatum, and nucleus accumbens in rats injected with MK-801 (0.5 mg/kg/day) for 6 days57. However, a significant reduction in glycolysis and impairment of mitochondrial enzymes was observed only in FCX, which is thought to be related to the duration of exposure to MK-801. However, our study found that lactate levels in both serum and FCX were significantly higher in the MK group compared to the HC group (P < 0.001). Similarly, lactate levels in both serum (P < 0.01) and FCX (P < 0.001) were significantly higher in the MK group than in the CLO group. These results suggest that chronic MK-801 intervention can abnormally increase endogenous lactate levels in the serum and FCX. Our findings are consistent with previous research. In the MK-801-induced SCZ mouse model, glycolysis and lactylation were enhanced in hippocampal tissues. 2-deoxy-D-glucose, a glycolysis inhibitor, effectively prevented the abnormal behaviors induced by MK-80165. Furthermore, lactic acid accumulated in MK-801-treated primary hippocampal neurons. The lactate receptor (HMGB1) levels were abnormally elevated in the culture medium, inducing apoptosis in primary hippocampal neurons. We also found that chronic MK-801 intervention increased pyruvate levels and LDH activity in serum and FCX (P < 0.001). These changes suggest that energy metabolism processes related to glycolysis and the tricarboxylic acid cycle were disturbed in the MK-801-induced SCZ model. Kolar D et al. suggested that repeated inhibition of NMDA receptor activity in specific regions can lead to progressive metabolic dysregulation66. They found that three days after a single injection of MK-801, LDH activity in the striatum was decreased. However, two hours after repeated injections of MK-801, LDH activity in the striatum was increased. Pinacho R et al. suggested that in schizophrenia, abnormal NMDA-dependent glycogenolysis causes a temporary energy deficit at the glial-neuronal junction, and this deficit leads to disturbed lactate metabolism in schizophrenic patients44. They demonstrated that this is a dynamic process. In the MK-801-induced model of SCZ, glycogen phosphorylase (PYGM) levels were abnormally elevated, leading to the breakdown of glycogen in the brain into lactate for energy. Interestingly, PYGM levels were found to correlate with the duration of schizophrenia. The longer the duration of the disease, the lower the PYGM levels. A study by Halim ND et al. found that chronic intraperitoneal injection of both clozapine and haloperidol in healthy adult male SD rats significantly increased lactate levels in the postmortem prefrontal cortex28. Another study showed that clozapine significantly reduced glucose-related metabolism in several brain regions. This effect was especially pronounced in the cerebral cortex of healthy Wistar rats67. In our study, there was no significant difference in lactate levels in the serum or FCX between the CLO group and the HC group (P > 0.05). This suggests that clozapine itself did not cause abnormal lactate metabolism in the serum or FCX. Additionally, we found that lactate levels in the FCX of rats in the MK + CLO group were significantly lower than those in the MK group (P < 0.001). On day 4 of MK-801 treatment (0.2 mg/kg/d for 4 days), the extracellular lactate concentration in the medial prefrontal cortex of adult male Wistar rats was elevated above baseline levels68. High-dose tandospirone reduced abnormally elevated lactate levels and improved cognitive deficits in the SCZ.
Aberrant activation of NLRP3/Caspase-1/IL-1β inflammatory pathway in the MK-801-induced SCZ model
Zhao J et al. performed RNA sequencing of the prefrontal cortex (PFC) in male mice exposed to MK-801, and they found that differentially expressed genes in the SCZ model were associated with immunomodulatory processes as well as impaired postsynaptic transmission69. Behavioral changes in mice after acute injection of MK-801 (0.1 mg/kg) were significantly correlated with microglia density in the HPC and PFC. Depletion of microglia improved schizophrenia-like behavior in mice, which was significantly associated with genes related to the regulation of brain immunity, including NLRP370. Interestingly, glutamate excitotoxicity leads to upregulation of key proteins that activate the NLRP3 inflammasome, including NLRP3, pro-Caspase-1, cleaved Caspase-1, and IL-1β71. Our study found that glutamate levels in serum and FCX of rats in the MK group were significantly higher than those in the HC group (P < 0.001). The protein expression levels of NLRP3 and Caspase-1 in FCX of rats in the MK group were significantly higher than those in the HC group (P < 0.01). In addition, the levels of IL-1β and IL-18 in serum and FCX were significantly higher in the MK group compared with the HC group (P < 0.05). Kim JY et al. found that MK-801 treatment induced an increase in phosphorylated-NF-κB expression, which in turn increased the levels of IL-6 and TNF-αexpression in the PFC72. Zhang Z et al. suggested that neuroinflammation and cognitive deficits induced by ketamine were associated with NLRP3/Caspase-1 axis-dependent hippocampal apoptosis73. Our results showed that NLRP3 protein expression was not significantly elevated in the FCX of SD rats in the CLO group compared with the HC group (P > 0.05), while Caspase-1 protein expression was abnormally elevated (P<0.01). Consistent with our findings, clozapine induced NLRP3 inflammation-dependent Caspase-1 activation and IL-1β release in undifferentiated and differentiated human monocytes74. In addition, we found that Caspase-1 protein expression in the FCX of rats in the MK + CLO group was significantly lower than that in the MK group (P < 0.01). This suggests that clozapine, while slightly increasing Caspase-1 protein levels in the FCX of healthy rats, significantly reduced the MK-801-induced overexpression of Caspase-1 in the FCX. Giridharan VV et al. found that clozapine reduced the expression of the NLRP3 inflammasome by 57% in Poly(I: C)-activated microglia75. They suggested that clozapine could exert anti-inflammatory effects by inhibiting NLRP3 inflammasome. Importantly, clozapine had a better anti-inflammatory effect than haloperidol and risperidone.
Interaction between SCZ lactate metabolism disorders and aberrant activation of NLRP3/Caspase-1/IL-1β inflammatory pathways
In the lipopolysaccharide (LPS)-induced maternal immune activation model, alterations in the CD200-CD200R and/or CX3 CL1-CX3 CR1 pathways may be associated with the etiology of SCZ76. Interestingly, LPS-induced early inflammatory changes promote glycolysis, including increased glucose uptake and lactate release77. Microglial cells exert a pro-inflammatory role in the brain, which can influence cerebral energy metabolism. LPS can stimulate astrocytes, thereby activating the TLR4 signaling pathway (NLRP3/Caspase-1). This process leads to an increased release of cytokines such as IL-6 and TNF-α, heightened tissue energy demands, and enhanced lactate production78. Our study found a significant positive correlation between serum lactate and serum IL-1β (r = 0.760, P = 0.000). A similar correlation was observed between serum lactate and IL-18 (r = 0.781, P = 0.000). Additionally, serum lactate showed a significant positive correlation with LDH activity (r = 0.744, P = 0.000) and pyruvate levels (r = 0.765, P = 0.000). It has been shown that activation of NLRP3-inflammasome requires glycolysis to produce lactate rather than pyruvate oxidation. Interestingly, phosphorylated protein kinase R (p-PKR) can act as a bridge between NLRP3-inflammasome and lactate (see Fig. 8). And inhibition of LDH not only reduces glycolytic lactate production but also decreases PKR and Caspase-1 activity and IL-1β maturation79. In rat microglia, p-PKR activates inflammatory signaling via NF-κB80. Additionally, lactate and pyruvate can be interconverted by LDHA/B. LDHA not only drives the conversion of pyruvate to lactate, but also mediates histone lactylation and induces inflammation and sepsis by targeting HMGB1 (see Fig. 8). Interestingly, HMGB1 was significantly elevated in SCZ patients compared to healthy controls81. Abnormally elevated HMGB1 can produce neurotoxic effects, including effects on the WNT/β-catenin signaling pathway and the BBB. In cells treated with oxygen-glucose deprivation/reoxygenation, LDHA, lactate, histone lactylation, and HMGB1 were upregulated. Knockdown of LDHA decreased IL-1β and IL-18 levels and inhibited Caspase-1 cleavage82. LDHB promotes the conversion of lactate to pyruvate. LDHB (-/-) mice show upregulated expression of apoptotic mediators, such as Caspase-3 and cytochrome C. In addition, LDHB deficiency induces neurogliosis, increases inflammatory mediators (e.g., NF-κB, TNF-α), and leads to cognitive deficits in mice83. Interestingly, the inflammatory factor IL-2 promotes aerobic glycolysis, strongly inducing lactate and LDH production84. We found a significant positive correlation between serum lactate and glutamate (r = 0.694, P = 0.000). It has been shown that aberrant glutamate stimulation in SCZ can contribute to LDH release, mitochondrial damage, and a loss of mitochondrial membrane potential. This stimulation also increases the number of cells containing autophagic lysosomes, ultimately leading to a decrease in cell viability and an increase in the rate of apoptosis85.
Pathological mechanisms of the interaction between lactate metabolism disorders and neuroinflammation in the SCZ brain. Abnormal activation of microglia in SCZ can trigger the activation of NLRP3 inflammasomes. Additionally, the abnormal accumulation of lactate in these cells can activate NLRP3 inflammasomes through protein kinase R phosphorylation (p-PKR). In SCZ neuronal cells, excessive lactate accumulation can lead to NLRP3 activation via histone lactylation, facilitated by LDHA. Mitochondrial dysfunction in SCZ induces oxidative stress and activates neuroinflammation, which damages neurons and astrocytes. This damage further disrupts lactate metabolism. Microglial activation also increases astrocyte activity, contributing to central nervous system damage. This process represents a dynamic progression from compensation to decompensation20,70,79,82. This figure was created by Figdraw2.0, https://www.figdraw.com.
Among the genes upregulated in early-onset schizophrenia, genes related to the LPS and NF-κB signaling pathways were enriched. In contrast, genes associated with glucocorticoids (GCs) were downregulated86. However, GCs may undergo dynamic changes during the pathogenesis of schizophrenia. Goldwaser EL et al. found elevated long-term GCs in schizophrenia, which were associated with cerebral white matter deficits87. Zhang Q et al. also demonstrated that PANSS scores in patients with schizophrenia were correlated with disease duration and GCs×IL-8×TNF-α, suggesting that abnormally elevated glucocorticoids and aberrant immune activation may have an impact on cognitive deficits in SCZ patients88. Interestingly, excess GCs can impair mitochondrial oxidation and energy production, leading to increased lactate production89. Furthermore, dysregulation of the classical WNT/β-catenin pathway is responsible for altered metabolic thermodynamics, interestingly, up/down-regulation of this pathway can lead to altered glucose aerobic glycolysis. Upregulation of the WNT/β-catenin pathway promotes glucose aerobic glycolysis. However, down-regulation of the pathway leads to oxidative stress and cell death through inactivation of LDHA, glucose transporter, monocarboxylate transporter-1, pyruvate kinase M2, pyruvate dehydrogenase kinase 1 and activation of the pyruvate dehydrogenase complex90. The interaction between oxidative stress and neuroinflammation is regulated by the WNT/β-catenin pathway, which is downregulated in schizophrenia and interacts with the NF-κB pathway91. Taken together, the above studies suggest a possible link between disturbances in endogenous lactate metabolism and aberrant activation of the NLRP3/Caspase-1/IL-1βinflammatory pathway in schizophrenic. And, PKR phosphorylation, histone lactylation, abnormalities in LDHA/B and glucocorticoids, dysregulation of the WNT/β-catenin pathway, and mitochondrial dysfunction may serve as a regulatory bridge between the two.
Limitations of our study
Despite the findings of our study, there are still some aspects that need to be studied in depth and improved. Firstly, only patients with schizophrenia who were hospitalized in Yunnan Province Psychiatric Hospital in between April and December 2022 were included in this study. In order to improve the generalizability of the study results, our team will collaborate with several other psychiatric hospitals. A multicenter, big data clinical study will be conducted by collecting, organizing, and analyzing the hospitalization data of SCZ patients from various hospitals throughout the year 2024 and monitoring the dynamic changes of these parameters during the course of treatment. Secondly, this study reveals an association between dysregulated lactate metabolism, aberrant activation of the NLRP3/Caspase-1/IL-1β inflammatory pathway, and SCZ, but direct causality validation experiments are lacking. We will observe their subsequent effects on SCZ-related symptoms and neuropathological changes by directly modulating lactate levels or the NLRP3 inflammatory pathway. Additionally, disturbed lactate metabolism in schizophrenia may be linked to astrocyte-neuron lactate shuttling disorders, as lactate is a key energy source for the brain. However, our experiments were conducted solely in vivo. The mechanism of lactate metabolism dysfunction in schizophrenia will be further explored using in vitro cellular experiments. Finally, we summarized potential molecular mechanisms for the interaction between the aberrant NLRP3 inflammatory pathway and SCZ lactate levels, however, no validation of these mechanisms was performed. Our team will validate the interactions between these mechanisms through molecular biology approaches to further support causal evidence for the interactions between these hypotheses.
Conclusions
In conclusion, serum lactate levels were significantly higher than the normal range in patients with schizophrenia, and they were significantly and positively correlated with both length of hospitalization and serum triglyceride levels. In addition, the MK-801-induced SCZ animal model exhibited impaired cognitive function, abnormally elevated levels of lactate, pyruvate, glutamate, IL-1β, IL-18, and LDH activity in serum and FCX, and significantly abnormally elevated levels of NLRP3 and Caspase-1 proteins in FCX. There may be a link between elevated serum lactate in schizophrenia and aberrant activation of the NLRP3/Caspase-1/IL-1βinflammatory pathway. And, mediators between the two may include protein kinase R phosphorylation, histone lactylation, abnormal LDHA/B and glucocorticoid levels, dysregulation of the WNT/β-catenin pathway, and mitochondrial dysfunction. In future studies, modulation of serum/brain lactate levels and the NLRP3/Caspase-1/IL-1βpathway in SCZ patients may serve as potential targets for improving cognitive impairment in SCZ.
Data availability
Data for this study is provided within the manuscript or supplementary information files.
References
Crawford, P., Go, K. V. & Schizophrenia Am. Fam Physician 106(4): 388–396. (2022).
Ouyang, X. et al. Cortical morphological heterogeneity of schizophrenia and its relationship with glutamatergic receptor variations. Eur. Psychiatry. 66 (1), e38 (2023).
Suárez Santiago, J. E., Roldán, G. R. & Picazo, O. Ketamine as a Pharmacological tool for the preclinical study of memory deficit in schizophrenia. Behav. Pharmacol. 34 (2–3), 80–91 (2023).
Javitt, D. C. Cognitive impairment associated with schizophrenia: from pathophysiology to treatment. Annu. Rev. Pharmacol. Toxicol. 63, 119–141 (2023).
Białoń, M. & Wąsik, A. Advantages and limitations of animal schizophrenia models. Int. J. Mol. Sci. 23(11). (2022).
Lee, G. & Zhou, Y. NMDAR hypofunction animal models of schizophrenia. Front. Mol. Neurosci. 12, 185 (2019).
Matousova, M. et al. Pregn-5-en-3β-ol and androst-5-en-3β-ol Dicarboxylic acid esters as potential therapeutics for NMDA hypofunction: in vitro safety assessment and plasma stability. Steroids 147, 4–9 (2019).
Faure, A. et al. Dissociated features of social cognition altered in mouse models of schizophrenia: focus on social dominance and acoustic communication. Neuropharmacology 159, 107334 (2019).
Skupienski, R., Steullet, P., Do, K. Q. & Xin, L. Developmental changes in cerebral NAD and neuroenergetics of an antioxidant compromised mouse model of schizophrenia. Transl Psychiatry. 13 (1), 275 (2023).
Cuenod, M. et al. Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol. Psychiatry. 27 (4), 1886–1897 (2022).
Do, K. Q. Bridging the gaps towards precision psychiatry: mechanistic biomarkers for early detection and intervention. Psychiatry Res. 321, 115064 (2023).
Glausier, J. R., Enwright, J. F. 3rd & Lewis, D. A. Diagnosis- and cell Type-Specific mitochondrial functional pathway signatures in schizophrenia and bipolar disorder. Am. J. Psychiatry. 177 (12), 1140–1150 (2020).
Calì, C., Tauffenberger, A. & Magistretti, P. The strategic location of glycogen and lactate: from body energy reserve to brain plasticity. Front. Cell. Neurosci. 13, 82 (2019).
Roosterman, D. & Cottrell, G. S. Astrocytes and neurons communicate via a Monocarboxylic acid shuttle. AIMS Neurosci. 7 (2), 94–106 (2020).
Takado, Y. et al. Hyperpolarized (13)C magnetic resonance spectroscopy reveals the Rate-Limiting role of the Blood-Brain barrier in the cerebral uptake and metabolism of l-Lactate in vivo. ACS Chem. Neurosci. 9 (11), 2554–2562 (2018).
Veloz Castillo, M. F., Magistretti, P. J. & Calì, C. l-Lactate: food for thoughts, memory and behavior. Metabolites 11(8). (2021).
Hagihara, H. et al. Decreased brain pH as a shared endophenotype of psychiatric disorders. Neuropsychopharmacology 43 (3), 459–468 (2018).
Zhang, Y. et al. Progress in the research of lactate metabolism disruption and Astrocyte-Neuron lactate shuttle impairment in schizophrenia: A comprehensive review. Adv. Biol. (Weinh) : e2300409. (2024).
Rahman, T. et al. N-Methyl-d-Aspartate receptor and inflammation in dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 240, 61–70 (2022).
Almeida, P., Nani, J. V., Oses, J. P., Brietzke, E. & Hayashi, M. Neuroinflammation and glial cell activation in mental disorders. Brain Behav. Immun. Health. 2, 100034 (2020).
Chen, D. B. et al. Quinones as preventive agents in alzheimer’s diseases: focus on NLRP3 inflammasomes. J. Pharm. Pharmacol. 72 (11), 1481–1490 (2020).
Zhang, W. J., Chen, S. J., Zhou, S. C., Wu, S. Z. & Wang, H. Inflammasomes and fibrosis. Front. Immunol. 12, 643149 (2021).
Zhou, Z. et al. Paeonia lactiflora pall. Polysaccharide alleviates depression in CUMS mice by inhibiting the NLRP3/ASC/Caspase-1 signaling pathway and affecting the composition of their intestinal flora. J. Ethnopharmacol. 316, 116716 (2023).
Hou, C. et al. Xinmaikang (XMK) tablets alleviate atherosclerosis by regulating the SREBP2-mediated NLRP3/ASC/Caspase-1 signaling pathway. J. Ethnopharmacol. 319 (Pt 2), 117240 (2024).
Zhang, Y. et al. The role of Nucleotide-Binding oligomerization Domain-Like receptor 3 inflammasome and related pathways in schizophrenia and its potential as a therapeutic target. Chin. J. Psychiatry. 57 (07), 419–425. https://doi.org/10.3760/cma.j.cn113661-20230925-00094 (2024).
Rowland, L. M. et al. Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl Psychiatry. 6 (11), e967 (2016).
Regenold, W. T. et al. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol. Psychiatry. 65 (6), 489–494 (2009).
Halim, N. D. et al. Increased lactate levels and reduced pH in postmortem brains of schizophrenics: medication confounds. J. Neurosci. Methods. 169 (1), 208–213 (2008).
Huang, W. et al. Effects of the co-administration of MK-801 and clozapine on MiRNA expression profiles in rats. Basic. Clin. Pharmacol. Toxicol. 128 (6), 758–772 (2021).
She, N. et al. NLRP3 inflammasome regulates astrocyte transformation in brain injury induced by chronic intermittent hypoxia. BMC Neurosci. 23 (1), 70 (2022).
Kim, S. et al. Searching for biomarkers in schizophrenia and psychosis: Case-control study using capillary electrophoresis and liquid chromatography time-of-flight mass spectrometry and systematic review for biofluid metabolites. Neuropsychopharmacol. Rep. 42 (1), 42–51 (2022).
Ravanfar, P. et al. In vivo 7-Tesla MRI investigation of brain Iron and its metabolic correlates in chronic schizophrenia. Schizophrenia (Heidelb). 8 (1), 86 (2022).
Sullivan, C. R. et al. Measurement of lactate levels in postmortem brain, iPSCs, and animal models of schizophrenia. Sci. Rep. 9 (1), 5087 (2019).
Elmorsy, E., Shahda, M., el-HM, M., Rakha, S. A. & Shoaib, M. Blood lactate levels as a biomarker of antipsychotic side effects in patients with schizophrenia. J. Psychopharmacol. 30 (1), 63–68 (2016).
Park, H. J., Choi, I. & Leem, K. H. Decreased brain pH and pathophysiology in schizophrenia. Int. J. Mol. Sci. 22(16). (2021).
Huang, X. et al. Histogenous hypoxia and acid retention in schizophrenia: changes in venous blood gas analysis and SOD in acute and stable schizophrenia patients. Front. Psychiatry. 12, 792560 (2021).
Huang, J. T. et al. CSF metabolic and proteomic profiles in patients prodromal for psychosis. PLoS One. 2 (8), e756 (2007).
Beasley, C. L. et al. Metabolic abnormalities in fronto-striatal-thalamic white matter tracts in schizophrenia. Schizophr Res. 109(1–3): 159 – 66. (2009).
Wijtenburg, S. A. et al. Metabolite alterations in adults with schizophrenia, first degree relatives, and healthy controls: A Multi-Region 7T MRS study. Front. Psychiatry. 12, 656459 (2021).
Orešič, M. et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 3 (3), 19 (2011).
Béland-Millar, A., Larcher, J., Courtemanche, J., Yuan, T. & Messier, C. Effects of systemic metabolic fuels on glucose and lactate levels in the brain extracellular compartment of the mouse. Front. Neurosci. 11, 7 (2017).
Schlaaff, K. et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav. Immun. 88, 497–506 (2020).
Casas, B. S. et al. Schizophrenia-derived HiPSC brain microvascular endothelial-like cells show impairments in angiogenesis and blood-brain barrier function. Mol. Psychiatry. 27 (9), 3708–3718 (2022).
Pinacho, R. et al. The glial phosphorylase of glycogen isoform is reduced in the dorsolateral prefrontal cortex in chronic schizophrenia. Schizophr Res. 177 (1–3), 37–43 (2016).
Silva, E., Brito, L., Yuzawa, M. D. & Rosenstock, J. Mitochondrial dysfunction and changes in High-Energy compounds in different cellular models associated to hypoxia: implication to schizophrenia. Sci. Rep. 9 (1), 18049 (2019).
Srivastava, R., Faust, T., Ramos, A., Ishizuka, K. & Sawa, A. Dynamic changes of the mitochondria in psychiatric illnesses: new mechanistic insights from human neuronal models. Biol. Psychiatry. 83 (9), 751–760 (2018).
Yesilkaya, U. H., Gica, S., Ilnem, M. C., Sen, M. & Ipekcioglu, D. Evaluation of IGF-1 as a novel theranostic biomarker for schizophrenia. J. Psychiatr Res. 140, 172–179 (2021).
Roosterman, D. & Cottrell, G. S. The two-cell model of glucose metabolism: a hypothesis of schizophrenia. Mol. Psychiatry. 26 (6), 1738–1747 (2021).
Bryll, A. et al. Oxidative-Antioxidant imbalance and impaired glucose metabolism in schizophrenia. Biomolecules 10(3). (2020).
Tian, Y. et al. Obesity in Chinese patients with chronic schizophrenia: prevalence, clinical correlates and relationship with cognitive deficits. Schizophr Res. 215, 270–276 (2020).
Morland, C. et al. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J. Neurosci. Res. 93 (7), 1045–1055 (2015).
Brown, T. P. & Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 206, 107451 (2020).
van den Lee, J., Nithianantharajah, J. & Jones, N. C. Acute NMDA receptor antagonism impairs working memory performance but not attention in rats-Implications for the NMDAr hypofunction theory of schizophrenia. Behav. Neurosci. 134 (4), 323–331 (2020).
Wang, X. et al. Effects of sodium Nitroprusside in the acute Dizocilpine (MK-801) animal model of schizophrenia. Brain Res. Bull. 147, 140–147 (2019).
Unal, G., Sirvanci, S. & Aricioglu, F. α7 nicotinic receptor agonist and positive allosteric modulators differently improved schizophrenia-like cognitive deficits in male rats. Behav. Brain Res. 397, 112946 (2021).
Maleninska, K., Jandourkova, P., Brozka, H., Stuchlik, A. & Nekovarova, T. Selective impairment of timing in a NMDA hypofunction animal model of psychosis. Behav. Brain Res. 419, 113671 (2022).
Eyjolfsson, E. M. et al. Altered 13 C glucose metabolism in the cortico-striato-thalamo-cortical loop in the MK-801 rat model of schizophrenia. J. Cereb. Blood Flow. Metab. 31 (3), 976–985 (2011).
Hung, C. C., Lin, C. H. & Lane, H. Y. Cystine/Glutamate antiporter in schizophrenia: from molecular mechanism to novel biomarker and treatment. Int. J. Mol. Sci. 22(18). (2021).
Vila, È. et al. Inhibition of Prolyl oligopeptidase restores prohibitin 2 levels in psychosis models: relationship to cognitive deficits in schizophrenia. Int. J. Mol. Sci. 24(7). (2023).
Qi, A. et al. Essential protein PHB2 and its regulatory mechanisms in Cancer. Cells 12(8). (2023).
Zhou, X. et al. Modulating NMDA receptors to treat MK-801-induced schizophrenic cognition deficit: effects of clozapine combining with PQQ treatment and possible mechanisms of action. BMC Psychiatry. 20 (1), 106 (2020).
Yu, K. et al. Mechanism of cognitive impairment and white matter damage in the MK-801 mice model of schizophrenia treated with quetiapine. Behav. Brain Res. 461, 114838 (2024).
Koo, B. et al. A botanical drug composed of three herbal materials attenuates the sensorimotor gating deficit and cognitive impairment induced by MK-801 in mice. J. Pharm. Pharmacol. 72 (1), 149–160 (2020).
Uehara, T. et al. Effect of transient Blockade of N-methyl-D-aspartate receptors at neonatal stage on stress-induced lactate metabolism in the medial prefrontal cortex of adult rats: role of 5-HT1A receptor agonism. Synapse 66 (5), 408–417 (2012).
Xie, J., Hong, S., Zhang, X., Li, Y. & Xie, R. Inhibition of Glycolysis prevents behavioural changes in mice with MK801-induced SCZ model by alleviating lactate accumulation and lactylation. Brain Res. 1812, 148409 (2023).
Kolar, D. et al. Glycolytic and Krebs cycle enzymes activity in rat prefrontal cortex, hippocampus, and striatum after single and repeated NMDA Inhibition by MK-801. Neurotoxicology 90, 35–47 (2022).
Rocha, A. et al. Clozapine induces astrocyte-dependent FDG-PET hypometabolism. Eur. J. Nucl. Med. Mol. Imaging. 49 (7), 2251–2264 (2022).
Uehara, T., Matsuoka, T., Sumiyoshi, T. & Tandospirone A 5-HT1A partial agonist, ameliorates aberrant lactate production in the prefrontal cortex of rats exposed to Blockade of N-methy-D-aspartate receptors; toward the therapeutics of cognitive impairment of schizophrenia. Front. Behav. Neurosci. 8, 291 (2014).
Zhao, J., Liu, X., Huo, C., Zhao, T. & Ye, H. Abnormalities in prefrontal cortical gene expression profiles relevant to schizophrenia in MK-801-Exposed C57BL/6 mice. Neuroscience 390, 60–78 (2018).
Ni, R. J. et al. Depletion of microglia with PLX3397 attenuates MK-801-induced hyperactivity associated with regulating inflammation-related genes in the brain. Zool. Res. 44 (3), 543–555 (2023).
Liu, M., Li, H., Yang, R., Ji, D. & Xia, X. GSK872 and necrostatin-1 protect retinal ganglion cells against necroptosis through Inhibition of RIP1/RIP3/MLKL pathway in glutamate-induced retinal excitotoxic model of glaucoma. J. Neuroinflammation. 19 (1), 262 (2022).
Kim, J. Y. et al. Effect of D-pinitol on MK-801-induced schizophrenia-like behaviors in mice. Phytother Res. 37 (12), 5904–5915 (2023).
Zhang, Z. et al. Blockade of the NLRP3/caspase-1 axis attenuates ketamine-induced hippocampus pyroptosis and cognitive impairment in neonatal rats. J. Neuroinflammation. 18 (1), 239 (2021).
Sernoskie, S. C. et al. Clozapine induces an acute Proinflammatory response that is attenuated by Inhibition of inflammasome signaling: implications for idiosyncratic Drug-Induced agranulocytosis. Toxicol. Sci. 186 (1), 70–82 (2022).
Giridharan, V. V. et al. Clozapine prevents Poly (I:C) induced inflammation by modulating NLRP3 pathway in microglial cells. Cells 9(3). (2020).
Chamera, K. et al. The prenatal challenge with lipopolysaccharide and polyinosinic:polycytidylic acid disrupts CX3CL1-CX3CR1 and CD200-CD200R signalling in the brains of male rat offspring: a link to schizophrenia-like behaviours. J. Neuroinflammation. 17 (1), 247 (2020).
Vizuete, A. et al. Early effects of LPS-induced neuroinflammation on the rat hippocampal glycolytic pathway. J. Neuroinflammation. 19 (1), 255 (2022).
Chausse, B., Lewen, A., Poschet, G. & Kann, O. Selective Inhibition of mitochondrial respiratory complexes controls the transition of microglia into a neurotoxic phenotype in situ. Brain Behav. Immun. 88, 802–814 (2020).
Lin, H. C. et al. Lactic acid fermentation is required for NLRP3 inflammasome activation. Front. Immunol. 12, 630380 (2021).
Lee, J. H. et al. Double-stranded RNA-activated protein kinase is required for the LPS-induced activation of STAT1 inflammatory signaling in rat brain glial cells. Glia 50 (1), 66–79 (2005).
Al-Dujaili, A. H., Mousa, R. F., Al-Hakeim, H. K. & Maes, M. High mobility group protein 1 and Dickkopf-Related protein 1 in schizophrenia and Treatment-Resistant schizophrenia: associations with Interleukin-6, symptom domains, and neurocognitive impairments. Schizophr Bull. 47 (2), 530–541 (2021).
Yao, X. & Li, C. Lactate dehydrogenase A mediated histone lactylation induced the pyroptosis through targeting HMGB1. Metab. Brain Dis. 38 (5), 1543–1553 (2023).
Park, J. S. et al. LDHB deficiency promotes mitochondrial dysfunction mediated oxidative stress and neurodegeneration in adult mouse brain. Antioxid. (Basel) 11(2). (2022).
Hermans, D. et al. Lactate dehydrogenase Inhibition synergizes with IL-21 to promote CD8(+) T cell stemness and antitumor immunity. Proc. Natl. Acad. Sci. U S A. 117 (11), 6047–6055 (2020).
Xie, D. et al. Moschus ameliorates glutamate-induced cellular damage by regulating autophagy and apoptosis pathway. Sci. Rep. 13 (1), 18586 (2023).
Kübler, R., Ormel, P. R., Sommer, I., Kahn, R. S. & de Witte, L. D. Gene expression profiling of monocytes in recent-onset schizophrenia. Brain Behav. Immun. 111, 334–342 (2023).
Goldwaser, E. L. et al. White matter in prolonged glucocorticoid response to psychological stress in schizophrenia. Neuropsychopharmacology 46 (13), 2312–2319 (2021).
Zhang, Q. et al. Analysis of cognitive impairment in schizophrenia based on machine learning: interaction between psychological stress and immune system. Neurosci. Lett. 760, 136084 (2021).
Jaszczyk, A. & Juszczak, G. R. Glucocorticoids, metabolism and brain activity. Neurosci. Biobehav Rev. 126, 113–145 (2021).
Vallée, A., Lecarpentier, Y., Guillevin, R. & Vallée, J. N. Thermodynamics in neurodegenerative diseases: interplay between canonical WNT/Beta-Catenin Pathway-PPAR gamma, energy metabolism and circadian rhythms. Neuromolecular Med. 20 (2), 174–204 (2018).
Vallée, A. Neuroinflammation in schizophrenia: the key role of the WNT/β-Catenin pathway. Int. J. Mol. Sci. 23(5). (2022).
Funding
We are grateful to these grant programs for funding our experiments: Yunnan Provincial Science and Technology Department-Science and Technology Program (202203AC100007; 202301AY070001-145); National Natural Science Foundation of China (82160269;82360275;82160272); Yunnan Provincial Science and Technology Department-Kunming Medical University Joint Fund for Basic Research Excellent Young Talent Cultivation Project (202101 AY070001-033); Yunnan Provincial Science and Technology Department-Kunming Medical University Joint Fund for Basic Research Project (202201 AY070001-212); “Chuncheng Plan” High-Level Talent Development Program for Spring City Prominent Talents Project (C201914016).
Author information
Authors and Affiliations
Contributions
Yingying Zhang, Yanjun Wang and Haoran Xing share the first authorship. Yingying Zhang, Yanjun Wang and Haoran Xing wrote the main manuscript text. Mier Li, Haiqiang Zhao and Luanmei Ding assisted in the experiment. Yuncheng Bai, Weiwei Wang and Tianhao Bao provided research design and guidance. Corresponding Author: Weiwei Wang* (Email: doctor_b@whu.edu.cn) and Tianhao Bao* (Email: doctor@kmmu.edu.cn). All authors revised the paper critically for intellectual content and gave final approval of the version to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Ethical Review Committee for Animal Experiments of Kunming Medical University and conformed to the standards (No. kmmu20230164).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Wang, Y., Xing, H. et al. Association between endogenous lactate accumulation and dysregulated activation of the NLRP3 inflammasome pathway in schizophrenia. Sci Rep 15, 19609 (2025). https://doi.org/10.1038/s41598-025-04823-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04823-6