Abstract
In a translationally relevant ovine model of gestational hyperandrogenism we have previously reported cardiometabolic disruption in female offspring (males were not extensively studied). We hypothesized that gestational hyperandrogenism would lead to sex-specific disruption in offspring’s growth, cytokine and metabolic milieu, potential mediators of cardiometabolic disease (CMD). 100 mg Testosterone propionate (T) or vehicle (C) was administered intramuscularly twice weekly between gestational days (GD) 30–90. Two cohorts of offspring were generated, including both males and females. Fetal weight and systemic metabolomics were analyzed in the fetal cohort (GD120). Growth trajectory, systemic cytokines, and metabolomics were analyzed in the postnatal cohort. Data was analyzed using mixed model ANOVA, student T-test, and Cohen D (d) analysis. T excess led to (1) a reduction in fetal weight at GD 120 in both sexes and sex-specific perturbations in the fetal metabolome, (2) T-Female had a growth trajectory similar to males and significantly different from C-Females (age x treatment interaction p = 0.028) and (3) sex-specific alterations in cytokine milieu at birth and metabolome in the pre-pubertal period. Altered sex-specific postnatal metabolic milieu and growth trajectory, notable for catch-up growth in T-Female, could have implications for sex-specific impact on the cardiometabolic function in the lambs.
Similar content being viewed by others
Introduction
The developing fetus is highly susceptible to alterations in the gestational environment. While there may be beneficial fetal adaptation to such alterations, various maladaptive fetal responses leading to adverse postnatal outcomes are well-documented1. Numerous clinical and pre-clinical studies have shown that an adverse in-utero environment is associated with poor fetal growth and increased risk for cardiovascular disease (CVD) and metabolic derangements in offspring later in life1,2,3,4. Other studies have found that the increased rate of weight gain in early childhood is also associated with increased risk for CMD later in life5. For instance, newborns with low birth weight and rapid weight gain after birth were associated with the highest mortality rates related to coronary heart disease6. Another study found that irrespective of birth weight, those who experienced the fastest weight gain during the initial three months of life exhibited the most unfavorable cardiovascular and metabolic outcomes7. Overall, both growth restriction during fetal life and enhanced postnatal growth rate appear to be related to adverse cardiometabolic outcomes and emphasize the need not only to monitor early growth trajectory but also to gain an understanding of the mediators.
The contributors to the altered fetal growth and postnatal growth trajectory include offspring cytokine and metabolic milieu8,9,10,11,12. For instance, alterations in cytokines at birth have been noted in IUGR fetuses and are reported to contribute to altered growth trajectory in the postnatal period13,14 .Similarly, blood and urine metabolite composition have offered valuable information about the metabolic pathways contributing to altered growth and adverse phenotypic outcomes and have the potential to serve as biomarkers15,16. Of developmental importance, perturbation in the metabolome in cord blood and urine samples of neonates is associated with intrauterine growth restriction (IUGR) and early growth perturbations, respectively8,9. A study using untargeted metabolomics found a correlation between cord blood metabolites and rapid weight gain after birth17. As such, profiling metabolome during fetal growth restriction and postnatal growth period will help identify potential mediators and biomarkers for alterations in sexually dimorphic growth patterns.
Animal models offer exceptional opportunities for relating fetal and offspring metabolome changes to their growth trajectory. For instance, in the well-validated and translationally relevant ovine model of gestational hyperandrogenism, prenatal testosterone excess induced IUGR and catch-up growth in female offspring as well as cardiometabolic perturbations in the female offspring18,19,20. While extensive studies have been carried out with female offspring of gestational T excess in terms of IUGR and postnatal growth, the impact of prenatal T excess in males has not undergone such intense investigation20,21,22,23. Of note, gestational hyperandrogenism is frequently reported in pregnancies impacted by polycystic ovarian syndrome (PCOS), congenital adrenal hyperplasia, and preeclampsia24,25,26,27,28. Our recent study addressing the metabolic milieu using untargeted lipidomics did find a sex-specific alteration in the lipidome at birth in the prenatal-T-treated offspring29. Because lipids supply essential lipid-soluble vitamins and polyunsaturated fatty acids needed in high amounts during early growth and are the major energy source, the sexual dimorphism in lipidomics evident at birth is suggestive of alterations in the trajectory of constituent lipids that may begin during fetal life and continue during the postnatal growth phase and result in sexually dimorphic growth impact. In addition, apart from lipids, branched-chain systemic amino acids, and other essential metabolites are also important for growth, and the impact of prenatal T excess on other metabolites has not been explored in the offspring. Utilizing the well-validated and translationally relevant sheep model of gestational hyperandrogenism, the objectives of the current study were twofold: (1) assess the impact of gestational T excess on late gestational fetal weight and metabolome in both sexes (2) determine the contribution of postnatal cytokine milieu and pre-pubertal metabolome on postnatal growth trajectory in both sexes from birth till post-pubertal age.
Results
Study 1 (fetal cohort): Impact of gestational T excess on fetal metabolome and relation to fetal weight:
Fetal weights: Fig. 1B shows the GD120 fetal weights. There was an overall treatment effect of prenatal T excess on fetal weight (p = 0.014). T treatment significantly reduced fetal weight at GD120 in both sexes.
Fetal metabolome: Sex differences in the metabolome in C fetuses: Enrichment analysis of polar metabolites measured in C fetuses at GD 120 showed that the main-class metabolites that differed between C-Males (CM) and C-Females (CF) (p < 0.05 and enrichment ratio > 1.5) mapped to glycine, serine, and threonine metabolism, glycerolipid metabolism, arginine and proline metabolism, cysteine and methionine metabolism, glyoxylate and dicarboxylate metabolism, lipoic acid metabolism and D amino acid metabolism (Fig. 2A). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 1. Enrichment analysis of the lipidomics data showed the main-class metabolites that differed between CM and CF (p < 0.05 and enrichment ratio > 1.5) included sphingomyelins, glycerophosphocholines, and sphingolipids (Fig. 2B).
Sex differences in metabolome profile of control fetuses and overall effect of prenatal T treatment at GD 120 (A) Polar metabolites main class responsible for sex differences between control males and females (male n = 9 and female n = 10) based on enrichment analysis (B) Lipid metabolite main class responsible for sex differences between control males and females (male n = 9 and female n = 10) based on enrichment analysis. (C) Overall effect of prenatal T treatment on polar metabolites main class responsible for differences between control (C) and testosterone (T) treated fetuses (control n = 19 and T treatment n = 16) based on enrichment analysis (D) Overall effect of prenatal T treatment on Lipid metabolite main class responsible for differences between control (C) and testosterone(T) treated fetuses (control n = 19 and T treatment n = 16) based on enrichment analysis.
Impact of T excess on the systemic fetal metabolome: Enrichment analysis of polar metabolites showed the main-class metabolites that differed between T vs C fetuses (p < 0.05 and enrichment ratio > 1.5) are involved in galactose metabolism, D-amino acid metabolism, fructose and mannose metabolism, glyoxylate and dicarboxylate metabolism, starch and sucrose metabolism, neomycin, kanamycin and gentamicin, pantothenate and CoA biosynthesis, amino sugar and nucleotide sugar metabolism, inositol phosphate metabolism and porphyrin metabolism between groups (Fig. 2C). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 2. Enrichment analysis of the lipidomics data showed the main-class metabolites that differed between T vs C fetuses (p < 0.05 and enrichment ratio > 1.5) included sphingomyelins, sphingolipids, glycerophosphocholines, glycerophospholipids, glycosphingolipids (Fig. 2D).
Sex-specific impact of T on systemic fetal metabolome: Enrichment analysis of polar metabolites showed the main-class metabolites that differed between CF and T-Female (TF) (p < 0.05 and/or enrichment ratio > 1.5) included galactose metabolism, valine, leucine, isoleucine biosynthesis and degradation, D-amino acid metabolism, porphyrin metabolism, fructose and mannose metabolism, starch and sucrose metabolism, phenylalanine metabolism, tyrosine and tryptophan metabolism (Fig. 3A). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 3. Lipidomic enrichment analysis (p < 0.05 and/or enrichment ratio > 1.5) showed that the main-class metabolites that were enriched between CF and TF included glycerophosphocholines, sphingomyelins, glycerophospholipids, glycerophosphoethanolamines, glycosphingolipids (Fig. 3B).
Sex-specific effect of prenatal T treatment on metabolome profiles at GD120. (A) Polar metabolites main-classes responsible for the separation of C and prenatal T-treatment based on enrichment analysis in females. (B) Lipid metabolite main classes responsible for the separation of C and prenatal T-treatment based on enrichment analysis in females. (C) Polar metabolites main classes responsible for the separation of C and prenatal T-treatment based on enrichment analysis in males. (D) Lipid metabolite main classes responsible for separation of C and prenatal T-treatment based on enrichment analysis in males.
In males’ main class, polar metabolites enriched (p < 0.05 and/or enrichment ratio > 1.5) between CM vs. T-Male (TM) included D-amino acid metabolism, glycerophospholipid metabolism, sphingolipid metabolism, pentose phosphate pathway, amino sugar, and nucleoside sugar metabolism, fructose and mannose metabolism (Fig. 3C). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 4. Lipidomic enrichment analysis (p < 0.05 and/ or enrichment ratio > 1.5) in males showed that the main class metabolites that were enriched between CM and TM included sphingomyelins, sphingolipids, fatty esters, glycerophosphocholines, glycerophospholipids, glycosphingolipids (Fig. 3D).
Study 2 (postnatal cohort): Impact of prenatal T excess on postnatal growth trajectory cytokine profile, and the metabolome
Postnatal growth trajectory: TF had a growth trajectory similar to TM and CM and significantly different from CF from birth till 24 weeks with a significant treatment, sex, and age interaction (p = 0.028) (Fig. 4). Since males were castrated at the time of weaning, we also analyzed weight trajectory with the same sex cohorts from birth till adulthood (40 weeks of age), and there was no significant difference in the growth curve between CM and TM (Fig. 5A). In contrast, TF gained weight faster compared to CF after 8 weeks of life till 40 weeks (p = 0.028) (Fig. 5B).
Impact of prenatal T excess on cytokine profile at birth: There was a significant elevation in TNFα level pg/mL in TM compared to CM (p = 0.04, d = 1.46; Fig. 6A). There was also a non-significant large magnitude increase in IL-1α in TM compared to CM (p = 0.17, d = 0.94; Fig. 6B). Moreover, there was a non-significant large magnitude decrease in MIP1β (p = 0.08, d = 0.99; Fig. 6C) in TM compared to CM. In contrast, no significant changes were seen in the cytokine milieu in the female offspring with T treatment compared to CF. There was a non-significant large magnitude increase in IL-4 (p = 0.16, d = 1.08; Fig. 7L) and a non-significant large magnitude decrease in IL-8 (p = 0.22, d = 0.84; Fig. 7A) and IL-1α (p = 0.28, d = 0.95; Fig. 7B) in TF compared to CF.
Impact of prenatal T excess on the pre-pubertal metabolome: Sex differences in control lambs: Enrichment analysis showed that the polar metabolite that differed between CM and CF (p < 0.05 and/or enrichment > 1.5) were histidine metabolism, valine, leucine, isoleucine biosynthesis and degradation, taurine and hypotaurine metabolism as well as alterations in beta-alanine metabolism, galactose metabolism, tryptophan metabolism, glycerolipid metabolism, primary bile acid biosynthesis and tyrosine metabolism (Fig. 8A). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 5. Enrichment analysis performed to identify systemic changes in the lipidome for CF and CM groups found lipid main class responsible for the separation between groups were sphingomyelins and ceramides (p = 0.05 and/or enrichment > 1.5) (Fig. 8B).
Sex differences in metabolome profile of control fetuses and overall effect of prenatal T treatment in prepubertal lambs (A) Polar metabolites main class responsible for sex differences between control males and females (male n = 9 and female n = 10) based on enrichment analysis (B) Lipid metabolite main class responsible for sex differences between control males and females (male n = 9 and female n = 10) based on enrichment analysis. (C) Overall effect of prenatal T treatment on polar metabolites main class responsible for differences between control (C) and testosterone(T) treated lambs (control n = 12 and T treatment n = 14) based on enrichment analysis (D) Overall effect of prenatal T treatment on Lipid metabolite main class responsible for differences between control (C) and testosterone(T) treated lambs (control n = 12 and T treatment n = 14) based on enrichment analysis.
Impact of T excess on the systemic metabolome of prepubertal lambs: Enrichment analysis of polar metabolites (p < 0.05 and enrichment > 1.5) comparing T vs. C identified glycerolipid metabolism, galactose metabolism, arginine biosynthesis, arginine and proline metabolism, biotin metabolism, butanoate metabolism, tyrosine metabolism, nicotinate and nicotinamide metabolism, glutathione metabolism, propanoate metabolism, glycine, serine, threonine metabolism, D-amino acid metabolism as metabolic pathways altered by prenatal T excess (Fig. 8C). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 6. Enrichment analysis undertaken to investigate lipidome profile identified glycosphingolipids, sphingolipids, ceramides, steroids, and steroid derivatives responsible for the separation (p < 0.05 between control and T excess groups (Fig. 8D).
Sex-specific impact of T on the systemic metabolome of prepubertal control lambs: Enrichment analysis (p < 0.05 and enrichment > 1.5) of polar metabolites showed the metabolites responsible for the separation between CF and TF included glycerolipid metabolism, galactose metabolism, butanoate metabolism, pantothenate and CoA biosynthesis, propanoate metabolism, pyrimidine metabolism, arginine, and proline metabolism, valine, leucine, isoleucine biosynthesis and degradation, nitrogen metabolism, glycerophospholipid metabolism and beta-alanine metabolism (Fig. 9A). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 7. Lipidomic enrichment analysis (p < 0.05 and/or enrichment > 1.5) showed that the main-class metabolites that were enriched between CF and TF included fatty acyls, glycerophosphoethanolamines glycerophosphocholines, glycosphingolipids, glycerophosphoinositols and fatty esters (Fig. 9B).
Sex-specific effect of prenatal T treatment on prepubertal metabolome profiles in lambs (A) Polar metabolites main-classes responsible for separation of C and prenatal T-treated based on enrichment analysis in females. (B) Lipid metabolite main classes responsible for separation of C and prenatal T-treated based on enrichment analysis in females. (C) Polar metabolites main-classes responsible for separation of C and prenatal T-treated based on enrichment analysis in males. (D) Lipid metabolite main classes responsible for separation of C and prenatal T-treated based on enrichment analysis in males.
In males’ main class polar metabolites enriched (p < 0.05 and enrichment ratio > 1.5) between CM vs TM included glycerolipid metabolism and galactose metabolism, D-amino acid metabolism, glutathione metabolism, phenylalanine metabolism, arginine biosynthesis, phenylalanine, tyrosine and tryptophan metabolism, nicotinate and nicotinamide metabolism, ubiquinone and other terpenoids, biotin metabolism, arginine and proline metabolism and pyrimidine metabolism (Fig. 9C). The specific metabolites that differed within enriched pathways mentioned above are listed in supplemental table 8. Enrichment analysis (p < 0.05 and/or enrichment > 1.5) of lipidomic profile in males showed glycosphingolipids, ceramides, steroid and steroid derivatives, and glycerolipids as the lipid classes responsible for the separation between CM vs TM groups (Fig. 9D).
Discussion
Our findings are suggestive of sex-specific adverse programming of growth and metabolic milieu as early as fetal life and in the postnatal period, with exposure to prenatal T excess during early to mid-gestation. Specifically, low fetal weight was noted in late gestation, and the rate of weight gain in the TF was comparable to that of the males in the postnatal period. Moreover, we noted prenatal T excess led to sex-specific disruption in both polar and non-polar metabolites that are important for growth in both the fetal and postnatal periods. Interestingly, specific lipid metabolites altered in fetal life due to prenatal T excess (FC cohort) were also altered at birth and persisted into the prepubertal time in the prenatal T-treated animals (PS cohort)29. Overall, it could be postulated that the altered systemic metabolome starting in fetal life and persisting into the postnatal period could contribute to the altered growth trajectory noted in the TF offspring.
Impact of prenatal T excess on offspring growth
The increased rate of weight gain in the control male compared to the control female lambs corroborates previous findings in sheep of higher postnatal weight gain in males compared to females30,31. Of interest, relative to the impact of prenatal T excess on offspring growth, our current study and previous study have found growth restriction in fetal life in both sexes, a risk factor for altered postnatal growth and cardiometabolic disease5,20. In contrast, Gill &Hosking reported increased growth in only female fetuses when a single injection of 200 mg T (2 ml of 100 mg/ml) was administered on gestational day 6032,33,34,35. Building upon our findings in early postnatal life of the sexual dimorphic impact of prenatal T excess on the rate of weight gain (between 0 and 2 months and 2–4 months), the current study provided a detailed assessment of postnatal growth pattern from birth till post-pubertal period20,36. These findings document that the increased growth rate persists until 10 months of age in the TF relative to CF and is comparable between CM and TM. Previous studies utilizing prenatal T exposure at a different time point during mid-gestation compared to what was used in our studies have also reported an increase in postnatal growth rate in T-treated female offspring compared to controls suggesting T exposure during mid-gestation programs postnatal growth trajectory in a sex-specific fashion34,35,37. Furthermore, a study conducted in Chile focusing on the impact of prenatal T excess (40 mg IM testosterone from gestational day 30–120 days; lower dose compared to our treatment group) on only male offspring noted no difference in body weights between control and prenatal T treated males except at a 20-week time-point38. In contrast, we found no differences in weight between T-treated and C males at any time points. There could be a combination of factors accounting for these differences, such as dosing and timing of exposure between the two studies. Moreover, since our male lambs were orchiectomized around the time of weaning while the earlier study involved gonad-intact animals, one could postulate that the difference in weight gain between the two studies could be due to differences in duration of T exposure, our animals not seeing T after weaning vs. the Chilean study seeing endogenous T throughout. Of interest, the males in our study showed an increased growth rate compared to females despite being orchiectomized at weaning. Of relevance, a study comparing body weight gain at birth and 16 weeks of age between testis intact and orchiectomized lambs found no differences in weight gain between the two groups39. Similarly, a study assessing the impact of early exposure to prenatal T in sheep between gestational days 20–65 did not note any difference in weight trajectory in testes intact and orchiectomized twins40. However, the aforementioned study did not note any differences in postnatal growth trajectory in both sexes with prenatal T exposure between GD 20–65, which is in contrast to our finding of increased growth rate in the T-treated females and could be due to differing timing and dose of prenatal T treatment40 suggesting that presence of testosterone in later prepubertal years is not driving the increase in weight gain. Although the exact etiology for adverse programming of offspring growth is unclear, postnatal perturbation in the insulin-like growth factor axis that occurs during the first month of postnatal life in the prenatal T-animals20 may have played a role in the persistence of altered postnatal growth.
Impact of prenatal T excess on cytokine profile at birth
The lambs’ cytokine profile can contribute to the altered growth trajectory. Although we did not find any significant changes in cytokine levels in T females at birth, we did note a significant increase in TNF alpha, a proinflammatory cytokine, in T males. Of relevance, TNF alpha is increased in IUGR offspring41,42. In our current study, growth was restricted in late gestation in T males; however, in the postnatal period, T excess did not perturb postnatal growth in T males. Similarly, a previous study in rodents noted that IUGR male offspring and not females had increased TNF alpha suggestive of sex-specific impact of the adverse prenatal environment on the pro-inflammatory milieu of the offspring41.
Impact of prenatal T excess on the systemic metabolome
In recent years, alterations in systemic metabolome have been reported in various human studies and animal models of IUGR43,44,45. Therefore, early determination of changes in systemic metabolome could serve as important biomarkers in predicting the IUGR and postnatal growth outcomes, as evidenced in our model. Our current study notes perturbation in the systemic lipidome in T-treated IUGR fetuses. Specifically, we note an increase in overall sphingolipid metabolism in the T-male fetuses with enrichment of sphingomyelins in both sexes with T treatment, suggesting that sphingolipids may play an essential role in T-induced IUGR in this model. In fact, perturbation in sphingolipids, as seen in T-treated fetuses, has been reported in pregnancies with other adverse pregnancy outcomes, including preeclampsia, and correlates with intrauterine growth retardation46,47,48. Moreover, we note sex-specific alteration in glycerophosphocholines and glycerophospholipids in our IUGR T females only and these metabolites are altered in cord blood of IUGR offspring, implicating their role in fetal growth49. Similarly, we note sex-specific alteration in fatty acyls (acylcarnitine CAR 16:1 and CAR 18:1) in IUGR T-male fetuses, and since fatty acids are important components of growth and metabolism changes in these lipids could be additional contributors to the IUGR noted in T male fetuses50. Interestingly, although we did not see alteration in fatty acids in T female fetuses, we did note increased enrichment in fatty acyls in T-prepubertal females, suggesting that this metabolite may play an important role in catch-up growth noted in the female offspring. Like during fetal life, both glycerophosphocholines and glycerophospholipids are enriched in T prepubertal offspring as well, and the persistence of these metabolites into the postnatal period indicates they play a crucial role in the T-induced alteration of growth and development in the female offspring.
Apart from alterations in lipidome, we also note alterations in polar metabolites with prenatal T excess. Specifically, we note that prenatal T excess alters galactose metabolism in T-treated female offspring in fetal life that persists into the prepubertal period. Galactose is an important nutrient for childhood growth and nutrition, and alteration in its metabolism could contribute to the altered growth trajectory seen in our T lambs51. Furthermore, we note enrichment in branched-chain amino acids (BCAAs) in fetal and prepubertal T females. Interestingly, BCAAs positively correlate with small for gestation age and postnatal increase in body mass index (BMI)52,53. BCAAs also play crucial roles as foundational components for muscle protein synthesis54. Among these BCAAs, leucine, which was enriched in our T female fetuses, offers numerous growth-related benefits, including the prevention of muscle loss, improvement of insulin sensitivity, augmentation of fat metabolism, and facilitation of recovery55. Therefore, alterations in leucine and BCAAs during a crucial time of growth may be underlying the perturbed growth noted in the T-treated female offspring. Hence, although we note low fetal weight and no change in postnatal growth in the T males compared to control males, underlying changes in amino acid composition could alter the biological function of the T males and the impact of T excess on some of the crucial biological functions listed above in our T males remains to be determined.
Finally, sex-specific changes in the systemic metabolome seen in our cohort during the prepubertal period in this study have also been noted at birth29. Specifically, our earlier finding of alterations in certain lipid metabolites, including fatty acyls/esters, glycerophosphoethanolamines, and glycerophosphocholines in T female offspring during fetal life and at birth, seems to have persisted into a prepubertal period in female offspring (Fig. 10)29. Similarly, apart from lipids, metabolites, including BCAAs, were altered in fetal life and seem to have persisted in the postnatal period in female offspring (Fig. 11). This suggests the sex-specific organizational impact of prenatal T excess on the metabolic milieu in this model. Moreover, persistent changes in the systemic metabolomic milieu can have long-term adverse cardiometabolic outcomes.
Strengths and limitations: The major strengths of our study include (1) Utilization of a translationally relevant precocial large animal model, (2) Sex-specific detailed characterization during the growth phase, and (3) Simultaneous evaluation of cytokine and metabolic milieu, known mediators of growth and development. Importantly, we are able to compare sex-specific perturbations in systemic lipidome in fetal (this study), at birth (previously published,29) and prepubertal period (this study), providing a more thorough understanding of how T adversely programs the metabolic milieu from late fetal life till prepuberty in this novel model.
Limitations include the inability to study the postnatal growth of gonad-intact males in the pubertal period, thereby, the inability to assess if gonadal steroids alter the weight trajectory in the males during the pubertal period. Furthermore, since T aromatizes to estrogen, it is not clear if the adverse postnatal growth and metabolic programming seen in our model is exclusively in response to androgen excess or secondary to estrogen programming, since T can be aromatized to estrogen.
Translational relevance: The prenatal T-excess ovine model mimics pregnancies impacted by gestational hyperandrogenism, including those impacted by PCOS, a reproductive disorder affecting > 100 million women worldwide56. Women with PCOS have a higher proportion of small for gestational age (SGA) babies who are also at risk for cardiometabolic disorders later in life57,58,59. Our sex-specific lifespan study encompasses the assessment of postnatal growth along with systemic cytokine and metabolome impacted by gestational hyperandrogenism and risk factors for cardiovascular disease. Our findings can serve as a platform for designing future mechanistic studies and early intervention in the postnatal period targeting growth and/or systemic metabolome milieu, thereby preventing the onset of adverse cardiometabolic outcomes documented in offspring of PCOS pregnancies.
Finally, our study demonstrates that prenatal T excess adversely impacts growth and the systemic metabolome in a sex-specific fashion in this novel ovine model. Moreover, the perturbations in the systemic metabolome in the offspring beginning as early as fetal life could contribute to the sex-specific cardiometabolic perturbation that occurs during the postnatal period in this ovine model of prenatal T excess. Further in-depth studies are required to determine the extent to which altered systemic metabolome can program the postnatal growth trajectory.
Material and methods
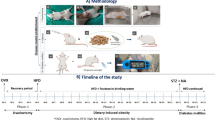
Animal studies: All study procedures, including studies conducted in fetal and postnatal offspring, were approved by the Institutional Animal Care and Use Committee at the University of Michigan, and all study procedures and methods were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. The study was carried out in compliance with ARRIVE guidelines. Experiments in this study used Suffolk sheep, housed at the University of Michigan Sheep Research Facility (Ann Arbor, MI) and met the National Research Council’s recommendations. Two separate cohorts were generated and are reported in this study. Study one, fetal cohort (FC), addressed the relationship between fetal metabolome and fetal weight at GD120 (term 147 days) (Fig. 1A). Animals from the FC have been used in previous studies60,61. Study two, postnatal cohort (PS), addressed the impact of prenatal T excess on the growth trajectory and its relationship to cytokines at birth and prepubertal metabolome. We have previously shown sex-specific alteration in lipidome at birth of T-treated lambs from this cohort29, and control animals from this cohort have also been used in another study62.
For both studies, time-mated pregnant Suffolk sheep were purchased and randomly assigned to the control (C) group or T group. Intramuscular injections of 100 mg T propionate (Sigma-Aldrich, St. Louis, MO) were administered twice weekly in 2 ml corn oil (vehicle) from days 30 to 90 of gestation (Term = 147 days). The same volume of vehicle in same frequency and duration was administered to C ewes. Time-mated pregnant ewes were housed under a natural photoperiod in sheep-specific housing facilities at the University of Michigan Sheep Research Facility and group-fed with a daily maintenance diet of 1.25 kg alfalfa/brome mix hay/ewe. Six weeks prior to lambing, when maximal fetus growth occurs, pregnant ewes were group-fed daily with 0.5 kg shelled corn, 2 kg alfalfa hay/ewe.
In the FC, 30 days post cessation of T injections (120 ± 5 days), ewes were humanely euthanized using barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI) in accordance with the AVMA’s guidelines for the Euthanasia of Animals63. The fetuses were removed, fetal weights were obtained (22 control from 12 mothers and 50 T-treated offspring from 22 mothers) and cord blood was obtained from the umbilical artery for metabolomic analysis.
In the PS study time-mated Suffolk sheep were treated with T or vehicle as described above. Control dams gave birth to 6 males and 4 female lambs, and T- treated dams gave birth to 8 females and 11 male lambs. 2 control lambs and 3 T-treated lambs died soon after delivery, with unclear etiology. Considering the need to use a dam as the experimental unit in these developmental studies to increase sample size, 6 additional C female and 5 C male offspring were obtained from the original source flock at 8 weeks of age and housed with the others. Male lambs were castrated to enable them to be housed with females and prevent pregnancies. The date of birth and sex of offspring were recorded. The sex of the fetuses of T-treated mothers was confirmed by conducting SRY gene analysis and visual examination of the offspring’s external genitalia, as female offspring of T-treated mothers are virilized and visually present with an empty scrotum. Blood sample was collected at pre-pubertal age for metabolomics analysis.
Assessment of fetal weight and offspring growth trajectory: For the FC cohort, fetal weight was obtained at harvest (GD120 ± 5 days) and analyzed using a mixed model with a random intercept for the mother and adjusting for the birth count. Sex was included as a covariate in the full model.
For the PS cohort, animals were weighed weekly on the same day from birth till 23 weeks of age (pre-pubertal period). The 6 additional control female and 5 male offspring moved to the sheep research facility are missing the first 8 weeks’ weight data. After 23 weeks (post-pubertal period), lambs were measured once a month till 40 weeks (early adulthood). To evaluate differences in lamb growth by sex and treatment over time, a generalized linear mixed model with a spline for age (weeks) was used to best fit the data. The spline model resulted in 7 equidistant knots. Random effects were included to adjust for repeated measurements for the lamb and lambs of the litter. Interactions between sex, treatment, and weekly age were tested. Due to differential growth after puberty, male and female lambs were only compared within the same model in the pre-pubertal period (up to 24 weeks). Additionally, lamb growth from birth to 40 weeks was compared between the treatment and control groups separately for both sexes using the spline mixed model. The models for female and male growth rates (weight change per week) included an intercept for the mother as a subject-level random effect to adjust for variability in initial conditions at birth. The data from the PS cohort was first analyzed with and without the addition of external control animals and found to be no different. Only composite data from all animals are presented.
Assessment of fetal cord blood and prepubertal plasma metabolomics/lipidomics: Disrupted metabolome has been previously reported in IUGR offspring and those with disrupted growth8,9,15,16. Therefore, metabolomic studies were conducted in both FC and PS cohort to assess their contribution to the altered growth in the offspring. Blood samples were collected for metabolomics and lipidomics analysis at 120 ± 5 days FC cohort and around 16 weeks of age in the PS cohort. To extract metabolites and lipids from plasma, 50 μL plasma was loaded onto a solid-phase-extraction (SPE) system CAPTIVA-EMR Lipid 96-well plate. Ultra-high-performance LC (UHPLC)/MS was performed with a Thermo Scientific Vanquish Flex UHPLC system interfaced with a Thermo Scientific Orbitrap ID-X Mass Spectrometer. Polar metabolites were separated on a HILICON iHILIC-(P)-Classic column (100 × 2.1 mm, 5 μm). The mobile-phase solvents were composed of: A = 20 mM ammonium bicarbonate, 2.5 μM medronic acid, 0.1% ammonium hydroxide in water: acetonitrile 95:5; and B = water:acetonitrile 5:95. The column compartment was maintained at 45 °C. The following linear gradient was applied at a flow rate of 0.25 mL min-1: 0–1 min, 90% B; 12 min, 35% B; 12.5 – 14.5 min, 25% B; 15 min, 90% B. Lipids were separated on a Waters Acquity Premier HSS T3 column (100 × 2.1 mm, 1.8 μm). The mobile-phase solvents were composed of A: 0.1% formic acid, 10 mM ammonium formate, 2.5 μM medronic acid in 60:40 acetonitrile:water; and B = 0.1% formic acid, 10 mM ammonium formate in 90:10 2-propanol: acetonitrile. The column compartment was maintained at 60 °C. The following linear gradient was applied at a flow rate of 0.25 mL min-1: 0–2 min, 30% B; 17 min, 75% B; 20 min, 85% B; 23–26 min, 100% B. The injection volume 4 μL for all polar and lipids analysis. Data was acquired in both positive and negative ion mode. LC/MS data were processed and analyzed with the open-source Skyline software, MetaboAnalyst 6.0, and Compound Discoverer 3.364,65. For lipidome analysis, data is grouped by chemical structures and superclass, main class, and subclass method in MetaboAnalyst. Polar metabolites were grouped by KEGG pathway analysis system in MetaboAnalyst.
Assessment of newborn inflammasome: Disruption in cytokine milieu at birth can contribute to postnatal growth disruption in offspring; hence, we analyzed newborn inflammasome in the PS cohort. Blood samples were collected at two days of life from all PS lambs born at the sheep research facility (purple cap -EDTA tubes, Becton, Dickinson and Company, NJ), centrifuged at 3000 g for 15 min, plasma separated and stored at − 20 °C till cytokine analysis. The levels of IL-1a, IL-8, IL-10, IL-36RA, IFN-γ, IP10, VEGF-A, and MIP-1a in the plasma were assessed using the MILLIPLEX® Ovine Cytokine/Chemokine Magnetic Bead Panel (SCYT1-91 K- MilliporeSigma, Burlington, MA), analyzed using a Luminex® 200™, HTS, FLEXMAP 3D® following the manufacturer’s guidelines at the MDRC Chemistry Laboratory at the University of Michigan. The reported data reflect the average mean fluorescent intensity values obtained from the duplicate measurements. Values resulted as pg/ml. The cytokine levels were examined in all groups using GraphPad Prism (Prism 10.0, GraphPad Software, San Diego, CA). Within each sex, cytokines were analyzed using the Student T-test. In addition, given the potential concern of the sole use of p-values in studies involving small sample sizes66,67,68,69, effect size analyses were undertaken. Cohen’s d values ranging from 0.6 to 0.8 indicate a moderate effect size, and > 0.8 indicates a large effect size.
Data availability
Data sharing is applicable to this article and metabolomic data set generated in the current study will be available online at https://www.metabolomicsworkbench.org/.
References
Barker, D. J. The developmental origins of adult disease. J. Am. Coll. Nutr. 23, 588S-595S. https://doi.org/10.1080/07315724.2004.10719428 (2004).
Fowden, A. L. & Forhead, A. J. Endocrine mechanisms of intrauterine programming. Reproduction 127, 515–526. https://doi.org/10.1530/rep.1.00033 (2004).
Padmanabhan, V., Cardoso, R. C. & Puttabyatappa, M. Developmental programming, a pathway to disease. Endocrinology 157, 1328–1340. https://doi.org/10.1210/en.2016-1003 (2016).
Hanson, M. A. & Gluckman, P. D. Early developmental conditioning of later health and disease: Physiology or pathophysiology?. Physiol. Rev. 94, 1027–1076. https://doi.org/10.1152/physrev.00029.2013 (2014).
Mericq, V. et al. Long-term metabolic risk among children born premature or small for gestational age. Nat. Rev. Endocrinol. 13, 50–62. https://doi.org/10.1038/nrendo.2016.127 (2017).
Eriksson, J. G. et al. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 318, 427–431. https://doi.org/10.1136/bmj.318.7181.427 (1999).
Leunissen, R. W., Kerkhof, G. F., Stijnen, T. & Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 301, 2234–2242. https://doi.org/10.1001/jama.2009.761 (2009).
Favretto, D. et al. Cord blood metabolomic profiling in intrauterine growth restriction. Ana. Bioanal. Chem. 402, 1109–1121. https://doi.org/10.1007/s00216-011-5540-z (2012).
Dessi, A. et al. Metabolomics in newborns with intrauterine growth retardation (IUGR): Urine reveals markers of metabolic syndrome. J. Matern. Fetal Neonatal. Med. 24(2), 35–39. https://doi.org/10.3109/14767058.2011.605868 (2011).
Stallmach, T. et al. Cytokine production and visualized effects in the feto-maternal unit. Quantitative and topographic data on cytokines during intrauterine disease. Lab. Invest. 73, 384–392 (1995).
Pickup, J. C., Chusney, G. D., Thomas, S. M. & Burt, D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 67, 291–300. https://doi.org/10.1016/s0024-3205(00)00622-6 (2000).
Lourenco, B. H., Cardoso, M. A. & Team, A. S. C-reactive protein concentration predicts change in body mass index during childhood. PLoS ONE 9, e90357. https://doi.org/10.1371/journal.pone.0090357 (2014).
Krajewski, P. et al. Assessment of interleukin-6, interleukin-8 and interleukin-18 count in the serum of IUGR newborns. J. Matern. Fetal Neonatal. Med. 27, 1142–1145. https://doi.org/10.3109/14767058.2013.851186 (2014).
Perrin, E. M. et al. Elevations of inflammatory proteins in neonatal blood are associated with obesity and overweight among 2-year-old children born extremely premature. Pediatr. Res. 83, 1110–1119. https://doi.org/10.1038/pr.2017.313 (2018).
Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 12, 668–679. https://doi.org/10.1038/nrendo.2016.98 (2016).
Holmes, E. et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 453, 396–400. https://doi.org/10.1038/nature06882 (2008).
Isganaitis, E. et al. Associations of cord blood metabolites with early childhood obesity risk. Int. J. Obes. (Lond) 39, 1041–1048. https://doi.org/10.1038/ijo.2015.39 (2015).
Cardoso, R. C. & Padmanabhan, V. Prenatal Steroids and Metabolic Dysfunction: Lessons from Sheep. Annu. Rev. Anim. Biosci. 7, 337–360. https://doi.org/10.1146/annurev-animal-020518-115154 (2019).
Padmanabhan, V. & Veiga-Lopez, A. Sheep models of polycystic ovary syndrome phenotype. Mol. Cell Endocrinol. 373, 8–20. https://doi.org/10.1016/j.mce.2012.10.005 (2013).
Manikkam, M. et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 145, 790–798. https://doi.org/10.1210/en.2003-0478 (2004).
Steckler, T., Wang, J., Bartol, F. F., Roy, S. K. & Padmanabhan, V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 146, 3185–3193. https://doi.org/10.1210/en.2004-1444 (2005).
Padmanabhan, V., Manikkam, M., Recabarren, S. & Foster, D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol. Cell. Endocrinol. 246, 165–174. https://doi.org/10.1016/j.mce.2005.11.016 (2006).
Crespi, E. J., Steckler, T. L., Mohankumar, P. S. & Padmanabhan, V. Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep. J. Physiol. 572, 119–130. https://doi.org/10.1113/jphysiol.2005.103929 (2006).
Kjerulff, L. E., Sanchez-Ramos, L. & Duffy, D. Pregnancy outcomes in women with polycystic ovary syndrome: A metaanalysis. Am. J. Obstet. Gynecol. 204(558), e551-556. https://doi.org/10.1016/j.ajog.2011.03.021 (2011).
Hakim, C., Padmanabhan, V. & Vyas, A. K. Gestational hyperandrogenism in developmental programming. Endocrinology 158, 199–212. https://doi.org/10.1210/en.2016-1801 (2017).
Dokras, A., Spaczynski, R. Z., Behrman, H. R. & Duleba, A. J. Testosterone levels in pregnant women correlate with the insulin response during the glucose tolerance test. Fertil. Steril. 79, 492–497. https://doi.org/10.1016/s0015-0282(02)04764-7 (2003).
Ficicioglu, C. & Kutlu, T. The role of androgens in the aetiology and pathology of pre-eclampsia. J. Obstet. Gynaecol. 23, 134–137. https://doi.org/10.1080/0144361031000074637 (2003).
Acromite, M. T., Mantzoros, C. S., Leach, R. E., Hurwitz, J. & Dorey, L. G. Androgens in preeclampsia. Am. J. Obstet. Gynecol. 180, 60–63. https://doi.org/10.1016/s0002-9378(99)70150-x (1999).
Saadat, N., Ciarelli, J., Pallas, B., Padmanabhan, V. & Vyas, A. K. Sex-specific perturbation of systemic lipidomic profile in newborn lambs impacted by prenatal testosterone excess. Endocrinology https://doi.org/10.1210/endocr/bqad187 (2023).
Owens, J. A. et al. Sex-specific effects of placental restriction on components of the metabolic syndrome in young adult sheep. Am. J. Physiol. Endocrinol. Metab. 292, E1879-1889. https://doi.org/10.1152/ajpendo.00706.2006 (2007).
De Blasio, M. J., Gatford, K. L., Robinson, J. S. & Owens, J. A. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R875-886. https://doi.org/10.1152/ajpregu.00430.2006 (2007).
Gill, J. W. & Hosking, B. J. Acute prenatal androgen treatment increases birth weights and growth rates in lambs. J. Anim. Sci. 73, 2600–2608. https://doi.org/10.2527/1995.7392600x (1995).
Hosking, J. G. & a. B.,. Effects of prenatal steroid environment on fetal size and morphology in twin sheep. Austr. J. Agri. Res. 47, 1315–1322 (1996).
Klindt, J., Jenkins, T. G. & Ford, J. J. Prenatal androgen exposure and growth and secretion of growth hormone and prolactin in ewes postweaning. Proc. Soc. Exp. Biol. Med. 185, 201–205. https://doi.org/10.3181/00379727-185-42535 (1987).
Jenkins, T. G., Ford, J. J. & Klindt, J. Postweaning growth, feed efficiency and chemical composition of sheep as affected by prenatal and postnatal testosterone. J. Anim. Sci. 66, 1179–1185. https://doi.org/10.2527/jas1988.6651179x (1988).
Shahkhalili, Y., Moulin, J., Zbinden, I., Aprikian, O. & Mace, K. Comparison of two models of intrauterine growth restriction for early catch-up growth and later development of glucose intolerance and obesity in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R141-146. https://doi.org/10.1152/ajpregu.00128.2009 (2010).
Klindt, J. J. F. J. in Animal Growth Regulation Ch. 14, 317–336 (1989).
Carrasco, A. et al. Insulin sensitivity in male sheep born to ewes treated with testosterone during pregnancy. J. Dev. Orig. Health Dis. 12, 456–464. https://doi.org/10.1017/S2040174420000665 (2021).
Wankowska, M. Influence of testicular hormones on the somatostatin-GH system during the growth promoted transition to puberty in sheep. Theriogenology 77, 615–627. https://doi.org/10.1016/j.theriogenology.2011.08.038 (2012).
Tarttelin, M. F. Early prenatal treatment of ewes with testosterone completely masculinises external genitalia of female offspring but has no effects on early body weight changes. Acta Endocrinol. (Copenh) 113, 153–160. https://doi.org/10.1530/acta.0.1130153 (1986).
Riddle, E. S. et al. Intrauterine growth restriction increases TNF alpha and activates the unfolded protein response in male rat pups. J. Obes. 2014, 829862. https://doi.org/10.1155/2014/829862 (2014).
Visentin, S. et al. Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother-child couple. Biomed. Res. Int. 2014, 401595. https://doi.org/10.1155/2014/401595 (2014).
Alexandre-Gouabau, M. C. et al. Offspring metabolomic response to maternal protein restriction in a rat model of intrauterine growth restriction (IUGR). J. Proteome Res. 10, 3292–3302. https://doi.org/10.1021/pr2003193 (2011).
Lin, G. et al. Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J. Nutr. 142, 990–998. https://doi.org/10.3945/jn.111.153411 (2012).
Chassen, S. S. et al. Altered Cord Blood Lipid Concentrations Correlate with Birth Weight and Doppler Velocimetry of Fetal Vessels in Human Fetal Growth Restriction Pregnancies. Cells https://doi.org/10.3390/cells11193110 (2022).
Korkes, H. A. et al. Lipidomic assessment of plasma and placenta of women with early-onset preeclampsia. PLoS ONE 9, e110747. https://doi.org/10.1371/journal.pone.0110747 (2014).
Chen, Q., Francis, E., Hu, G. & Chen, L. Metabolomic profiling of women with gestational diabetes mellitus and their offspring: Review of metabolomics studies. J. Diabetes Compl. 32, 512–523. https://doi.org/10.1016/j.jdiacomp.2018.01.007 (2018).
Jaaskelainen, T. et al. A non-targeted LC-MS metabolic profiling of pregnancy: Longitudinal evidence from healthy and pre-eclamptic pregnancies. Metabolomics 17, 20. https://doi.org/10.1007/s11306-020-01752-5 (2021).
Chao de la Barca, J. M. et al. A Metabolomic Profiling of Intra-Uterine Growth Restriction in Placenta and Cord Blood Points to an Impairment of Lipid and Energetic Metabolism. Biomedicines https://doi.org/10.3390/biomedicines10061411 (2022).
Kabaran, S. & Besler, H. T. Do fatty acids affect fetal programming?. J Health Popul Nutr 33, 14. https://doi.org/10.1186/s41043-015-0018-9 (2015).
Kliegman, R. M. & Sparks, J. W. Perinatal galactose metabolism. J Pediatr 107, 831–841. https://doi.org/10.1016/s0022-3476(85)80173-6 (1985).
Schupper, A. et al. Metabolic biomarkers of small and large for gestational age newborns. Early Hum. Dev. 160, 105422. https://doi.org/10.1016/j.earlhumdev.2021.105422 (2021).
Handakas, E. et al. A systematic review of metabolomic studies of childhood obesity: State of the evidence for metabolic determinants and consequences. Obes. Rev. 23(1), 13384. https://doi.org/10.1111/obr.13384 (2022).
Polidori, N., Grasso, E. A., Chiarelli, F. & Giannini, C. Amino acid-related metabolic signature in obese children and adolescents. Nutrients https://doi.org/10.3390/nu14071454 (2022).
Duan, Y. et al. Nutritional and regulatory roles of leucine in muscle growth and fat reduction. Front. Biosci. (Landmark Ed) 20, 796–813. https://doi.org/10.2741/4338 (2015).
Padmanabhan, V. Polycystic ovary syndrome–"A riddle wrapped in a mystery inside an enigma". J. Clin. Endocrinol. Metab. 94, 1883–1885. https://doi.org/10.1210/jc.2009-0492 (2009).
Sir-Petermann, T. et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum. Reprod. 20, 2122–2126. https://doi.org/10.1093/humrep/dei009 (2005).
Wilde, M. A. et al. Cardiovascular and metabolic health of 74 children from women previously diagnosed with polycystic ovary syndrome in comparison with a population-based reference cohort. Reprod. Sci. 25, 1492–1500. https://doi.org/10.1177/1933719117749761 (2018).
Hart, R. Generational health impact of PCOS on women and their children. Med Sci (Basel) https://doi.org/10.3390/medsci7030049 (2019).
Saadat, N., Pallas, B., Ciarelli, J., Vyas, A. K. & Padmanabhan, V. Gestational testosterone excess early to mid-pregnancy disrupts maternal lipid homeostasis and activates biosynthesis of phosphoinositides and phosphatidylethanolamines in sheep. Sci Rep 14, 6230. https://doi.org/10.1038/s41598-024-56886-6 (2024).
Halloran, K. M. et al. Developmental programming: Testosterone excess masculinizes female pancreatic transcriptome and function in sheep. Mol. Cell Endocrinol. 588, 112234. https://doi.org/10.1016/j.mce.2024.112234 (2024).
Thangaraj, S. V. et al. Comparative lipidome study of maternal plasma, milk, and lamb plasma in sheep. Sci. Rep. 14, 7401. https://doi.org/10.1038/s41598-024-58116-5 (2024).
Association), A. A. V. M. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. (Schaumburg, Illinois, 2020).
Pang, Z. et al. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 52, 398–406. https://doi.org/10.1093/nar/gkae253 (2024).
Adams, K. J. et al. Skyline for small molecules: A unifying software package for quantitative metabolomics. J. Proteome. Res. 19, 1447–1458. https://doi.org/10.1021/acs.jproteome.9b00640 (2020).
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Nakagawa, S. & Cuthill, I. C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 82, 591–605. https://doi.org/10.1111/j.1469-185X.2007.00027.x (2007).
Sullivan, G. M. & Feinn, R. Using effect size-or why the P value is not enough. J. Grad. Med. Educ. 4, 279–282. https://doi.org/10.4300/JGME-D-12-00156.1 (2012).
Amrhein, V., Greenland, S. & McShane, B. Scientists rise up against statistical significance. Nature 567, 305–307. https://doi.org/10.1038/d41586-019-00857-9 (2019).
Acknowledgements
Research reported in this publication was supported by National Institutes of Health (NIH) awards R01 HL139639 and R21 HD095501
Funding
National Heart, Lung, and Blood Institute, HL139639, HL139639, National Institute of Child Health and Human Development, R21 HD095501.
Author information
Authors and Affiliations
Contributions
E.T: formal analysis, writing original draft, J.C, S.D., B.P., A.G.: responsible for all animal experimentation aspects of study undertaken at the university of Michigan sheep research facility, M.A., C.G.: assisted with statistical analysis of growth data, K.C. , LP.S. and GJ. P.: Metabolomic core team that assisted with processing and analyzing fetal and prepubertal metabolomic data, V.P.: supervised the animal experimentation aspects of study carried out at University of Michigan sheep research Facility, participated in writing original draft, edited and revised manuscript, AK.V.: funding acquisition, supervised animal experimentation aspects of the study, conceived and designed the study, supervision of analytical aspects and statistical and bioinformatic analyses of data, writing original draft, edited and revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Topaktas, E., Ciarelli, J., Domke, S. et al. Sexually dimorphic impact of prenatal hyperandrogenism on offspring growth trajectory in sheep. Sci Rep 15, 20394 (2025). https://doi.org/10.1038/s41598-025-04892-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04892-7