Abstract
Dimethyl disulfide (DMDS) is an important volatile organic sulfur compound with diverse applications and substantial demand in the chemical, agricultural, and food industries. The challenges associated with their chemical synthesis have spurred exploration of biological synthesis as an alternative route. Nevertheless, there is limited research on the biosynthesis of DMDS, and the yield remains low. In this study, strain W2-3, exhibiting high DMDS production, was isolated from aged tobacco leaves collected in Hubei Province, China. Based on morphological characteristics, 16S rRNA gene sequencing and phylogenetic analysis, the strain was identified as Alcaligenes faecalis. Through single-factor experimental methods, an initial pH of 7 and temperature ranging from 19.5 to 30 °C were demonstrated to be the optimal fermentation conditions for DMDS production. Under these optimized conditions, the maximum yield of DMDS reached 213.49 mg/L, which was a 1.34-fold increase from the pre-optimization yield of 159.86 mg/L. Moreover, optimization of sulfur sources and concentrations showed that increasing methionine concentration led to higher DMDS production. Under sufficient Met conditions, W2-3 achieved a peak of 2440.71 mg/L DMDS, representing an increase of 11.43-fold compared to the control group without methionine supplementation. This yield significantly surpassed the highest yield reported in the literature (40.06 mg/L) by 60.93-fold, establishing a new record for the highest yield currently known. This study identifies A. faecalis W2-3 as a promising microbial chassis for the biosynthesis of DMDS, characterized by a high yield and strong temperature adaptability. It offers insights into the metabolic pathways and fermentation conditions for industrial-scale DMDS production. As the first systematic optimization research aimed at enhancing DMDS production, it fills a gap in the literature. Our findings pave the way for sustainable and efficient DMDS biosynthesis, with significant implications for promoting its application in agriculture, industry, and environmental management.
Similar content being viewed by others
Introduction
The continuous cultivation of cash crops has exacerbated soil-borne disease, leading to the widespread use of fumigants such as methyl bromide (MB) and dazomet (DZ) for effective control of weeds, fungi, and pests. However, studies have revealed that the application of MB fumigants has negative impacts on the stratospheric ozone layer, the environment and human health, resulting in their prohibition1,2,3. DZ fumigants can alter the predominant soil bacterial community, causing a notable reduction in microbial diversity and promoting the growth of drug-resistant pathogens, which can disrupt soil function and health4. Given the limitations and environmental concerns associated with MB and DZ, there is an urgent need for safer and more sustainable alternatives. Dimethyl disulfide (DMDS) is a highly effective broad-spectrum fumigant that exhibits significant inhibitory effects against various nematode species, such as Meloidogyne spp., soilborne fungi including Fusarium spp. and Phytophthora spp., and weed seeds like Abutilon theophrasti and Digitaria sanguinalis5,6. Moreover, studies indicate that the application of DMDS for soil sterilization enhances the activity of functional microorganisms involved in nitrogen cycling, thereby promoting nitrogen transformation7. DMDS fumigants have the advantages of low harm and high efficiency8, making them the most promising alternatives to MB and DZ fumigants, and potentially addressing the shortage of environmentally friendly and cost-effective fumigants7. Beyond agricultural applications, DMDS is also used in the chemical and food industries. In the chemical industry, it can be used in the petroleum and rubber sectors, serving as a solvent, regenerating agent, softener, and plasticizer9. In the food industry, DMDS can be added as a flavor enhancer to impart a unique taste and aroma to various food, such as cooked cabbage10, cooked meat11, and cheese12. Furthermore, it is also an important sulfur source for enhancing coffee aroma in baking13. Additionally, DMDS has potential anti-inflammatory and vasodilatory properties; for instance, DMDS in onions can inhibit cellular inflammatory responses induced by lipopolysaccharides, promoting vasodilation and reducing hypertension14.
Currently, the production of DMDS primarily relies on chemical synthesis methods, such as methanethiol oxidation, halogenated hydrocarbons, and dimethyl sulfate9. However, these traditional chemical synthesis approaches often come with drawbacks like harsh reaction conditions, low product purity, difficulty in purification, elevated cost, generation of toxic by-products, and environmental pollution. These limitations hinder their industrial application. Consequently, the top priority is to identify more sustainable and efficient production methods. Microbial processes typically involve mild reaction conditions, low production costs, eco-friendly operations with no toxic by-products15,16,17, and hold potential for diverse applications spanning industries, healthcare, and environmental protection18. To conserve resources and enhance the efficiency of product synthesis, microbial fermentation has emerged as a viable alternative for DMDS production19,20.
There are few studies using microorganisms to synthesize DMDS. The strains capable of producing DMDS mainly include Tuber melanosporum, Bacillus, Massilia putida, Serratia marcescens, and Burkholderia, among them, Burkholderia has received more extensive research attention21,22,23,24. These studies primarily examine the mechanisms by which DMDS-producing microorganisms regulate plant growth and enhance disease resistance, while research on the specific metabolic pathways involved in the microbial synthesis of DMDS and its yield regulation remains relatively limited25,26,27. Previous studies have demonstrated that these microorganisms primarily synthesize DMDS through metabolic pathways involving sulfur-containing amino acids, such as methionine. The biosynthetic process mainly consists of demethylation and sulfur transfer reactions related to methionine, with key enzymes including methionine γ-lyase and sulfur transferases28. However, the complete metabolic pathway for DMDS synthesis has not yet been fully characterized, and the functional validation of key enzymes and their encoding genes remains inadequate, significantly limiting the application of metabolic engineering for the efficient synthesis of DMDS. Additionally, resources for efficient DMDS-producing strains are relatively scarce, and there is a lack of systematic screening methods, leading to slow progress in strain development. Furthermore, the known natural strains typically yield low amounts of DMDS and are highly susceptible to environmental factors, making it challenging to meet the demands of large-scale industrial production.
Microbial synthesis represents a more environmentally friendly and potentially more effective method for producing DMDS29. Since DMDS yield is crucial for its industrial applications30, this study aims to select efficient strains for the synthesis of DMDS, elucidate the underlying metabolic pathways involved in DMDS biosynthesis, and enhance DMDS production through the optimization of fermentation conditions, with the ultimate goal of developing a cost-effective and scalable DMDS bioproduction platform.
Materials and methods
Chemicals and media
Methionine and cysteine were purchased from Shanghai Macklin Biochemical Co., Ltd., while other common chemicals were purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd. or Oxoid Corporation. Primer and bacterial genomic DNA extraction kits were purchased from Beijing Qingke Biological Technology Co., Ltd. Selective medium (per liter): 13.3 g K2HPO4·3H2O, 4 g KH2PO4, 0.2 g MgSO4·7H2O, 1 g nicotine, 1 g Met, 0.5 mL microelements. Microelements: 0.05 g CaCl2·2H2O, 0.05 g CuCl2·2H2O, 0.008 g MnSO4·H2O, 0.004 g FeSO4·7H2O, 0.1 g ZnSO4·H2O, 0.1 g Na2MoO4·2H2O, 0.05 g Na2WO4·2H2O21,31. For the selective solid medium, 1.5% agar was added. Fermentation Luria–Bertani (LB) medium (per liter): 10 g peptone, 10 g NaCl, 5 g yeast extract. High-pressure sterilization was performed at 115 °C for 30 min. Met or Cys were added according to the indicated concentrations.
Screening strains that produce DMDS
Five grams of tobacco leaf samples were pulverized and placed in 50 mL of selective liquid medium, shaken at 37 °C and 200 rpm for 40 min, then let it stand for 10 min. The supernatant from the upper layer was spread after gradient dilution onto the solid selective medium plates, and incubated at 37 °C for 1–2 days. Following the growth of individual colonies, those showing distinct differences in morphology, color, and size were selected from the plates, and streaked for purification on LB agar plates. Subsequently, the selected strains were activated and inoculated in LB medium, and then incubated at 37 °C and 200 rpm for 10–12 h to obtain the seed liquid. The seed liquid was then inoculated into 50 mL of fermentation medium, ensuring an initial OD600 of 0.05. The same volume of sterile water was inoculated into the fermentation medium as a blank control. Each saline vial was sealed with a rubber stopper. After 10 h of cultivation, 1 mL of headspace gas was sampled for DMDS determination by gas chromatography-mass spectrometry (GC–MS). The strains identified as capable of producing DMDS were utilized as the experimental strains in subsequent experiments.
Identification strain
The genome of strain W2-3 was extracted using a bacterial genomic DNA extraction kit , and sent to Beijing Tsingke Biotechnology Co., Ltd, for 16 S rRNA sequencing using Sanger sequencing technology and identification. Amplified with 16S rRNA universal primers (forward primer 27F: 5'-AGAGTTTGATCCTGGCTCAG-3', reverse primer 5’-ACGGCTACCTTGTTACGACTT-3'). The PCR amplification program comprised an initial denaturation at 95 °C for 5 min, followed by denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, extension at 72 °C for 90 s for a total of 30 cycles, and a final extension at 72 °C for 5 min. Beijing Tsingke Biotechnology Co., Ltd conducted the PCR product splicing, testing and sequencing. Comparison for similarity was carried out in NCBI GenBank, utilizing the most similar (96–100%) known reference strain as a benchmark. MEGA 6.0 software was employed to construct a phylogenetic tree and identify the species information of the strain. Cell morphology was examined using a scanning electron microscope under cryo-field emission32. For detailed experimental methods, please refer to the Supplementary Information.
Detection method for DMDS
For quantitative analysis of DMDS production in bacterial strains, an in situ extraction method was employed to mitigate the volatilization of DMDS due to the gases’ high diffusion and instability. Common extractants, including n-hexane, cyclohexane, n-heptane, n-decane, n-dodecane, and dichloromethane were carefully selected and optimized33. Upon reaching an OD600 value of 0.2–0.3 in the fermentation broth, 10 mL of each organic solvent extractant was added separately to the fermentation medium, and the culture was continued for 48 h. Every 24 h, the upper organic layer was sampled and centrifuged at 14,000 rpm for 20 min, and 1 μL of the upper layer was subjected to gas chromatography analysis. Simultaneously, a control group (without extractant addition) was established to compare the growth status of the strains. Chromatographic conditions: HP-INNOWAX column (30 m × 0.25 mm × 0.25 μm); helium carrier gas; flow rate of 1.0 mL/min; injection port temperature set at 250 °C, and a temperature program starting at 50 °C, holding for 3 min, with a gradual increase of 10 °C/min up to 240 °C, followed by a 2 min hold, and further increased at a rate of 20 °C/min up to 250 °C with a 5 min hold. For mass spectrometry analysis, the conditions include an EI ion source with an ionization energy of 70 eV, an ion source temperature of 230 °C, quadrupole temperature at 150 °C, full scan mode, and a mass scan range of 30 to 400 amu. The mass spectrometry library is NIST 08.
Exploration of the optimal fermentation conditions for strain W2-3
The seed liquid was inoculated into the fermentation medium with various initial pH values ranging from 3 to 11, while ensuring an initial OD600 of 0.05. Once the OD600 of the fermentation broth reached 0.2 to 0.3, 10 mL of dodecane was added. Measurements of strain growth and DMDS production were taken at 24 and 48 h, respectively. Temperature optimization trials (12.5–40.5 °C) were carried out within the optimal pH range. Furthermore, optimization of sulfur source types (Met and Cys) and their concentrations was performed under the ideal temperature and pH conditions, with amino acid levels varying from 0 to 30 g/L (Met) and 0–20 g/L (Cys), respectively. All fermentation experiments were conducted in triplicate, and the data were presented as the mean ± standard deviation. The conversion rate of DMDS in the fermentation broth was calculated using Eq. (1) to evaluate the effect of adding amino acids on DMDS production.
Data analysis
The results were analyzed and column charts were made by GraphPad Prism and SAS. A one-way analysis of variance (ANOVA) was conducted to assess the significance of differences among the various experimental groups. All results were expressed as mean ± standard deviation (SD). Mean values with different lowercase letters indicate significant differences at p < 0.05, while those with the same lowercase letters indicate no significant difference at p > 0.05.
Results
Isolation of DMDS-producing strains and extractant optimization
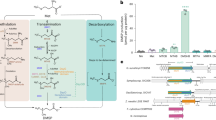
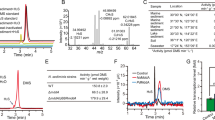
Using selective medium, over 100 strains were isolated from the aged tobacco leaves. Among these strains, three exhibited an aroma resembling the DMDS standard during cultivation, which was characterized by a sulfur gas odor. These strains were designated W2-1, W2-3, and W2-4. Subsequently, the three selected strains were fermented in salt bottles, and the headspace gas compositions were analyzed using GC–MS to determine the production and quantity of DMDS generated. Notably, strains W2-1 and W2-4 were found to contain 12 different compounds each, whereas W2-3 displayed a simpler product profile, with CO2 and DMDS as the main products. Comparative analysis revealed that W2-3 demonstrated the highest capacity for DMDS production and yielded the greatest amount of product (Fig. 1). As a result, W2-3 was chosen for further investigations.
To enhance the efficiency of product extraction and ensure precise quantification, an in situ recovery method was employed, using various organic solvents for the optimization of extractants. As shown in Table 1, in comparison to the control group, n-hexane, cyclohexane, and dichloromethane exhibited significant inhibitory effects on the growth of W2-3, indicating potential toxicity towards this bacterial strain. Although n-heptane showed a weaker inhibitory effect on W2-3, its extraction efficacy was subtle, resulting in a flat peak in detection. Both n-decane and n-dodecane showed minimal impact on the growth of W2-3. However, n-decane was deemed unsuitable as an extraction agent because its peak time overlapped with that of DMDS. In contrast, n-dodecane demonstrated superior extraction efficiency, with a peak time that did not conflict with that of DMDS. Therefore, considering the impact on the strain’s growth and the peak timings of the extraction agents, n-dodecane was chosen as the extractant for subsequent experiments.
Identification of strain W2-3
The morphological characteristics of strain W2-3 are presented (Fig. 2). When cultivated on LB agar plates for 24 h, W2-3 formed light yellow colonies. With extended cultivation to 48 h, the colony color progressively darkened. The individual colonies exhibit a round shape, semi-transparency, an irregular periphery, and a slight protrusion at the center (Fig. 2a). Scanning electron microscopy revealed rod-shaped cells with blunt rounded ends, measuring approximately 0.6–1.8 μm in length and 0.1–0.3 μm in width (Fig. 2b).
The 16S rRNA sequence database and genetic algorithm are now prominent tools for the classification and identification of bacteria32. Comparative analysis of the 16S rRNA sequence of strain W2-3 against the GenBank database revealed over 99% similarity with multiple Alcaligenes faecalis strains. The phylogenetic tree, constructed using the Neighbor-Joining method (Fig. 3) for homology comparison, shows that strain W2-3 is closely related to Alcaligenes faecalis strain NBRC 13111. Following routine bacterial culture tests and biochemical identification, and phylogenetic analysis, strain W2-3 was definitively classified as A. faecalis.
Exploration of optimal fermentation conditions for A. faecalis W2-3
To enhance the production of DMDS more efficiently, we investigated the optimal fermentation conditions for the DMDS-producing strain A. faecalis W2-3. The initial pH optimization of the culture medium was carried out first, and the optimization of pH results are presented (Fig. 4a,b). Strain W2-3 exhibited no growth in fermentation media with initial pH values of 3, 4, 5, 10, and 11, and did not produce DMDS. At an initial pH of 7, the DMDS titers at 24 h and 48 h were significantly higher compared to other pH conditions, reaching the highest values of 154.53 mg/L at 24 h and 159.86 mg/L at 48 h. Initial pH of 8 and 9 showed lower DMDS production, with no significant difference between them. Despite similar growth of strains at initial pH of 7 and 8, there was a significant difference in DMDS titers, necessitating further investigation. Much lower DMDS production was observed at an initial pH of 6. Consequently, the following optimizations were conducted at an initial pH of 7.
DMDS production and growth of A. faecalis W2-3 under different fermentation conditions. (a) DMDS production under different pH conditions. (b) The growth of W2-3 under different pH conditions. (c) DMDS production under different temperature conditions. (d) The growth of W2-3 under different temperature conditions. e DMDS production with different concentrations of methionine. (f) The growth of strains under different concentrations of methionine. g DMDS production with different concentrations of cysteine. (h) The growth of W2-3 with different concentrations of cysteine. Error bars represent the SD. Mean values with different lowercase letters are significantly different at p < 0.05, and those with the same lowercase letters are not significantly different at p > 0.05.
Temperature is the most critical environmental variable influencing the composition of functional genes in microorganisms34, and plays a significant role in microbial metabolism35. We examined DMDS production at varying temperatures and identified the optimal temperature for DMDS production. The results of temperature optimization are depicted (Fig. 4c,d). Strain W2-3 demonstrated rapid and comparable growth across temperatures ranging from 19.5 to 40.5 °C, indicating strong temperature adaptability. Slower growth was observed at 12.5 °C and 16 °C. After 24 h of growth, the highest production of DMDS was observed at temperatures of 23 °C and 26.5 °C, with no significant differences noted. Similarly, after 48 h of growth, the highest DMDS production was recorded between 19.5 and 30 °C, with no significant variations. The highest production was 193.39 mg/L at 24 h, and 213.49 mg/L at 48 h under the 23 °C condition. The production of DMDS by W2-3 did not strictly correlate with its growth, as the optimal growth temperature range (19.5–40.5 °C) did not align with the high DMDS production temperature range (19.5–30 °C). Strain grown at 40.5 °C exhibited a cell density comparable to that grown at the high DMDS production temperature range. However, its DMDS production was significantly lower. It is preliminarily speculated that this discrepancy may be related to decreased enzyme activity for DMDS synthesis at this temperature. Notably, at temperatures between 19.5 and 30 °C after 48 h, the differences in DMDS production were negligible, indicating the strain’s robust temperature adaptability for DMDS fermentation. Therefore, the industrial fermentation of DMDS using strain W2-3 does not require a specific temperature, rather maintaining a range of 19.5 °C to 30 °C can efficiently synthesize DMDS, conserving energy and reducing costs. Given the highest DMDS production at 23 °C, sulfur source optimization was conducted at this cultivation temperature.
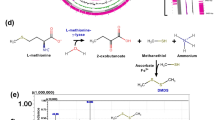
Exploration of metabolic pathways revealed that Met is a vital substrate for DMDS synthesis in most microorganisms, while Cys can also be degraded to produce DMDS. Regarding experimental costs and amino acid solubility, the maximum concentrations were set at 30 g/L for Met and 20 g/L for Cys. Strain growth was unaffected by Met concentration, with growth tendency remaining similar across all Met concentration levels (Fig. 4e,f). However, DMDS production was significantly influenced. DMDS production increased along with rising Met concentrations, demonstrating a positive correlation. Notably, at a Met concentration of 30 g/L, DMDS production reached 1892.16 mg/L at 24 h and 2440.71 mg/L at 48 h, representing the highest titer reported to date. In contrast, elevated Cys concentrations (5–20 g/L) inhibited both strain growth and DMDS production (Fig. 4g,h). DMDS titers and cell density decreased in conjunction with the increasing concentrations of Cys, with no DMDS detected at 15 g/L and 20 g/L, indicating a negative correlation.
The conversion rate of DMDS produced by W2-3 at varying Met concentrations was calculated using formula (1), with the results presented in Table 2. Although DMDS production increased with higher Met concentrations, the conversion rate diminished at elevated Met levels. The optimal conversion rate of 32.49% was achieved at a Met concentration of 5 g/L after 48 h of fermentation. Balancing production yield and conversion rate should be a focal point for future research.
Discussion
Current research on DMDS predominantly centers on its functional characteristics and applications (Table 3). In contrast, our study represents the first comprehensive report on the microbial biosynthesis and production enhancement of DMDS. The Alcaligenes species is reported for the first time to be capable DMDS. This finding enriches the available strain resources. Subsequently, fermentation conditions were systematically optimized, which included optimizing extractants, adjusting the initial pH of the culture medium, and determining the fermentation temperature and sulfur sources. As a result, DMDS production gradually increased. These efforts filled an existing data gap in microbial-mediated DMDS yield enhancement. Eventually, A. faecalis W2-3 achieved a high DMDS production of 2440.71 mg/L, which is 60.93-fold higher than the previously reported maximum21, highlighting the substantial potential of W2-3 as an industrial production strain. Notably, the W2-3 strain attains high-level DMDS production without genetic modification, positioning it as an ideal platform for green biomanufacturing. This discovery not only elevates the industrial application value of A. faecalis but also provides essential technical support for developing novel agricultural biopesticides and environmentally friendly fragrance production processes. As such, it demonstrates significant scientific and applied potential within the realm of green biomanufacturing.
The pH and temperature of the growth environment significantly influence the physiological and biochemical processes of the microbial cells, thereby affecting the productivity of the desired product. The study found that an initial pH of approximately 7.0 was optimal for DMDS production in A. faecalis W2-3 (Fig. 4). DMDS production was observed across a pH range of 6.0 to 9.0. Both excessively high and low pH levels inhibit cell growth and seriously affect DMDS synthesis. In contrast, W2-3 exhibited a broad temperature range for DMDS production (16–40.5 °C), with the optimal range between 19.5 and 30 °C, indicating its strong temperature adaptability. These characteristics are consistent with previously reported optimal pH and temperature ranges for A. faecalis growth and the production of certain metabolites41,42,43. The underlying mechanism may involve complex regulatory processes in microbial metabolism, including the effects of pH and temperature on cell membrane permeability, which regulate substrate uptake efficiency, as well as the modulation of the quantity, conformation, and activity of key enzymes, such as methionine γ-lyase (MGL)44. Notably, this strain’s strong temperature adaptability (16–40.5 °C) exceeds that of conventional industrial bacterial strains, such as Escherichia coli and Corynebacterium glutamicum, which typically have an optimal temperature range of 25–35 °C for compound production. This broad temperature adaptability endows A. faecalis W2-3 a unique advantage, potentially reducing energy consumption for temperature control during fermentation and indicating significant potential for industrial application45.
DMDS is produced via the degradation of Met or Cys12. The Met-derived DMDS metabolic pathways are relatively well understood, yet some regulatory mechanisms remain unclear. Conversely, the Cys-derived pathway requires further investigation. Microbial synthesis of DMDS through Met degradation mainly involves three main pathways (Fig. 5). The first pathway begins with the conversion of Met to methanethiol (MTL), along with the generation of equimolar amounts of α-ketobutyrate and ammonia (Fig. 5a)46. This reaction is catalyzed by MGL28 or C-S lyase (cysteine β- or γ-lyase, CBL or CGL)47, and the enzymes involved and the amount of MTL produced vary among different microorganisms28. MTL is then further auto-oxidized to DMDS. The second pathway comprises two catalytic reactions initiated by the conversion of Met to 4-methylthio-2-oxobutyric acid (KMBA) by aminotransferase. Subsequently, KMBA is converted to MTL catalyzed by C-S lyases (Fig. 5b). The amount of MTL generated by this second route is limited47. The third pathway branches from the second, converting KMBA to 3-Methylthiopropanal (MSDS) through a spontaneous degradation or decarboxylation reaction (Fig. 5c). MSDS is then transformed into MTL via an unknown mechanism. Currently, there are no comprehensive reports detailing the enzymes involved in the metabolic pathways of DMDS biosynthesis in A. faecalis. The yield of DMDS increased significantly with the addition of exogenous Met, indicating that Met serves as a key precursor for the biosynthesis of DMDS in A. faecalis W2-3. Given the high yield of DMDS produced in W2-3, it is possible that one or multiple pathways may coexist simultaneously48. While some microorganisms can synthesize DMDS through the breakdown of the C-S bond in Cys49, the supplementation of exogenous Cys in this study did not elevate DMDS production; rather, it resulted in a decrease. This suggests a potential absence of a Cys-derived DMDS metabolic pathway in strain W2-3 or an inhibitory effect of Cys on Met degradation. Alternatively, the elevated concentration of Cys may affect the enzymes MGL or C-S lyases directly48, leading to reduced DMDS production. These findings further emphasize the uniqueness of this strain in efficiently synthesizing DMDS via the methionine pathway and offer valuable insights for more in-depth studies of its metabolic network.
Microbial genome sequencing is essential for uncovering significant functional modules and metabolic pathways50,51,52,53, and is beneficial for promoting the high-level production of novel natural compounds54. Future investigations may delve deeper into the genomics of A. faecalis W2-3, elucidate the metabolic pathway of DMDS, and employ genetic engineering to enhance DMDS production more effectively at the molecular level. For instance, the enzyme STR3, which catalyzes the direct conversion of Met and its transaminase product KMBA into MTL, has been shown to improve DMDS production through overexpression55. This approach could also be applied to A. faecalis W2-3 to further increase DMDS yields. Additionally, strategies can be developed to enhance the conversion rate of the precursor while simultaneously reducing production costs, thereby facilitating industrial production processes.
Conclusion
In this study, a high-yield strain producing DMDS, A. faecalis W2-3, was isolated from aged tobacco leaves. Its optimal growth conditions were explored, and its robust temperature adaptability was revealed. The optimal pH for DMDS production by W2-3 is 7, with the most suitable temperature range being 19.5 to 30 °C. The strain exhibited the capability to produce elevated levels of DMDS across a wide temperature range, with the titers increasing in correlation with methionine concentrations, suggesting its promising potential as an industrial chassis. Under sufficient Met conditions, W2-3 produced 2440.71 mg/L of DMDS, representing a 60.93-fold increase compared to findings from previous studies. A. faecalis W2-3 could serve as a valuable model microorganism for DMDS production. Future research focusing on the metabolic and genetic mechanisms underlying high production in the DMDS strain has the potential to significantly enhance the microbial synthesis capacity of DMDS. Additionally, it is anticipated that higher productions may be attained through fermentation processes in bioreactors.
Data availability
The datasets generated during the current study are available in the NCBI repository, the relevant accession number for this strain is PQ671090 [https://www.ncbi.nlm.nih.gov/search/all/?term=PQ671090].
Abbreviations
- DMDS:
-
Dimethyl disulfide
- MB:
-
Methyl bromide
- DZ:
-
Dazomet
- ISR:
-
Induced systemic resistance
- MGL:
-
Methionine γ-lyase
- CBL:
-
Cysteine β-lyase
- CGL:
-
Cysteine γ-lyase
- Met:
-
Methionine
- MTL:
-
Methanethiol
- KMBA:
-
4-Methylthio-2-oxobutyric acid
- MSDS:
-
3-Methylthiopropanal
- Cys:
-
Cysteine
- LB:
-
Luria–Bertani
- GC-MS:
-
Gas chromatography–mass spectrometry
References
Xue, J. et al. Dimethyl disulfide (DMDS) as an effective soil fumigant against nematodes in China. PLoS ONE 14, e0224456. https://doi.org/10.1371/journal.pone.0224456 (2019).
Gullino, M. L., Clini, C., Garibaldi, A. & Garibaldi, A. Life without methyl bromide: The Italian experience in replacing the fumigant. Commun. Agric. Appl. Biol. Sci. 70, 13–25 (2005).
Xie, H. et al. Evaluation of methyl bromide alternatives efficacy against soil-borne pathogens, nematodes and soil microbial community. PLoS ONE 10, e0117980 (2015).
Chen, R. et al. An emerging chemical fumigant: Two-sided effects of dazomet on soil microbial environment and plant response. Environ. Sci. Pollut. Res. 29, 3022–3036. https://doi.org/10.1007/s11356-021-15401-4 (2021).
Wang, F. Y. et al. Effects of dimethyl disulfide on microbial communities in protectorate soils under continuous cropping. Chin. J. Eco-Agric. 19, 890–896. https://doi.org/10.3724/SP.J.1011.2011.00890 (2011).
Mao, L. et al. Evaluation of the combination of dimethyl disulfide and dazomet as an efficient methyl bromide alternative for cucumber production in China. J. Agric. Food Chem. 62, 4864–4869. https://doi.org/10.1021/jf501255w (2014).
Wang, Q. X. et al. Advances in the studies on new soil fumigant dimethyl disulfide. J. Plant Protect. 50, 32–39. https://doi.org/10.13802/j.cnki.zwbhxb.2023.2021045 (2023).
Wang, D. et al. Production of methyl sulfide and dimethyl disulfide from soil-incorporated plant materials and implications for controlling soilborne pathogens. Plant Soil 324, 185–197. https://doi.org/10.1007/s11104-009-9943-y (2009).
Cheng, Z. L. et al. Application of activated carbon supported cobalt(II) tetraaminophthalocyanine towards preparation of dimethyl disulfide. J. Porphyrins Phthalocyanines 23, 267–278. https://doi.org/10.1142/S108842461950010X (2019).
Song, X. et al. Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics. LWT 146, 111427. https://doi.org/10.1016/j.lwt.2021.111427 (2021).
Li, L., Belloch, C. & Flores, M. A comparative study of savory and toasted aromas in dry cured loins versus dry fermented sausages. LWT 173, 114305. https://doi.org/10.1016/j.lwt.2022.114305 (2023).
Hanniffy, S. B. et al. Heterologous production of methionine-γ-lyase from Brevibacterium linens in Lactococcus lactis and formation of volatile sulfur compounds. Appl. Environ. Microbiol. 75, 2326–2332. https://doi.org/10.1128/aem.02417-08 (2009).
Uekane, T. M., Costa, A. C. P., Pierucci, A. P. T. R., da Rocha-Leão, M. H. M. & Rezende, C. M. Sulfur aroma compounds in gum Arabic/maltodextrin microparticles. LWT 70, 342–348. https://doi.org/10.1016/j.lwt.2016.03.003 (2016).
Chu, C. C. et al. The anti-inflammatory and vasodilating effects of three selected dietary organic sulfur compounds from allium species. J. Funct. Biomater. 8, 5. https://doi.org/10.3390/jfb8010005 (2017).
Ajikumar, P. K. et al. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 5, 167–190. https://doi.org/10.1021/mp700151b (2008).
Zhang, G. et al. Production of alkaline pectinase: A case study investigating the use of tobacco stalk with the newly isolated strain Bacillus tequilensis CAS-MEI-2-33. BMC Biotechnol. 19, 45. https://doi.org/10.1186/s12896-019-0526-6 (2019).
Wang, Q. et al. Biosynthesis of 2-phenylethanol using tobacco waste as feedstock. Biocatal. Biotransform. 31, 292–298 (2013).
Han, J. et al. Microbial-derived gamma-aminobutyric acid: Synthesis, purification, physiological function, and applications. J. Agric. Food Chem. 71, 14931–14946. https://doi.org/10.1021/acs.jafc.3c05269 (2023).
Ye, Z. et al. Revolution of vitamin E production by starting from microbial fermented farnesene to isophytol. Innovation 3, 100228 (2022).
Wang, F. et al. Tobacco as a promising crop for low-carbon biorefinery. Innovation 5, 100687 (2024).
Liu, R. S. et al. Metabolism of L-methionine linked to the biosynthesis of volatile organic sulfur-containing compounds during the submerged fermentation of Tuber melanosporum. Appl. Microbiol. Biotechnol. 97, 9981–9992. https://doi.org/10.1007/s00253-013-5224-z (2013).
Gong, A. D. et al. Inhibitory effect of dimethyl disulfide from Burkholderia pyrrocinia WY6-5 on Aspergillus flavus and aflatoxins in peanuts during storage period. Scientia Agricultura Simica 52, 2972–2982. https://doi.org/10.3864/j.issn.0578-1752.2019.17.006 (2019).
Liu, A. et al. Volatile organic compounds of endophytic Burkholderia pyrrocinia strain JK-SH007 promote disease resistance in poplar. Plant Dis. 104, 1610–1620. https://doi.org/10.1094/Pdis-11-19-2366-Re (2020).
Lin, Y. T. et al. Fungicidal activity of volatile organic compounds emitted by Burkholderia gladioli strain BBB-01. Molecules https://doi.org/10.3390/molecules26030745 (2021).
Huang, C. J. et al. Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag. Sci. 68, 1306–1310. https://doi.org/10.1002/ps.3301 (2012).
Meldau, D. G. et al. Dimethyl disulfide produced by the naturally associated Bacterium bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 25, 2731–2747. https://doi.org/10.1105/tpc.113.114744 (2013).
Meldau, D. G. et al. Dimethyl disulfide produced by the naturally associated Bacterium bacillus sp. B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 25, 2731–2747. https://doi.org/10.1105/tpc.113.114744 (2013).
Ferchichi, M., Hemme, D., Nardi, M. & Pamboukdjian, N. Production of methanethiol from methionine by Brevibacterium linens CNRZ 918. J. Gen. Microbiol. 131, 715–723. https://doi.org/10.1099/00221287-131-4-715 (1985).
Immethun, C. M., Hoynes-O’Connor, A. G., Balassy, A. & Moon, T. S. Microbial production of isoprenoids enabled by synthetic biology. Front. Microbiol. 4, 75. https://doi.org/10.3389/fmicb.2013.00075 (2013).
Chen, G. et al. Optimization and characterization of pullulan production by a newly isolated high-yielding strain Aureobasidium melanogenum. Prep. Biochem. Biotechnol. 49, 557–566. https://doi.org/10.1080/10826068.2019.1591988 (2019).
Najme, R., Zhuang, S., Qiu, J. & Lu, Z. Identification and characterization of nornicotine degrading strain Arthrobacter sp. NOR5. Sci. Total Environ. 764, 142894. https://doi.org/10.1016/j.scitotenv.2020.142894 (2021).
Han, J. et al. Isolation and identification of an osmotolerant Bacillus amyloliquefaciens strain T4 for 2, 3-butanediol production with tobacco waste. Prep. Biochem. Biotechnol. 52, 210–217. https://doi.org/10.1080/10826068.2021.1925912 (2021).
Zhang, Z. T., Taylor, S. & Wang, Y. In situ esterification and extractive fermentation for butyl butyrate production with Clostridium tyrobutyricum. Biotechnol. Bioeng. 114, 1428–1437. https://doi.org/10.1002/bit.26289 (2017).
Chen, S. et al. Depth-related microbial communities and functional genes in alpine permafrost. Innov. Life 2, 100081. https://doi.org/10.59717/j.xinn-life.2024.100081 (2024).
Liu, L. et al. Sequential production of secondary metabolites by one operon affects interspecies interactions in Enterobacter sp. CGMCC 5087. Innov. Life 1, 100023. https://doi.org/10.59717/j.xinn-life.2023.100023 (2023).
Luo, H., Riu, M., Ryu, C. M. & Yu, J. M. Volatile organic compounds emitted by Burkholderia pyrrocinia CNUC9 trigger induced systemic salt tolerance in Arabidopsis thaliana. Front. Microbiol. 13, 1050901. https://doi.org/10.3389/fmicb.2022.1050901 (2022).
Gong, A. D. et al. Inhibitory effect of volatiles emitted from Alcaligenes faecalis N1–4 on Aspergillus flavus and aflatoxins in storage. Front. Microbiol. 10, 1419. https://doi.org/10.3389/fmicb.2019.01419 (2019).
Feng, G. D., Yang, S. Z., Li, H. P. & Zhu, H. H. Massilia putida sp. nov., a dimethyl disulfide-producing bacterium isolated from wolfram mine tailing. Int. J. System. Evolut. Microbiol. 66, 50–55. https://doi.org/10.1099/ijsem.0.000670 (2016).
Gong, A. et al. Dual activity of Serratia marcescens Pt-3 in phosphate-solubilizing and production of antifungal volatiles. BMC Microbiol. 22, 26. https://doi.org/10.1186/s12866-021-02434-5 (2022).
Chen, X. et al. Burkholderia ambifaria H8 as an effective biocontrol strain against maize stalk rot via producing volatile dimethyl disulfide. Pest Manag. Sci. 80, 4125–4136. https://doi.org/10.1002/ps.8119 (2024).
Kesik, M., Blagodatsky, S., Papen, H. & Butterbach-Bahl, K. Effect of pH, temperature and substrate on N2O, NO and CO2 production by Alcaligenes faecalis p. J. Appl. Microbiol. 101, 655–667 (2006).
Zheng, R. et al. A newly isolated Alcaligenes faecalis ANSA176 with the capability of alleviating immune injury and inflammation through efficiently degrading Ochratoxin A. Toxins (Basel) 14, 569 (2022).
Zhang, H., Zhang, Y., Yin, T., Wang, J. & Zhang, X. Heterologous expression and characterization of a novel ochratoxin a degrading enzyme, N-acyl-L-amino acid Amidohydrolase, from Alcaligenes faecalis. Toxins (Basel) 11, 518 (2019).
Amarita, F. et al. Identification and functional analysis of the gene encoding methionine-γ-lyase in Brevibacterium linens. Appl. Environ. Microbiol. 70, 7348–7354. https://doi.org/10.1128/AEM.70.12.7348-7354.2004 (2004).
Wu, Z., Chen, T., Sun, W., Chen, Y. & Ying, H. Optimizing Escherichia coli strains and fermentation processes for enhanced L-lysine production: A review. Front. Microbiol. 15, 1485624 (2024).
Bonnarme, P., Lapadatescu, C., Yvon, M. & Spinnler, H. E. L-methionine degradation potentialities of cheese-ripening microorganisms. J. Dairy Res. 68, 663–674. https://doi.org/10.1017/s002202990100509x (2001).
Bustos, I. et al. Volatile sulphur compounds-forming abilities of lactic acid bacteria: C-S lyase activities. Int. J. Food Microbiol. 148, 121–127. https://doi.org/10.1016/j.ijfoodmicro.2011.05.011 (2011).
del Castillo-Lozano, M. L., Mansour, S., Tâche, R., Bonnarme, P. & Landaud, S. The effect of cysteine on production of volatile sulphur compounds by cheese-ripening bacteria. Int. J. Food Microbiol. 122, 321–327. https://doi.org/10.1016/j.ijfoodmicro.2007.12.025 (2008).
Martinez-Cuesta Mdel, C., Pelaez, C. & Requena, T. Methionine metabolism: Major pathways and enzymes involved and strategies for control and diversification of volatile sulfur compounds in cheese. Crit. Rev. Food Sci. Nutr. 53, 366–385. https://doi.org/10.1080/10408398.2010.536918 (2013).
Jiang, X. et al. In vitro assembly of multiple DNA fragments using successive hybridization. PLoS ONE 7, e30267. https://doi.org/10.1371/journal.pone.0030267 (2012).
Chen, H. et al. Directed evolution of mevalonate kinase in Escherichia coli by random mutagenesis for improved lycopene. RSC Adv. 8, 15021–15028. https://doi.org/10.1039/c8ra01783b (2018).
Zhou, Y. & Xu, C. Miniature genome editors derived from engineering Cas9 ancestor. Innov. Life 1, 100008. https://doi.org/10.59717/j.xinn-life.2023.100008 (2023).
Hu, Y. et al. Toward the generation of pure coral genomes with experimental and bioinformatic improvements. Innovation 5, 100643 (2024).
Li, M. et al. Improvement of isoprene production in Escherichia coli by rational optimization of RBSs and key enzymes screening. Microb. Cell Fact. 18, 4. https://doi.org/10.1186/s12934-018-1051-3 (2019).
Jia, K. Z. et al. Clonostachys rosea demethiolase STR3 controls the conversion of methionine into methanethiol. Sci. Rep. 6, 21920. https://doi.org/10.1038/srep21920 (2016).
Funding
This work was supported by National Natural Science Foundation of China, China (U24A20478, 32170084), Natural Science Foundation of Shandong Province, China (ZR2023MC163), Qingdao New Energy Shandong Laboratory Open Project (QNESL, OP202308).
Author information
Authors and Affiliations
Contributions
Pingle Wu : Article experiments, article writing and editing, and experimental design;Shuaijun Deng: Provide experimental ideas and guidance for experiments, and revise articles;Jian Li : Collect some data and survey information;XiaodiWei : Analyze relevant literature;Rong Zheng : Provide experimental ideas and suggestions;Xiao Men : Revise and review the article;Haibo Zhang: Article design, revision, and review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, P., Deng, S., Li, J. et al. Isolation and identification of Alcaligenes faecalis W2-3 with high-yield production of dimethyl disulfide. Sci Rep 15, 20830 (2025). https://doi.org/10.1038/s41598-025-04904-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-04904-6