Abstract
Soils developed in karst regions are widely reported to exhibit high geological backgrounds of heavy metals (HMs) globally. However, due to unique geological conditions, there are both mineralized and/or altered areas of polymetallic minerals in karst areas, and whether the HMs concentrations of regional soil are affected by these mineralized and/or altered areas is far from enough attention at present. This study investigated the accumulation and chemical speciation of HMs in soils from typical lead-zinc mineralized zones in karst areas of Southwest China, using random forest (RF) modeling to identify key driving factors. The results indicated significant variability in HMs concentrations. Cr, Ni, and Cu levels were comparable to Guizhou Province’s background values, while Cd, Pb, Zn, As, and Hg exceeded these values by 0.54 to 44.98 times. Notably, the accumulation of Cd, Pb, and Zn was particularly pronounced, with certain samples surpassing the risk control thresholds for agricultural land. Sequential extraction analysis revealed that Cd and Pb is predominantly found in reducible fractions, (40.61% and 36.49%, respectively), indicating strong bioavailability and potential mobility. RF analysis identified TFe₂O₃ and Al₂O₃ as key factors for the accumulation of As, Hg, Cr, Ni and Cu, while MnO and the distance to lead-zinc mineralization points (DL) significantly influenced Cd, Pb, and Zn levels. This study highlights that HMs accumulation is driven by carbonate rock parent materials and Cd, Pb, and Zn levels exacerbated by lead-zinc mineralization, offering a valuable insight for regional HMs management and control. Meanwhile, the research results can provide references for the geochemical accumulation mechanisms and risk management of soil HMs in mineralized areas (belts) worldwide.
Similar content being viewed by others
Introduction
Soil is an indispensable resource for human survival and ecosystem stability, playing a crucial role in maintaining ecosystem functions and supporting human production and livelihood1. The accumulation of Heavy metals(HMs) in soil is directly influenced by natural sources and anthropogenic activities2. Natural sources are linked to parent material, soil formation processes, and geological background. Soils in karst regions globally exhibit high HMs levels due to their unique geological conditions2,3,4,5. The accumulation of HMs in geological high-background area, determined by parent rock composition and geochemical processes like weathering and leaching, leads to significant HMs enrichment in karst soils. HMs are abnormally enriched in soils driven by natural geological backgrounds have garnered significant attention3,6,7. Although carbonate rocks (e.g., limestone, dolomite) typically have low HM content, their weathering can result in soil HM concentrations much higher than the parent material8,9,10. Soluble components like Ca and Mg are leached during weathering, while residual HMs are absorbed by clay or iron-manganese oxides, leading to long-term HM accumulation. Through long-term physical and chemical weathering processes, the concentration of HMs in the soil gradually increases, leading to significant heavy metal enrichment in the soils of karst areas5,11,12. Studies in regions such as southern Italy13, Okinawa, Japan14, and China (e.g., Guizhou, Yunnan, Guangxi) have shown that HM levels in karst soils are influenced by carbonate rock weathering and natural geological backgrounds. Researches show that the high heavy metal background in karst areas poses risks to the safety of local agricultural products, ecological security, and human habitation2,15,16. Therefore, it is essential to focus on the geochemical accumulation of HMs and their impacts in areas with naturally high geological backgrounds.
The southwestern karst region, centered around Guizhou, is the largest and most concentrated area of karst distribution in the world. The background levels of soil HMs are significantly higher than the national average, primarily due to the natural geological background17. According to the National Soil Pollution Survey Report, the distribution of HMs in China’s soil exhibits a clear regional pattern, with the southwestern region showing particularly high-background levels of HMs such as Cd, Zn, and Pb, with the natural enrichment of Cd being especially prominent. At the same time, these carbonate rock distribution areas are also important metallogenic regions for Pb-Zn, Hg, As, Sb, and other metal minerals18, and are among the key metallogenic belts in China. These metal mineral resources are often found in regional carbonate rock strata, and as a result, the ore-bearing carbonate formations typically contain high levels of heavy metal elements. Therefore, during prolonged hydrothermal alteration and/or weathering, the natural accumulation of HMs in the soil occurs in these mineralized areas19,20. However, current researches on ore-bearing formations in mineralized areas mostly focuses on the mineral resource potential, with limited studies addressing the natural accumulation, chemical forms, and pollution risks of metallogenic and/or associated HMs in the soil due to the weathering of ore-bearing formations under natural conditions20,21,22. Among which, the Cambrian Qingxudong Formation carbonate rock strata, located in eastern Guizhou, serve as the primary host layer for lead-zinc deposits in the region, forming a distinctive lead-zinc metallogenic belt23. These ore-bearing rock formations frequently contain elevated levels of HMs. A significant portion of the agricultural soils in this region may be influenced by mineralized and/or altered zones, putting these soils at risk of naturally high levels of HMs. However, to date, there is limited understanding of the accumulation of HMs in soils derived from the weathering of such ore-bearing formations, and the speciation and influencing factors of HMs in the region remain unclear.
Therefore, this study focuses on the overlying soils developed on the Qingxudong Formation strata in the karst region of eastern Guizhou. The objectives are to: (1) investigate the concentration and accumulation characteristics of HMs in soils overlying ore-bearing rock formations; (2) clarify the chemical speciation of HMs and assess their bioavailability and mobility; (3) explore the main driving factors influencing the accumulation and bioavailability of HMs in geologically high-background lead-zinc mineralized areas. The research findings will provide baseline data for regional HMs pollution prevention and control, and offer scientific references for soil HMs risk management in geological background areas with ore-bearing rock series and agricultural sustainable development in other regions worldwide.
Materials and methods
Study area
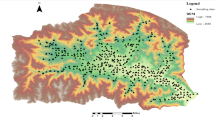
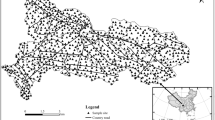
The Cambrian Qingxudong Formation carbonate rocks in eastern Guizhou cover approximately 3,527 km², accounting for about 3% of the total karst area in Guizhou, which is an ore-bearing formation for lead-zinc deposits24. The region features a subtropical monsoon climate, with average annual temperatures of 15–20 °C and annual rainfall of 1000–1400 mm. The Pb-Zn metallogenic belt in the study area is one of the important Pb-Zn resource bases in China. As shown in Fig. 1, the Kaili-Duyun area in eastern Guizhou is the most concentrated distribution area of Pb-Zn in Guizhou Province within this metallogenic belt, where Pb-Zn deposits (occurrences) are distributed in groups and zones. Currently, a few Pb-Zn deposits have been discovered, including 5 medium-sized deposits, 20 small-sized deposits, and several mineral occurrences.
Sample collection
Based on field investigations, soil samples were collected from the overlying soil in the outcrop distribution area of the Qingxudong Formation in the eastern region of Guizhou. The sampling sites are located between 26°13’24″–28°15’1″N and 107°37’35″–109°17’24″ E, showing in Fig. 1. In the selection of sampling sites, factors such as outcrops of carbonate rocks in the Qingxudong Formation, geological background, and distribution of Pb-Zn mineralization deposits (occurrences) were thoroughly considered. Soil sampling points were appropriately intensified near the concentrated distribution areas of Pb-Zn mineralization deposits (occurrences). At the same time, areas with anthropogenic pollution, such as industrial enterprises, mining activities, and highways, were avoided, and naturally developed soils were chosen for sample collection. Five subsamples were collected from each sampling site and combined to form a representative sample. After thorough mixing, the samples were bagged, with each sample weighing between 1.5 and 2.0 kg. A total of 87 surface soil samples (0–20 cm) were collected following the Technical Specification for soil Environmental monitoring (HJ/T 166–2004)25. The soil type was initially determined by the color, texture, and soil horizon feature. Acrisols are the major soil type following FAO5,26. The locations of sampling sites are listed in Table S1.

(Adapted from Chen et al.22).
Stratigraphic distribution of the Qingxudong Formation in Guizhou Province and the location of the sampling sites.
Chemical analysis
The soil samples were air-dried naturally, and impurities such as stones and roots were removed. The soil was then ground and sieved sequentially through 2.0 mm and 0.074 mm nylon sieves. The sieved soil samples were stored in sealed bags for subsequent chemical analysis. Soil pH was measured with a calibrated pH meter (PHS-3c, Leici, China) by mixing soil and ultrapure water (@18.2 MΩ cm) at a 1:2.5 ratio and letting it stand for 1 h27. Total organic carbon (TOC) was analyzed using potassium dichromate oxidation-reduction titration. The specific operational procedures are as follows: 5 mL of 0.800 mol/L potassium dichromate solution and concentrated sulfuric acid were sequentially added to the pre-weighed soil samples. The mixture was digested in an oil bath at 170–180 °C for 5 min. After cooling, using phenanthroline as an indicator, the unreacted potassium dichromate was titrated with a 0.2 mol/L ferrous sulfate standard solution28. Soil particle size and texture were assessed with a laser particle size analyzer (Malvern, Mastersizer 2000, UK), categorizing particles into sand (2–0.02 mm), silt (0.02 –0.002 mm), and clay (< 0.002 mm) based on international standards29. The specific testing procedures were performed with reference to the literature by Wang et al.30. Cation exchange capacity (CEC) was determined using the hexamminecobalt trichloride solution-spectrophotometric method, following Chinese Environmental Protection Agency guidelines31. After 50.00 mL of 1.66 cmol/L hexamminecobalt chloride solution was added to 3.50 g of soil sample, the mixture was shaken for 60 min. The suspension was then centrifuged, and the supernatant was collected. Absorbance was measured at 475 nm and 380 nm using a UV–Vis spectrophotometer, and the CEC was subsequently calculated. The major geochemical compositions (SiO₂, Al₂O₃, TFe₂O₃, CaO, K₂O, MgO, Na₂O) were determined using powder X-ray fluorescence spectroscopy (XRF). The procedure for determining the total concentration of soil HMs is as follows: Approximately 0.1000 (± 0.0002) g of the sample was added into a 10 mL polytetrafluoroethylene (PTFE) beaker with mixed acid (3 ml ultra-pure HNO₃ and 1 ml ultra-pure HF) and thoroughly mixed. Samples were digested with the mixture at 180 °C under high pressure for 48 h. After cooling, the mixture is evaporated on a hot plate at 120 °C until dry. Finally, the residue was diluted with 2% nitric acid to 50 mL and stored at 4 °C until analysis. Concentrations of Cr, Ni, Pb, Zn, Cu, and Cd were measured using inductively coupled plasma mass spectrometry (ICP-MS, NexION 300X, PerkinElmer, USA), while As and Hg were analyzed by atomic fluorescence spectrometry. (AFS-8510, Beijing Haiguang, China).
Blank samples, parallel samples, and Reference Materials of China (GBW07404 and GSS-4 for soil samples) were used for QA/QC. During the testing process, 10% of the samples were selected for repeated testing, with the relative standard deviation of the results below 8%. The recovery rates of Cd, Cr, Cu, Ni, Pb, and Zn in the reference standard materials ranged from 83.90 to 112.80%, while those of As and Hg were within the range of 93.50–105.80%. Additionally, to assess the analytical precision of the instrument, internal standards Rh and In were inserted to verify instrument stability, and a calibration standard solution with a concentration of 10 µg/L was analyzed after every 20 samples to ensure that the analytical error remained within ± 5%.
In this study, the chemical speciation of HMs in soil was extracted using the European Community Bureau of Reference (BCR) method. The speciation of HMs is classified into four categories: F1-F4. The extraction steps are presented in Table S2. It should be noted that due to the low concentrations of As and Hg in the soil of the study area, combined with the high volatility and speciation instability, the extraction recovery rates of As and Hg by BCR sequential chemical extraction are low. Specifically, the qualification rate for Hg speciation analysis is only 46%, while that for As is 58.4%, indicating that it is not suitable for BCR speciation analysis30. Therefore, the speciation of As and Hg was not considered in this study. The concentrations of the various extracted fractions of HMs were determined using ICP-MS. Compared to the total concentrations, the extraction rates for Cr, Ni, Pb, Zn, Cu, and Cd by sequential chemical extraction were 73.59 − 106.85%, 75.87 − 104.97%, 82.21 − 118.51%, 80.17 − 122.16%, and 81.34 − 110.67%, respectively, indicating overall satisfactory extraction efficiency.
Data processing and analysis
Assessment method of accumulation levels
The geo-accumulation index (Igeo) is a commonly used method for evaluating the accumulation degree of HMs in the soil of the study area. The Igeo comprehensively considers the influence of soil parent materials and human activities, and reflects the natural distribution and anthropogenic effects on heavy metal accumulation to a certain extent32. The calculation formula of Igeo is as follows:
where Cn is the measured mass concentration of heavy metal element n, in mg/kg; Bn is the geochemical background value of heavy metal element n, in mg/kg. In the present study, Bn is the corresponding background value of HMs in the surface soil of Guizhou Province33. The constant k is a background variation factor that accounts for differences in parent rock materials, typically assigned a value of 1.534. The classification standards for Igeo are shown in Table S3.
Mobility factor (MF) and potential mobile fractions (PMF)
It is generally accepted that the mobility and bioavailability of HMs in soil can governed by their chemical speciation patterns35,36. The HMs mobility factor (MF) and the potentially mobile fractions (PMF) are used to evaluate the mobility and bioavailability of heavy metal(loid)s in soil37,38, with calculation formulas provided in Eq. (2) and Eq. (3).
Random forest method (RF)
Random Forest (RF), an ensemble machine learning algorithm, offers advantages such as resistance to overfitting and strong generalization ability39. It can effectively handle complex nonlinear relationships between soil HMs concentrations and environmental factors, quantitatively identify the influence of different environmental variables on soil HMs, and improve the prediction accuracy of factor contributions to variables without being constrained by the assumption conditions of linear regression models. Therefore, it has been widely applied to studies on predicting influencing factors of soil HMs40,41,42.
In the current study, the “Random Forest” package in R (version 4.0.2) was employed to perform random forest regression for predicting factors influencing HMs accumulation. Fourteen variables, including soil pH, TOC, CEC, texture, and major elements, were selected as contributing factors for quantitative evaluation. A random data splitting technique was used to divide the dataset into a training dataset (80% for calibration) and a test dataset (20% for validation). The prediction accuracy of RF was evaluated using the coefficient of determination (R²), root mean square error (RMSE), and mean absolute error (MAE) to identify the optimal prediction model41. When the RF model for identifying influencing factors of each HMs exhibited minimal differences in R² values between the training and validation sets (both maintaining high levels) and low RMSE and MAE values42, it indicated that the RF model used in this study for identifying influencing factors of HMs possessed optimal performance and robustness under this parameter configuration, capable of providing reliable prediction results.
Statistical analysis
The concentration data of soil HMs were statistically analyzed using SPSS 23.0 (IBM Corporation, USA) and Microsoft Excel 2022 (Microsoft, USA). Following a normal distribution test, outliers were removed, and key statistical parameters - maximum (Max), minimum (Min), median, arithmetic mean, standard deviation (SD) and coefficient of variation (CV) - were computed. The distribution map of sampling points in the study area was conducted by ArcGIS (version 10.5, ESRI, USA). Data plots were created using Origin ver. 8.5.
Results and discussion
Concentrations of heavy metals and related properties in soils
The physicochemical parameters and concentrations of HMs in the topsoil samples (0–20 cm) from the study area are shown in Table 1. Soil pH ranges from 5.23 to 7.60, with an average of 6.76 ± 0.60. Over 70% of the soil samples have a pH between 6.50 and 7.60, indicating that the soils in the study area are generally neutral to slightly basic. The average values of the major soil elements SiO₂, Al₂O₃, TFe₂O₃, MgO, CaO, Na₂O, and K₂O are 52.89%, 15.35%, 7.06%, 3.21%, 3.67%, 0.14%, and 2.33%, respectively. When compared to the average abundance of the upper continental crust (UCC)43, the Al₂O₃ content in the soils of the study area is like that of the upper continental crust. However, the normalized values of major elements such as Na, K, Ca, and Si are all below 1.0, indicating that these elements were lost during the weathering process. The soil TOC and CEC levels range from 0.088 to 6.2% and from 0.79 to 13.39 cmol (+)/kg, with average values of 2.45% and 6.35 cmol (+)/kg, respectively The soil TOC in the study area was slightly higher than that in the typical karst basins of Guangxi, China: almost all samples were below 1% 44, while the CEC content was significantly lower than that in the karst areas of Hunan Province (ranging from 6.99 to 36.03 cmol·kg⁻¹45. In terms of soil texture, the samples collected in this study were dominated by particles of 0.02–0.002 mm, with an average proportion as high as 61.60% (ranging from 0.68 to 95.40%). This was followed by small particles of < 0.002 mm, with an average proportion of 20.57% and a maximum of 65.39%. This indicates that the soil samples were mainly silt loam and clay, possibly containing clay minerals, which may contribute to the adsorption and accumulation of HMs46. It is worth noting that the physicochemical parameters of the investigated soil, such as TOC, CEC, and texture, exhibited significant variations with high coefficients of variation (CVs) (Table 1). Specifically, the CVs of TOC and CEC were 58.5% and 45.3%, respectively, while the CVs of sand and clay were 137% and 66.1%, respectively. The above results indicate significant differences in the represented soil physicochemical properties and texture, which are related to the fact that soil samples were collected from different regions in eastern Guizhou Province.
The average concentrations of Cr, Ni, Cu, Zn, Pb, Cd, As, and Hg are 103 ± 126 mg/kg, 49.03 ± 27.16 mg/kg, 46.77 ± 59.27 mg/kg, 617 ± 760 mg/kg, 309 ± 364 mg/kg, 3.51 ± 4.38 mg/kg, 41.65 ± 25.07 mg/kg, and 0.50 ± 0.310 mg/kg, respectively. Except for Cr, The concentrations of Ni, Cu, Zn, Pb, Cd, As, and Hg exceed the background levels for surface soil in Guizhou33, and their average concentrations are 1.17, 1.15, 4.31, 6.08, 4.71, 1.99, and 4.45 times of the background values of Guizhou, respectively (Fig. 2 (a)). In addition, compared with the risk screening values in GB 15,618 − 2018 (Chinese Agricultural Land Soil Pollution Risk Control Standard), the over-standard rates of Cd, Pb and Zn were notably high, with exceedance multiples of 1.99, 2.39 and 9.02 times, respectively (Fig. 2 (b)). This suggests that natural or human factors are contributing to the accumulation of HMs in the soil. Numerous studies have shown significant differences in heavy metal content in soils developed from various parent materials in karst areas5,6,12,47. The average concentrations of HMs in soils developed in high geochemical background areas of various karst regions worldwide are presented in Table S4. It is evident that the surface soils developed from the Qingxudong Formation in the study area generally contain significantly higher levels of HMs compared to those in carbonate and clastic rock soils from the high geochemical background areas of Guangxi12. Additionally, they exceed the HMs concentrations in soils developed from black shales in Hunan and Chongqing, particularly in terms of Cd, Pb, Zn and Hg6,48. However, the HMs concentrations are comparable to that in soils developed from carbonate rocks in western Guizhou Province6. This suggests a significant accumulation of HMs in the soils during the weathering and soil formation processes of the Qingxudong Formation in the study area. The coefficient of variation (CV%) reflects the degree of dispersion among different samples. In the study area, the CV values of HMs in the surface soils are all greater than 36%, indicating a high degree of spatial heterogeneity and potential influence from certain human activities49,50.
Evaluation of heavy metals accumulation levels in soils
The geo-accumulation index (Igeo) for eight HMs was calculated using Guizhou Province’s background values as a reference. The Igeo classification for the HMs is presented in Fig. 3. The overall accumulation levels of the eight HMs, from highest to lowest, are as follows: Pb > Zn > Hg > Cd > As > Ni > Cu > Cr. Notably, 93% of the soils have an Igeo value for Cr below 0, indicating no accumulation. Additionally, 90% and 96% of Ni and Cu, respectively, range from non-accumulation to moderate accumulation levels. Overall, the Igeo values for Cr, Ni, and Cu in the soils of the study area are relatively low. Research indicates that Cr, Ni, and Cu in the soil are mainly influenced by natural factors driven by the parent material4. The Qingxudong Formation in the study area consists primarily of carbonate rocks such as limestone and dolomite, and the weathering of these natural parent rocks is considered the main source of Cr, Ni, and Cu in the soil. 80.5% of As falls within the “unaccumulation” to “unaccumulation to moderate accumulation” range, suggesting that As accumulation is not significant, with a relatively low influence from human activities.
However, as shown in Fig. 3, Pb, Zn, Hg, and Cd in the soil exhibit relatively high accumulation levels, with 72.4% of Pb, 68.9% of Hg, 55.2% of Zn, and 47.1% of Cd classified in the “moderate accumulation” to " heavily to extremely accumulation” range. Numerous studies have shown that soils formed by the weathering of carbonate rocks in karst regions exhibit abnormal enrichment of HMs such as Cd, Pb, Cu, Ni, Zn, and Cr5,6,12. The weathering and soil formation of carbonate rocks is the dominant factor driving the HMs enrichment. Although carbonate rocks typically contain low concentrations of trace metals, during the weathering and subsequent soil formation processes, over 97% of the parent carbonate materials is leached out, leading to significant enrichment of trace elements in the residual matter8,51,52. However, a comparison reveals that there is no significant difference in Cr, Ni, and Cu contents in soils developed from carbonate rocks and black shales across different region5,6,12,48, although Cr content is notably higher in soils developed from weathered carbonate rocks in western Guizhou6. It is worth noting that although soils developed from carbonate rocks, clastic rocks, and black shales in various regions show some degree of accumulation of Cd, Pb, Zn, and Hg5,6,12,48,53, the accumulation levels are far lower than those observed in the soils of the study area (Table 1).
The study area is a low-temperature hydrothermal mineralization zone, with the Cambrian Qingxudong Formation’s carbonate rocks being the most developed. These carbonate strata are the main ore-hosting layers for Pb and Zn, and the area is also rich in Hg, Cu, Fe, and Al deposits54. As a result, the weathering and soil formation processes of these strata have led to the accumulation of a large amount of ore-forming elements and their associated elements in the soil, causing significant accumulation of Pb, Zn, Cd, and Hg in the soils of the study area. A comparative analysis of Cd, Pb, and Zn concentrations in the exposed bedrock and overlying soils of the study area shows that soil Cd is well correlated with Pb and Zn in the rocks22, suggesting that Cd accumulation in the soil may be associated with the presence of lead-zinc deposits in the region. Studies indicate that in the lead-zinc deposits of varying sizes within the study area, Cd primarily occurs in an isomorphous form within sphalerite, with some Cd present as independent minerals like greenockite in the oxidation zones of the deposits1,55. This evidence confirms that the accumulation of Cd in the soils is mainly related to the weathering of carbonate rocks containing lead-zinc ore, with possible additional influence from anthropogenic factors such as regional agricultural activities. In addition, the strong correlation between Pb and Zn in the soil and the corresponding elements in the bedrock indicates that the concentrations of Pb and Zn in the soil are controlled by the parent rock and exhibit regional consistency22. As for the accumulation of Hg in the soil, on one hand, Hg is often associated with atmospheric deposition resulting from human activities, primarily influenced by industrial operations, coal combustion, and traffic pollution56,57. On the other hand, the study area is located in a county rich in mercury mineral resources, suggesting that soil Hg accumulation may also be influenced by the parent material and the atmospheric dispersion and deposition of Hg released during mercury mining operations.
Chemical speciation of heavy metals in soils and mobility assessment
In the BCR sequential extraction, the exchangeable fraction (F1) of HMs species is considered weakly bound, which are prone to leaching into water or absorbed by crops, posing environmental and health risks58,59. The HMs mobility factor (MF) depends on the binding strength between HMs and soil components, reflecting their mobility, bioavailability, and release potential50. By calculating the ratio of the F1 to the HMs content in all fractions (expressed as the MF), the bioavailability and mobility of HMs can be assessed. The higher the proportion of the exchangeable fraction, the greater the bioavailability and mobility of the HMs, and the higher the environmental risk37,60. In addition, non-residual fractions, such as those bound to Fe/Mn oxides or organic/sulfide compounds, can also become mobile under specific pH and Eh conditions, making them potentially mobile fractions (PMF), suggesting further investigation of their possible impact on biodiversity and the environmental health38. Residue states, characterized by structural incorporation within aluminosilicate mineral lattices (e.g., quartz and feldspars), exhibit exceptional geochemical stability under ambient pedogenic conditions. These chemically refractory forms demonstrate negligible remobilization potential and are consequently considered non-bioavailable with minimal ecotoxicological relevance and low environmental hazard potential21,61.
Different speciations of HMs have different bioavailabilities and migration capabilities. The proportion of each fraction, mobility factor (MF) and potential mobile fractions (PMF) Cr, Ni, Cu, Pb, Zn, and Cd in the soil of the study area are presented in Figs. 4 and 5. There are some differences in the proportion of different species of various HMs, among which Cr, Ni, Cu and Zn are predominantly found in the F4 fraction, with average proportions as high as 92.2%, 84.8%, 74.5%, and 87.5%, respectively,. The proportions of other species are relatively low, only the F3 of Cu reaches 12.9%. Meanwhile, the MF and PMF values of these HMs are also relatively low (Fig. 5). The obtained results indicate that these elements in the soil of the study area are primarily present in the lattices of silicate minerals, primary and secondary minerals62. This is attributed to natural geological weathering, and these elements exhibit stable chemical properties, making them difficult to release under natural conditions. Consequently, their bioavailability and mobility are low, and pose minimal ecological risks to human health and the environment.
However, an exception is observed in the distribution of Pb and Cd species in the soil, which significantly differs from other HMs. The proportion of Pb in the F4 fraction ranges from 18.18 to 69.84%, with an average of 46.15%, followed by the F2 fraction, which accounts for 6.24–65.33%, with an average proportion as high as 36.49% (Fig. 4). Numerous studies indicate that in high geological background areas of carbonate rocks and black shale, Pb in soil is predominantly found in the residual form, with the F1 and F2 fractions typically present at lower concentrations 5,48,63. In this study, however, the proportion of reducible Pb is significantly higher than that of other HMs, which is consistent with the findings of Wei et al. and Yang et al. regarding the speciation of Pb in soils around mining areas48,64. Additionally, a similar distribution of Pb speciation has been observed in the soil of a Sb mineralization area in Brazil, where Pb has the highest proportion in the reducible fraction (F2), with an average of 68.2%. The authors attribute this mainly to the release of Pb during hydrothermal alteration of rocks and the formation of minerals like galenite in the mineralization zone, with the released Pb being predominantly adsorbed or precipitated by Fe-Mn oxides.21. Studies have also shown that Fe-Mn oxides in soil have a particular affinity for Pb36,65. Therefore, the high F2 component of Pb in the soil of the study area is closely related to regional Pb-Zn mineralization. Nevertheless, the MF value of Pb in the soil reaches a maximum of only 14.94%, with an average of 2.84%, which is much lower than the MF of Pb in mining area soils64, indicating that Pb is relatively stable, even though its total concentration in the soil is high. However, the PMF value of Pb is relatively high, with an average of 49.59%, primarily because Pb is mainly present in the F2 fraction, which increases its potential mobility and ecological risk 66. The F2 fraction is mainly composed of Pb bound to Fe/Mn oxides, typically present as mineral coatings and fine particulate matter. This may be related to the weathering of Pb-bearing mineralized strata in the study area. Additionally, changes in soil pH and Eh have a significant impact on the F2 fraction. Higher pH and Eh values promote the formation of Fe/Mn oxides67,68. The chemical speciation of Pb in the study area may be related to its presence in aluminosilicate minerals and Fe/Mn oxides, primarily through substitution and adsorption48,65. Since the reducible form of Pb is highly sensitive to changes in external environmental conditions and can easily transform into bioavailable free forms65,66, leading to increased bioavailability, so more attention should be paid to it.
As for Cd, the proportions of F1, F2, F3, and F4 are 26.75%, 40.61%, 12.95%, and 19.69%, respectively, with higher percentages in the acid-extractable and reducible forms. This is similar to the distribution of Cd speciation observed in previous studies of soils from high-background carbonate geological areas in Guangxi, Anhui, and Guizhou11,69,70. These findings indicate that in carbonate geological high-background areas, a significant proportion of Cd in the soil exists in acid-extractable/exchangeable and reducible forms. From Fig. 5, it can be observed that both the MF and PMF of Cd are relatively high, mainly due to the higher proportions of the weak acid-soluble fraction (F1) and the reducible fraction (F2). This indicates that the mobility and potential mobility of Cd in the soil are strong. The F1 represents Cd primarily adsorbed onto the soil matrix, which is the most mobile and bioavailable portion in the soil71. The F2 fraction is strictly dependent on the presence of Fe, Mn, and Al oxides and/or hydroxides in the soil72,73. However, it is important to note that the binding of cadmium to iron/manganese oxides does not guarantee its immobilization in the soil, as soil pH and Eh change, especially under acidic or reducing conditions, the mobility of Cd increases, allowing for potential indirect bioavailability and posing environmental risks 5,11,59,68. Additionally, the proportion of residual Cd in the soil is relatively low, indicating that the presence of Cd in the soil may be influenced by anthropogenic activities in addition to natural sources43. In the studied soil, Cd exhibits relatively higher MF and PMF values, followed by Pb and Zn (Fig. 5). For the study area, anthropogenic sources related to Cd may be associated with regional agricultural activities. Studies have shown that the accumulation of Cd, Pb, and Zn in soil is linked to the intensive application of pesticides, chemical fertilizers, and livestock manure during agricultural planting57,74,75. Field investigations reveal that local farmers have applied substantial amounts of livestock manure, compound fertilizers, and pesticides in agricultural production to achieve higher yields, thereby causing annual residues of Cd, Pb, and Zn in the soil over time. Besides, the study area is an important Pb-Zn mineral resource distribution region in Southwest China. Although most Pb-Zn deposits (occurrences) remain unexploited, several large-scale Pb-Zn deposits within the region are still under mining. During Pb-Zn mining and smelting processes, Cd and other volatile metals are prone to depositing in soils through wet and dry deposition40,76. Therefore, anthropogenic sources of Cd, Pb, and Zn in regional soils may also be associated with Pb-Zn mining and smelting activities. However, this variation in Cd speciation could also be affected by the geochemical heterogeneity of the parent rock and/or different weathering processes across regions5.
Integrated analysis of the controlling factors of heavy metals accumulation and mobility
The important of factors influencing HMs accumulation ranked by RF model
From the above analysis, it can be concluded that the accumulation of HMs in the soil of the study area is related to the regional carbonate geological background, while the anomalous enrichment of Cd, Pb, and Zn may also be influenced by the distribution of regional Pb-Zn mineralization zones/points. To further investigate the impact of the distribution of these Pb-Zn mineralization zones/points on soil HMs accumulation, the Average Nearest Neighbor Analysis in ArcGIS 10.5 was used to calculate the distance between each sampling point and its nearest Pb-Zn mineralization point, represented as DL. Then, combined with soil physicochemical properties and the composition of major and trace elements, the RF model was used to determine the importance ranking of factors influencing HMs accumulation (Fig. 6). The percentage increase in mean squared error (IncMSE%) is typically used as an indicator to measure the importance of driving factors77. As shown in Fig. 6, TFe₂O₃ and Al₂O₃ are key factors in explaining the accumulation of Cr, Ni, Cu, As, and Hg in the soil. Additionally, SiO₂, CEC, CaO, MgO, and Na₂O are secondary factors influencing the accumulation of Cr, Ni, Cu, and Zn, while pH, clay, and TOC are relatively important factors affecting the As and Hg. Meanwhile, TFe₂O₃ is also an important factor influencing Pb and Zn. Generally, primary soil components such as aluminum, iron, manganese oxides, clay minerals, and organic matter play crucial roles in the distribution and accumulation of HMs78,79,80.
a) Major element oxides.
Studies have found that in high geological background areas, Fe and Al oxides are very common in the secondary products of carbonate rock weathering. In the developed soils, Fe₂O₃ and Al₂O₃ have a particularly significant impact on the enrichment of HMs such as Cr, Ni, Cu, Zn, Cd, and Pb 10,81. This is mainly because Fe and Al oxides have been proven to be the primary adsorption carriers for HMs in soils. Due to their large surface area and adsorption capacity, Fe and Al minerals exhibit strong adsorption and precipitation effects on HMs during their formation5,82,83. The weathering products of carbonate rocks present an alkaline environment, and when the pH exceeds 6.0, Fe and Al oxides compete with organic matter for metal adsorption78,84. As pH increases, the amount of metal adsorbed on their surfaces also increases85,86. Therefore, TFe₂O₃ and Al₂O₃ have a particularly significant impact on the accumulation of HMs in soils within karst12. In the study area, soils formed from weathered carbonate rocks mostly exhibit weakly alkaline pH, and Cr, Ni, Cu, Zn, Pb, Cd, As, and Hg are enriched to varying degrees, with Fe and Al oxides playing a critical role in their accumulation.
Surprisingly, MnO also shows a significant influence on Cd and Pb. Previous study has found that the spatial distribution of Cd in soils within the karst geological high-background area of Guangxi is highly consistent with the geochemical distribution of Mn in China5. Among the primary carriers of HMs in the soil, Mn oxides have a greater adsorption affinity for Cd and Pb compared to Fe oxides83. The co-enrichment of Cd and Mn in soils is commonly observed in karst geological high-background areas. This phenomenon may be attributed to the formation of iron-manganese nodules during the weathering of carbonate rocks, which have a strong adsorption capacity for Cd and Pb, leading to their unusually high concentrations5. However, whether the accumulation of Cd and Pb in the soils of the study area is related to the presence of iron-manganese nodules remains to be investigated further.
b) Soil pH, TOC and CEC.
Soil pH has been recognized as an important factor influencing the accumulation of HMs in soil87,88, primarily by influencing the solubility and speciation of HMs in soil, thereby altering their concentrations. Additionally, pH levels significantly impact the adsorption of heavy metal ions by soil minerals such as kaolinite, aluminum oxides, and silicon dioxide89,90. The weathered soils of carbonate rocks in the study area are mostly alkaline. However, due to relatively low weathering (with a Chemical Alteration Index (CAI) ranging from 71.3 to 87.3, averaging 78.2), the soils contain relatively high levels of carbonates, CaO, MgO, and Na2O, resulting in a relatively high pH (up to 7.6). When the pH value is neutral to weakly alkaline, the mobility of HMs in soil decreases, making them more likely to be retained in the surface soil and reducing their ecological risks 89. Consequently, the soil in the study area exhibits obvious accumulation characteristics of HMs. This also seems to confirm the controlling role of CaO, MgO, and Na2O on the accumulation of Cr, Ni, Cu, and Zn in the soil.
Additionally, studies have shown that soil properties such as TOC (or organic matter) and CEC are key factors influencing the adsorption and immobilization of HMs, and they have important impacts on the mobility and bioavailability of HMs in soil46,91. Researches have shown that soil organic matter can affect the speciation and mobility of HMs through processes such as adsorption, desorption, and complexation92,93. The primary reason is that soil organic matter contains numerous ligands and functional groups, such as hydroxyl and carboxyl groups, which can act as binding sites for HMs 64,94. Generally, the higher the TOC (total organic carbon) content in the soil, the more binding sites available for HMs, resulting in stronger immobilization of HMs in soil. However, in this study, no obvious influence or correlation of TOC with HMs in the soil was observed (Figs. 6 and 7). However, it can be seen from Fig. 6 that soil CEC has a certain influence on the accumulation of Cd, Cr, Ni and Zn in soil. CEC refers to the soil’s capacity to adsorb exchangeable cations, which is primarily affected by the content and type of soil clay minerals and organic matter. Higher CEC indicates a greater capacity for adsorbing cations like HMs64,95. Meanwhile, the relatively high TOC content in the studied soil may increase the negative charge content, thereby enhancing the content of base cations and further promoting cation exchange45, which in turn immobilizes HMs.
c) Soil particle size.
Soil particle size, particularly clay content, is a key factor influencing the distribution of HMs in soil96,97,98. However, in the present study, the correlation between particle size and HMs was not significant, with only a certain positive correlation observed for Cr, Ni, and Cu (Fig. 7). The particle size distribution did not exhibit corresponding importance for HMs accumulation. This may be partly because low-activity clays primarily exist in the clayey soils, while high-activity clays are found in sandy or loamy soils4, resulting in a weaker adsorption and fixation effect of clay on HMs. No correlation was found between HMs concentrations and clay and organic matter in the soil of one area of Brazil, which interpreted as the result of the dominant control of the parent material, i.e. the retention of metals in the soil is governed more by geological factors than by pedogenic processes99. On the other hand, although the parent material for soil formation in the study area is the Qingxudong Formation carbonate rocks, the wide distribution of the study area is influenced by pedogenic factors such as altitude, topography, and temperature. Additionally, the geochemical heterogeneity of the bedrock and the weathering processes result in variations in soil types, textures, and weathering degrees across the region. As a result, there are significant differences in the physical and chemical properties of soils in different geographical spaces and types5,48. Consequently, the spatial accumulation and distribution of various HMs show strong heterogeneity, and the clay content in the soil does not exhibit a clear controlling effect on the accumulation of HMs.
Overall, the controlling effects of soil TOC and CEC on HMs accumulation are limited, while soil pH and the contents of Fe, Mn, and Al oxides exhibit more prominent influences. This suggests that the accumulation of HMs in the surface soils of the study area is predominantly influenced by regional geological factors, particularly that secondary enrichment processes during carbonate rock weathering in the area may play a dominant role. Besides, the contents of physicochemical parameters such as TOC and CEC in the collected soils vary significantly, reflecting differences in soil texture. This is primarily attributed to the large spatial scope of soil sample collection and strong spatial heterogeneity, resulting in insignificant controlling effects on soil HMs.
d) Distance between each sampling point and its nearest Pb-Zn mineralization point (DL).
It is noteworthy that, as shown in Fig. 6, the DL holds a significant position in the importance ranking of factors influencing Pb and Cd, and it is also relatively important in the context of Zn, As, and Hg. However, its importance ranking for Cr, Ni, and Cu is relatively lower. In Sect. "Evaluation of heavy metals accumulation levels in soils", we analyzed that the low Igeo values of Cr, Ni, and Cu in the soil are mainly influenced by natural factors driven by the regional parent material. The parent material is an important factor causing the accumulation and spatial variation of HMs in soil. The carbonate rocks of the Qingxudong Formation in the study area serve as the main ore-bearing strata for Pb-Zn deposits, forming a widespread lead-zinc mineralization belt in the region. Additionally, the study area is located within a significant low-temperature hydrothermal mercury mineralization belt in China, where the stratigraphic rocks often contain elevated levels of metals such as Cd, As, and Hg54. Therefore, the chemical weathering of these high-metal-content rocks is a primary source of HMs in the soil, leading to increased enrichment of metals and metalloids such as Cd, Pb, Zn, As, and Hg20,21. Thus, the anomalous enrichment of HMs such as Cd and Pb in the soil of this study is closely related to the Pb-Zn mineralization in the carbonate strata of the Qingxudong Formation. In addition, based on the distribution of Pb-Zn mining points (Fig. 1), we categorized the soil samples into Class I sampling points, which are close to Pb-Zn mineralization points, and Class II sampling points, which are relatively far from them. We then analyzed the concentrations of Cd, Pb, and Zn in both categories of samples. The results showed that soil samples closer to Pb-Zn mineralization points exhibited significant variability in Cd, Pb, and Zn concentrations, with higher average values. Notably, the highest values were found among samples close to the lead-zinc mineralization points 22, whereas samples located further away exhibited less variation in HMs content. This further underscore the controlling effect of the distribution of Pb-Zn mineralization points in the regional carbonate strata on the accumulation of Cd, Pb, and Zn in the soil. Generally speaking, areas with a dense distribution of Pb-Zn mining points indicate a relatively strong mineralization process, with evident Pb-Zn mineralization in the strata100. Consequently, the long-term weathering and pedogenesis of these mineralized strata have led to a certain degree of HMs accumulation in the soils of this region, particularly for Cd, Pb, and Zn.
The controlling factors of the bioavailability and mobility of HMs analyzed by correlation analysis
To investigate the controlling factors of the bioavailability and potential mobility of HMs in soils of a karst geological high-background Pb-Zn mineralization area, correlations were analysed between total concentrations of Cr, Ni, Cu, Pb, Zn, Cd, soil physicochemical properties, migration coefficient and potential mobility. As can be seen from Fig. 7, there is no obvious correlation among soil physicochemical parameters, such as TOC, CEC, and texture, and the soil HMs concentrations, as well as the MF and PMF. This is similar to the result obtained from Fig. 6, that is, the physicochemical parameters of the soil, such as TOC, CEC, and texture, have an insignificant controlling effect on the accumulation of HMs. The possible reasons have been analyzed in the previous text. However, the total concentration of HMs, soil pH, and the contents of Fe₂O₃ and Al₂O₃ have a significant impact on the mobility of HMs. The PbMF, CdMF, ZnMF, and CuMF show strong positive correlations with their total concentrations but negative correlations with pH. Researches indicate that changes in soil pH can significantly affect the morphological distribution of HMs, particularly the exchangeable fractions, which are highly sensitive to variations in pH70,101. The soil pH directly controls the variability of surface charge on clay particles, the complexation between humic substances and HMs, and the adsorption of HMs by iron and manganese oxides. Under low pH conditions, the activation of HMs is promoted, and the increase in positive surface charge on soil colloids hinders the adsorption of heavy metal ions on the soil colloid surface 102,103. Non-specific adsorption of HMs is likely to exchange with H⁺ in the soil solution, leading to an increase in the proportion of exchangeable HMs61. This is consistent with the findings of most studies that indicate the activity of heavy metal ions is controlled by soil pH 5,36,70. Additionally, PbPMF, CrPMF, NiPMF, CuPMF, and ZnPMF all show a significant positive correlation with Fe₂O₃ and Al₂O₃. Previous research has shown that Fe and Al oxides in karst regions have strong adsorption and fixation capacities for HMs, primarily in the form of cation-specific adsorption. The more Fe and Al oxides present in the soil, the lower the activity and bioavailability of HMs, thereby reducing their mobility104. It can be inferred that HMs accumulation is primarily driven by carbonate rock weathering, and bioavailability and mobility are influenced by total concentrations, pH, TOC, and Fe/Al oxide adsorption. Fe and Al oxides both promote HM accumulation and reduce their bioavailability and mobility.
Conclusions
This study investigated the accumulation, chemical speciation and influencing factors of heavy metals in the overlying soils of lead-zinc mineralization zones in a typical carbonate rock region in Southwest China.
-
(1)
The elevated concentrations of most HMs in the soil exceed the corresponding background values for surface soil in Guizhou, with Cd, Pb, and Zn even surpassing the soil risk intervention values.
-
(2)
Cr, Ni, Cu, and Zn are dominantly present in the residual fraction, while Pb is primarily present in the residual (F4: 46.15%) and the reducible fraction (F2: 36.49%), Cd is mainly found in the reducible fraction (F2: 40.61%) and weak acid-soluble fraction (F1: 26.75%). The high CdMF and PbPMF suggesting bioavailability and mobility of Cd in the soil, while the potential mobility of Pb cannot be ignored.
-
(3)
TFe2O3 and Al2O3 are key factors in explaining the accumulation of Cr, Ni, Cu, Zn, As, and Hg in soils, while the total HMs concentration, pH, and TFe2O3 and Al2O3 all have a certain impact on the mobility and potential mobility of HMs in the soil. Key factors influencing the accumulation of Cd and Pb in soils are MnO and DL. Meanwhile, significant enrichment of Cd, Pb, and Zn is observed in soils near the Pb-Zn mineralization belts/points. This indicates that the secondary enrichment during the weathering of carbonate rocks from the Qingxudong Formation play an important role in soil HMs accumulation, especially as the distribution of Pb-Zn mineralization belts/points in the study area exacerbates the accumulation of Cd, Pb, and Zn in the soil. It is particularly noteworthy that the HMs in the soils of the distribution areas of these Pb-Zn mineralization belts/points possess high bioavailability and mobility, and the potential pollution risks thereof should be controlled.
This study elucidates the driving factors influencing the accumulation of HMs in soils within Pb-Zn mineralized areas under a carbonate geological background, providing a reference for identifying the causes of regional soil HMs accumulation and its pollution prevention and control.
Data availability
Data will be made available by contacting the corresponding author.pwu@gzu.edu.cn (Pan Wu).
References
Jin, S. et al. A discussion on the Pb-Zn mineralization regularity and ore prospecting targets in the Kaili-Duyun area, Guizhou province, China. Acta Mineral. Sinica. 38 (06), 675–683 (2018).
Lin, G. et al. High geological background concentrations of as and cd in karstic soils May not contribute to greater risks to human health via rice consumption. J. Hazard. Mater. 480, 135876 (2024).
Baize, D. & Sterckeman, T. Of the necessity of knowledge of the natural pedo-geochemical background content in the evaluation of the contamination of soils by trace elements. Sci. Total Environ. 264 (1), 127–139 (2001).
Zinn, Y. L., Faria J. A., Araujo, M. A. & Skorupa, A. L. A. Soil parent material is the main control on heavy metal concentrations in tropical highlands of Brazil. CATENA, 185, 104319 (2020).
Wen, Y., Li, W., Yang, Z., Zhang, Q. & Ji, J. Enrichment and source identification of cd and other heavy metals in soils ith high geochemical background in the karst region, Southwestern China. Chemosphere 245, 125620 (2020).
Chen, M. et al. Soil-forming accumulation of heavy metals in geological high background areas: constraints of structure, lithology, and overlying soil geochemistry. J. Geochem. Explor. 263, 107518 (2024).
Xia, X., Ji, J., Zhang, C., Yang, Z. & Shi H. Carbonate bedrock control of soil cd background in Southwestern china: its extent and influencing factors based on Spatial analysis. Chemosphere 290, 133390 (2022).
Zhao, F. J., Ma, Y., Zhu, Y., Tang, Z. & Mcgrath, S. P. Soil contamination in china: current status and mitigation strategies. Environ. Sci. Technol. 49 (2), 750 (2015).
Chen, L. et al. Black shale bedrock control of soil heavy metal typical high geological background in China Loushao basin: pollution characteristics, source and influence assessment based on Spatial analysis. J. Hazard. Mater. 477, 135072 (2024).
Quezada-Hinojosa, R. P. & Föllmi, K. B., Verrecchia, E. & Adatte, T. Speciation and multivariable analyses of Geogenic cadmium in soils at Le gurnigel, Swiss Jura mountains. CATENA 125, 10–32 (2015).
Xia, X. et al. Cadmium risk in the soil-plant system caused by weathering of carbonate bedrock. Chemosphere 254, 126799 (2020).
Yang, Q. et al. Distribution and secondary enrichment of heavy metal elements in karstic soils with high geochemical background in guangxi, China. Chem. Geol. 567, 120081 (2021).
Vingiani, S., Di Iorio, E., Colombo, C. & Terribile, F. Integrated study of red mediterranean soils from Southern Italy. CATENA 168, 129–140 (2018).
Yamasaki, S., Takeda, A., Nunohara, K. & Tsuchiya N. Red soils derived from limestone contain higher amounts of trace elements than those derived from various other parent materials. Soil. Sci. Plant. Nutr. 59 (5), 692–699 (2013).
Xu, R. et al. Pollution assessment and risk area delineation of potentially toxic elements in agricultural soils with high geological background through an integrated framework. J. Environ. Chem. Eng. 12 (6), 114177 (2024).
Li, X. et al. Priority areas identification for arable soil pollution prevention based on the accumulative risk of heavy metals. Sci. Total Environ. 954, 176440 (2024).
Zhao, J. et al. Valency distributions and geochemical fractions of arsenic and antimony in non-ferrous smelting soils with varying particle sizes. Ecotoxicol. Environ. Saf. 233, 113312 (2022).
Huang, Z. et al. A study on the Large-Scale Low-Temperature metallogenic domain in Southwestern China—Significance,History and new progress. Acta Mineral. Sinica. 31 (3), 6 (2011).
Voutsis, N., Kelepertzis, E., Tziritis, E. & Kelepertsis A. Assessing the hydrogeochemistry of groundwaters in ophiolite areas of Euboea island, greece, using multivariate statistical methods. J. Geochem. Explor. 159, 79–92 (2015).
Sungur, A., Vural, A., Gundogdu, A. & Soylak, M. Effect of antimonite mineralization area on heavy metal contents and geochemical fractions of agricultural soils in Gümüşhane Province, Turkey. CATENA, 184, 104255 (2020).
Muhammad, S., Shah, M., Tahir & Khan, S. Heavy metal concentrations in soil and wild plants growing around Pb–Zn sulfide terrain in the Kohistan region, Northern Pakistan. Microchem. J. 99 (1), 67–75 (2011).
Chen, Z., Wu, P., Meng, W. & Zeng, X. Effects of weathering of Qingxudong formation on heavy metal accumulation in soil in karst area. Chin. J. Ecol. 38 (12), 7 (2019).
Liu, J. et al. Sedimentary Environment and Lead-Zinc Mineralization of the Qingxudong Formation in the Early Cambrian in Songtao-Heku Area. Mineral Deposits(S1), 2 (2012.).
Chen, G., Qi, A. & Fan Y. Geologic characteristic of the Lead-Zinc deposits in the Eastern Guizhou and analysis on metallogenesis. Guizhou Geol. 22 (4), 8 (2005).
China Environmental Science, HJ/T. 166–2004 the Technical Specification for Soil Environmental Monitoring (HJ/T 166–2004 (China Environmental Science, 2004). (in Chinese).
FAO, World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. (2015). (2014).
Mosley, L. M., Rengasamy, P. & Fitzpatrick, R. Soil pH: techniques, challenges and insights from a global dataset. Eur. J. Soil. Sci., 75(6), e70021 (2024).
Nelson, D. W. & Sommers, L. E. Total Carbon, Organic Carbon, and Organic Matter Methods of Soil Analysis (pp. 961–1010). (1996).
Corral-Pazos-de-Provens, E., Rapp-Arrarás, Í. & Domingo-Santos, J. M. Estimating textural fractions of the USDA using those of the international system: A quantile approach. Geoderma 416, 115783 (2022).
Wang, Y. et al. Distribution of soil heavy metals and their speciation in typical farmlands in Jiangsu Province. Environ. Pollut. 56 (6), 1326–1338 (2024).
Chinese Environmental Protection Agency guidelines & Beijing HJ 889–2017 Soil quality—Determination of cation exchange capacity(CEC)—Hexamminecobalt trichloride solution-Spectrophotometric method (in Chinese). (2017).
Hasan, A. B. et al. Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund upazilla (Bhatiary–Kumira), chittagong, Bangladesh. J. Geochem. Explor. 125, 130–137 (2013).
China National Environmental Monitoring Centre. Elemental Background Values of Soils in China (Environmental Science Press of China, 1990).
Bhuiyan, M. A. H., Karmaker, S. C., Bodrud-Doza, M., Rakib, M. A. & Saha, B. B. Enrichment, sources and ecological risk mapping of heavy metals in agricultural soils of dhaka district employing SOM, PMF and GIS methods. Chemosphere, 263, 128339 (2021).
Ji, Z., Zhang, Y., Zhang, H., Huang, C. & Pei, Y. Fraction Spatial distributions and ecological risk assessment of heavy metals in the sediments of Baiyangdian lake. Ecotoxicol. Environ. Saf. 174, 417–428 (2019).
Rodríguez, L., Ruiz, E., Alonso-Azcárate, J. & Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manage. 90 (2), 1106–1116 (2009).
Madadi, R., Mejjad, N. & Gabriel, D. Enrique, geochemical speciation, ecological risk, and source identification of heavy metal(loid)s in sediments and waters from Musa estuary, Persian Gulf. Mar. Pollut. Bull. 190, 114836 (2023).
Sherene, T. Mobility and transport of heavy metals in polluted soil environment. (2010).
Breiman, L. Random Forests. Mach. Learn. 45(1), 5–32 (2001).
Xu, D., Fu, R., Liu, H. & Guo, X. Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: A critical review. J. Clean. Prod. 286, 124989 (2021).
Nie, S., Chen, H., Sun, X. & An, Y. Spatial distribution prediction of soil heavy metals based on random forest model. Sustainability 16 (11), 4358 (2024).
Zhou, R. et al. A data-driven framework to identify influencing factors for soil heavy metal contaminations using random forest and bivariate local moran’s I: A case study. J. Environ. Manage. 375, 124172 (2025).
Rudnick, R. & Gao, S. Composition of the Continental Crust. (2014).
Xiao, J. et al. New strategy for exploring the accumulation of heavy metals in soils derived from different parent materials in the karst region of Southwestern China. Geoderma 417, 115806 (2022).
Xing, Y. et al. Response relationships among CEC, mechanical compositions and mineral types in typical karst soil. CARSOLOGICA SINICA, 43(5), 1076–1087 (2024).
Lin, W. et al. Dissolved organic matter mediates the interactions between bacterial community and heavy metal fractionation in contaminated coal mine soils. Ecotoxicol. Environ. Saf. 297, 118237 (2025).
Liu, Xu. et al. Transfer mechanism and bioaccumulation risk of potentially toxic elements in soil–rice systems comparing different soil parent materials. Ecotoxicol. Environ. Saf. 216, 112214 (2021).
Wei, X. et al. A large and overlooked cd source in karst areas: the migration and origin of cd during soil formation and erosion. Sci. Total Environ. 895, 165126 (2023).
Wang, Y., Guo, G., Zhang, D. & Lei, M. An integrated method for source apportionment of heavy metal(loid)s in agricultural soils and model uncertainty analysis. Environ. Pollut. 276, 116666 (2021).
Kong, F., Chen, Y., Huang, L., Yang, Z. & Zhu, K. Human health risk visualization of potentially toxic elements in farmland soil: A combined method of source and probability. Ecotoxicol. Environ. Saf. 211, 111922 (2021).
Ji, H. et al. Geochemistry of red residua underlying dolomites in karst terrains of Yunnan-Guizhou plateau: I. The formation of the Pingba profile. Chem. Geol. 203 (1), 1–27 (2004).
Temur, S., Orhan, H. & Delikan, A. Geochemistry of the limestone of Mortas formation and related Terra rossa, seydisehir, konya, Turkey. Geochem. Int. 47, 67–93 (2009).
Bini, R., Hume, P. A. & Croft, J. L. Effects of bicycle saddle height on knee injury risk and cycling performance. Sports Med. 41 (6), 463–476 (2011).
Liu, L. et al. A preliminary study of genesis of Pb-Zn deposits in Guizhou Province. Acta Geologica Sichuan. 39 (01), 54–57 (2019).
Ye, L. et al. Trace and minor elements in sphalerite from base metal deposits in South china: A LA-ICPMS study. Ore Geol. Rev. 39 (4), 188–217 (2011).
Zhou, W. et al. Soil type data provide new methods and insights for heavy metal pollution assessment and driving factors analysis. J. Hazard. Mater. 480, 135868 (2024).
Li, X., Liu, H., Meng, W., Liu, N. & Wu, P. Accumulation and source apportionment of heavy metal(loid)s in agricultural soils based on GIS, SOM and PMF: A case study in superposition areas of geochemical anomalies and zinc smelting, Southwest China. Process Saf. Environ. Prot. 159, 964–977 (2022).
Kabala, C. & Singh, B. R. Fractionation and mobility of copper, lead, and zinc in soil profiles in the vicinity of a copper smelter. J. Environ. Qual. 30 (2), 485-92 (2001).
Dong, S., Li, Longrui, Chen, Weijie, Chen, Zhaoming, Wang, Y. & Wang, S. Evaluation of heavy metal speciation distribution in soil and the accumulation characteristics in wild plants: A study on naturally aged abandoned farmland adjacent to tailings. Sci. Total Environ. 917, 170594 (2024).
Chen, X., Wu, P., Liu, H. & Li, X. Source apportionment of heavy metal(loid)s in sediments of a typical karst mountain drinking-water reservoir and the associated risk assessment based on chemical speciations. Environ. Geochem. Health. 45 (11), 7585–7601 (2023).
Liu, G. et al. Partitioning and geochemical fractions of heavy metals from Geogenic and anthropogenic sources in various soil particle size fractions. Geoderma 312, 104–113 (2018).
Bradl, H. B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 277 (1), 1–18 (2004).
Wang, J., Xu, D., Fu, R. & Chen, J. Bioavailability assessment of heavy metals using various Multi-Element extractants in an Indigenous zinc smelting contaminated site, Southwestern China. Int. J. Environ. Res. Public Health. 18 (16), 8560 (2021).
Yang, J. et al. Cadmium, lead and arsenic contamination in an abandoned nonferrous metal smelting site in Southern china: chemical speciation and mobility. Ecotoxicol. Environ. Saf. 239, 113617 (2022).
Ghayoraneh, M. & Qishlaqi, A. Concentration, distribution and speciation of toxic metals in soils along a transect around a zn/pb smelter in the Northwest of Iran. J. Geochem. Explor. 180, 1–14 (2017).
Mouni, L., Belkhiri, L., Bouzaza, A. & Bollinger J.C. Interactions between cd, cu, pb, and Zn and four different mine soils. Arab. J. Geosci. 10 (4), 77 (2017).
Guo, G., Yuan, T., Wang, W., Li, D. & Zhou, P. Bioavailability, mobility, and toxicity of Cu in soils around the dexing Cu mine in China. Environ. Geochem. Health. 33 (2), 217–224 (2011).
Zornoza, R. et al. The effect of former mining activities on contamination dynamics in sediments, surface water and vegetation in El avenque stream, SE Spain. Water Air Soil. Pollution. 223 (2), 519–532 (2012).
Zhang, Q. & Han, G. Speciation characteristics and risk assessment of soil heavy metals from puding karst critical zone, Guizhou Province. Environ. Sci. 43 (06), 3269–3277 (2022).
Wang, Y. et al. Available heavy metals concentrations in agricultural soils: relationship with soil properties and total heavy metals concentrations in different industries. J. Hazard. Mater. 471, 134410 (2024).
Li, S., Li, M., Sun, H., Li, H. & Ma, L. Q. Lead bioavailability in different fractions of mining- and smelting-contaminated soils based on a sequential extraction and mouse kidney model. Environ. Pollut. 262, 114253 (2020).
Agnieszka, J. & Barbara, G. Chromium, nickel and vanadium mobility in soils derived from fluvioglacial sands. J. Hazard. Mater. 237–238, 315–322 (2012).
Wei, W., Ling, S., Wu, X. & Li, X. Geochemical accumulation and source tracing of heavy metals in arable soils from a black shale catchment, Southwestern China. Sci. Total Environ. 857, 159467 (2023).
Ke, X. et al. Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe river protected area, China. Chemosphere 175, 473–481 (2017).
Guan, Q. et al. Source apportionment of heavy metals in agricultural soil based on PMF: A case study in Hexi corridor, Northwest China. Chemosphere 193, 189–197 (2018).
Feng, X., Li, G. & Qiu, G. A preliminary study on mercury contamination to the environment from artisanal zinc smelting using Indigenous methods in Hezhang county, guizhou, China—Part 1: mercury emission from zinc smelting and its influences on the surface waters. Atmos. Environ. 38 (36), 6223–6230 (2004).
Zhang, H. et al. Use of machine-learning and receptor models for prediction and source apportionment of heavy metals in coastal reclaimed soils. Ecol. Ind. 122, 107233 (2021).
Gustafsson, J., Persson, I., Oromieh, A. G. & Schaik, J. W. J. Chromium(III) complexation to natural organic matter: mechanisms and modeling. Environ. Sci. Technol. 48 (3), 1753–1761 (2014).
Fischel, M. H. H., Clarke, C. E. & Sparks, D. L. Arsenic sorption and oxidation by natural manganese-oxide-enriched soils: reaction kinetics respond to varying environmental conditions. Geoderma 441, 116715 (2024).
Han, J. & Katz, L. E. Capturing the variable reactivity of goethites in surface complexation modeling by correlating model parameters with specific surface area. Geochim. Cosmochim. Acta. 244, 248–263 (2019).
Ni, S. et al. Enrichment of heavy metal elements and their adsorption on iron oxides during carbonate rock weathering process. Prog. Nat. Sci. 19 (9), 1133–1139 (2009).
Sipos, P., Németh, T., Kis, V. K. & Mohai, I. Association of individual soil mineral constituents and heavy metals as studied by sorption experiments and analytical electron microscopy analyses. J. Hazard. Mater., 168(2), 1512–1520 (2009).
Suda, A. & Makino, T. Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review. Geoderma 270, 68–75 (2016).
Shi, J., Zhao, D., Ren, F. & Huang, L. Spatiotemporal variation of soil heavy metals in china: the pollution status and risk assessment. Sci. Total Environ. 871, 161768 (2023).
Swedlund, P. J., Webster, Jenny, G. & Miskelly, G. M. Goethite adsorption of Cu(II), Pb(II), Cd(II), and Zn(II) in the presence of sulfate: properties of the ternary complex. Geochim. Cosmochim. Acta. 73 (6), 1548–1562 (2009).
Wang, C. et al. Effects of soil properties on the transfer of cadmium from soil to wheat in the Yangtze river Delta region, China—a typical Industry–Agriculture transition area. Biol. Trace Elem. Res. 148 (2), 264–274 (2012).
Tack, F. M. G., Vanhaesckebroe, T., Verloo, M. G., Rompaey, K. V. & Ranst, E. V. Mercury baseline levels in Flemish soils (Belgium). Environ. Pollut. 134 (1), 173–179 (2005).
Cipullo, S., Snapir, B., Campo, P., Prpich, G. & Coulon, F. Insights into mixed contaminants interactions and its implication for heavy metals and metalloids mobility, bioavailability and risk assessment. Sci. Total Environ. 645, 662–673 (2018).
Loganathan, P., Vigneswaran, S., Kandasamy, J. & Naidu, R. Cadmium sorption and desorption in soils: A review. Crit. Rev. Environ. Sci. Technol. 42, 489–533 (2012).
Nabuyanda, M. M., Kelderman, P., Sankura, M. G. & Irvine, K. Investigating the effect of Eh and pH on binding forms of co, cu, and Pb in wetland sediments from Zambia. J. Environ. Manage. 319, 115543 (2022).
Kou, B. et al. The relationships between heavy metals and bacterial communities in a coal gangue site. Environ. Pollut. 322, 121136 (2023).
Qu, C. et al. Heavy metal behaviour at mineral-organo interfaces: mechanisms, modelling and influence factors. Environ. Int. 131, 104995 (2019).
Chen, J. et al. Heavy metal(loid)s migration mechanisms during soil erosion: A systematic quantitative review. Int. Soil. Water Conserv. Res. 13 (2), 410–421 (2025).
Park, J. H. et al. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 185(2), 549–574 (2011).
Esfandbod, M., Forghani, A., Adhami, E. & Rashti, M. R. The role of CEC and pH in cd retention from soils of North of Iran. J. Soil. Contam. 20 (8), 908–920 (2011).
Marques, J. J., Schulze, D. G., Curi, N. & Mertzman, S. A. Trace element geochemistry in Brazilian Cerrado soils. Geoderma 121 (1), 31–43 (2004).
Gloaguen, T. V. & Passe, J. J. Importance of lithology in defining natural background concentrations of cr, cu, ni, Pb and Zn in sedimentary soils, Northeastern Brazil. Chemosphere 186, 31–42 (2017).
Fernandes, A., et al. Quality reference values and background concentrations of potentially toxic elements in soils from the Eastern Amazon, Brazil. J. Geochem. Explor. , 190, 453–463 (2018).
Guevara, M. et al. Soil Organic Carbon Across Mexico and the Conterminous United States (1991–2010). Global Biogeochemical Cycles. 34(3), eGB006219 (2020). (2019).
Kurtz, A. C., Derry, L. A., Chadwick, O. A. & Alfano M. J. Refractory element mobility in volcanic soils. Geology 28 (28), 279–282 (2000).
He, S., et al. Factors controlling cadmium and lead activities in different parent material-derived soils from the Pearl river basin. Chemosphere 182, 509–516 (2017).
Tahervand, S. & Jalali, M. Sorption and desorption of potentially toxic metals (Cd, cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 181, 148–159 (2017).
Luo, Z., He, J., Polle, A. & Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and Woody plants: paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 34 (6), 1131–1148 (2016).
Ji, W. et al. Potential ecological risk assessment of heavy metals in the Fe–Mn nodules in the karst area of guangxi, Southwest China. Bull. Environ Contam. Toxicol. 106 (1), 51–56 (2021).
Acknowledgements
This study was financially supported by the High-Level Talent Training Program in Guizhou Province (GCC [2023] 045).
Author information
Authors and Affiliations
Contributions
Zhenglu Yu: Data curation, Writing-original draft, Visualization. Xuexian Li: Validation, Writing-review & editing. Pan Wu: Validation, Visualization, Conceptualization, Writing-review & editing, Project administration, Funding acquisition. Zhiwei Han: Validation, Writing-review & editing. Jian Zhu: Validation, Writing-review & editing. Manzhi Chen: Writing-review & editing. Zhuo Chen: Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, Z., Li, X., Wu, P. et al. Effect of lead zinc mineralization area on heavy metals accumulation and geochemical fractions of agricultural soils in Southwest China. Sci Rep 15, 19196 (2025). https://doi.org/10.1038/s41598-025-04993-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04993-3