Abstract
Ischemic stroke has various etiologies, and its pathogenesis is influenced by a range of factors. In addition to systemic factors, such as hypertension and diabetes mellitus, blood flow and blood viscosity are also known to contribute to the development of ischemic stroke. The aim of this retrospective study was to investigate the impact of blood viscosity on ischemic stroke, according to subtypes. The medical records of 298 patients admitted to St. Vincent’s Hospital with acute ischemic stroke between January 2020 and May 2022 were analyzed. Comprehensive diagnostic procedures, including blood viscosity tests, brain imaging, and etiology workups, were conducted and reviewed. Clinical and laboratory factors, including diastolic blood viscosity, were compared among patients with different subtypes of ischemic stroke. Factors associated with diastolic blood viscosity were identified. The average age of the patients was 68.6 ± 13.4 years, and there was a higher proportion of females (54.7%). Diastolic blood viscosity was higher in patients with small vessel disease and those with old lacunar infarctions or cerebral microbleeds than in those with white matter hyperintense lesions or significant stenosis. However, multivariable analysis revealed no significant associations between diastolic blood viscosity and stroke subtype of index stroke or any imaging markers. Instead, diastolic blood viscosity was independently associated with hypertension and high levels of C-reactive protein. In patients with acute stroke, diastolic blood viscosity showed a statistically significant relation with hypertension and C-reactive protein and also tended to be associated with old lacunar infarction and cerebral microbleeds.
Similar content being viewed by others
Introduction
Ischemic stroke has diverse etiologies. It contributes significantly to global morbidity and mortality and imposes a substantial socioeconomic burden. Various factors influence the occurrence and prognosis of ischemic stroke. Risk factors, such as age, diabetes mellitus, hypertension, alcohol consumption, and smoking, are well established, regardless of etiology1. In addition to systemic factors, local vascular factors also contribute to the development of ischemic stroke. Notably, large artery and small vessel occlusions account for > 40% of ischemic strokes, despite being attributed to different pathophysiological mechanisms2.
Hemodynamic factors interact with the vascular endothelium, with endothelial dysfunction playing a crucial role in the development of atherosclerosis3. Endothelial cells respond to the mechanical forces acting on the blood vessels, leading to increased expression of adhesion molecules, decreased endothelial nitric oxide production, and increased superoxide anion production, all of which contribute to atherogenesis. The ejection of blood from the heart generates pulsatility, which can exacerbate endothelial oxidative stress4. Additionally, vascular geometry changes the direction of blood flow, creating larger atherogenic areas5. Regions with reduced hemodynamic shear stress have increased levels of reactive oxygen species, which promote inflammation. Moreover, a reduced rate of endothelial cell repair contributes to the formation of atherosclerotic plaques.
Blood viscosity is a critical factor influencing hemodynamic parameters. An increase in the number of blood cells or plasma components increases blood viscosity, thereby increasing the risk of thromboembolism6,7. Most research on the effects of hemodynamic parameters has focused on large cerebral arteries, such as the carotid artery8. The impact of the direction and force of blood flow on small cerebral vessels is believed to differ from that on large arteries; however, assessing and visualizing these effects in small vessels is more challenging. Previous studies9,10 have reported associations between blood viscosity and ischemic stroke, especially in cases of small vessel occlusion, as well as with early neurological deterioration. The aim of this study was to explore the relationship between blood viscosity and stroke subtypes, in addition to intima-media thickness (IMT), which is a marker of systemic atherosclerosis.
Materials and methods
Patients
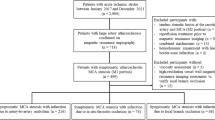
The medical records of patients admitted to the emergency department of St. Vincent’s Hospital, Suwon, South Korea, with acute ischemic stroke within one week of symptom onset between January 2020 and May 2022 were retrospectively analyzed. Patients who underwent brain imaging and blood viscosity testing were included. Patients lacking sufficient clinical or imaging data were excluded. All patients underwent standard evaluation for acute ischemic stroke, including blood tests, electrocardiography, and brain imaging.
The studies involving human participants were reviewed and approved by the Institutional Review Board of St. Vincent’s Hospital (VC22RASI0295). The requirement for written informed consent was waived owing to the retrospective nature of the study. All methods related to the study were performed in accordance with the guidelines and regulations of the institution.
Stroke imaging and etiology workups
Patients with acute ischemic stroke underwent brain magnetic resonance imaging, including diffusion-weighted imaging and magnetic resonance angiography, using a 3.0-T magnetic resonance imaging scanner (Ingenia, Philips Healthcare, Best, The Netherlands). Non-contrast computed tomography and computed tomography angiography (Discovery CT 750 HD; GE Healthcare, Chicago, IL, USA) were performed when magnetic resonance imaging and magnetic resonance angiography were not available.
The location of the acute ischemic lesion was identified by diffusion-weighted imaging. Stroke subtypes (large artery atherosclerotic stroke, cardioembolic stroke, small-vessel occlusion, or stroke of other determined etiology) were classified according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria11. To identify a cardioembolic source, transthoracic echocardiography and 24-hour electrocardiogram monitoring (Holter) were performed.
Imaging biomarkers of small vessel disease and large artery atherosclerosis, including large artery stenosis and IMT, were evaluated. Stenosis of > 50% in diameter on magnetic resonance angiography or computed tomography angiography was considered significant. Basilar artery, common carotid artery, internal carotid artery, vertebral artery, and anterior/middle/posterior cerebral artery stenosis was assessed. Carotid artery duplex ultrasonography was performed using an ACUSON S1000 Ultrasound System (Siemens, Munich, Germany). IMT was measured in the common carotid arteries, according to the Mannheim IMT Consensus12. The mean IMT of the bilateral common carotid arteries was calculated for analysis.
For the detection of small vessel disease, white matter hyperintensity and old lacunar infarctions (determined by T2-weighted imaging), and cerebral microbleeds (small round low signal intensity lesions in gradient-echo images or susceptibility-weighted images) were investigated.
Blood viscosity
Blood viscosity was measured using a computerized scanning capillary-tube viscometer (Hemovister, Pharmode Inc., Seoul, Republic of Korea). Approximately 3 mL of whole blood was collected from a peripheral vein into an ethylenediaminetetraacetic acid tube for testing. The viscometer operates by running the whole blood, which exhibits non-Newtonian characteristics, through a U-shaped tube and measuring viscosity based on the changes in the heights of the columns on both sides of the tube.
High shear rate viscosity (systolic blood viscosity [SBV]) was measured at 300 s⁻¹, whereas low shear rate viscosity (diastolic blood viscosity [DBV]) was measured at 5 s⁻¹. DBV was used for analysis.
Statistical analysis
The relationships between stroke subtypes and DBV were analyzed using one-way ANOVA. DBV was also analyzed according to the presence of large artery atherosclerosis or small vessel disease markers. Categorical variables are presented as n (%), while continuous variables are presented as mean ± SD. Pearson correlation analysis and multiple logistic regression analysis were used to examine factors associated with DBV. All statistical analyses were conducted using SPSS (version 27.0; IBM Corp., Armonk, NY, USA). P-values < 0.05 were considered statistically significant.
Results
Patient characteristics
During the study period, a total of 298 patients were enrolled. The mean age of the patients was 68.57 ± 13.43 years, with female patients (n = 163) comprising 54.7% of the cohort. Hypertension and diabetes mellitus were identified in 189 (63.9%) and 170 (57%) patients, respectively. White matter hyperintensity was observed in 201 (67.4%) patients. Old lacunar infarctions and cerebral microbleeds were observed in 164 (55%) and 120 (40.4%) patients, respectively. The subtype of stroke was small vessel occlusion in 105 (35.2%) patients, large artery disease in 102 (34.2%), cardiogenic embolism in 44 (14.8%), and undetermined in 47 (15.8%) (Table 1). The mean SBV was 3.86 ± 0.732 cP. The mean DBV was 11.63 ± 2.865 cP.
Blood viscosity according to stroke subtype and imaging biomarkers
DBV was highest in patients with small vessel occlusion (13.03 ± 3.199), followed by those with undetermined etiology (11.13 ± 2.865), large artery disease (11.06 ± 2.333), and cardiogenic embolism (10.13 ± 2.252) (P < 0.001; Table 2). There was no significant association between DBV and white matter hyperintensity (P > 0.05). However, DBV was found to be significantly higher in patients with old lacunar infarctions compared to those without such infarctions (12.19 ± 2.917 vs. 10.94 ± 2.63, respectively; P < 0.001). DBV was also found to be significantly higher in patients with cerebral microbleeds compared to those without such microbleeds (12.32 ± 3.020 vs. 11.16 ± 2.672, respectively; P = 0.001). There was no association between DBV and large artery stenosis, regardless of the presence or absence of stenosis.
Factors associated with DBV
Statistically significant associations were observed between DBV and SBV (r = 0.965; P < 0.05), HbA1c (r = 0.227; P < 0.05), and creatinine (r=-0.123; P < 0.05). Total cholesterol (r=-0.213; P < 0.05), low-density lipoprotein (r = 0.203; P < 0.05), and high-density lipoprotein (r = 0.118; P < 0.05) were also significantly associated with DBV. No association was observed between DBV and IMT (Table 3).
In multivariable analysis, DBV was independently associated with SBV (P < 0.001), C-reactive protein (P < 0.001), and hypertension (P = 0.047). No significant associations were observed between DBV and stroke subtypes or brain imaging findings (Table 4).
Discussion
Blood viscosity is closely related to blood components, such as the aggregation of red blood cells and hematocrit13. Blood viscosity in large vessels affects flow direction and force, which affects the occurrence and progression of large vessel atherosclerosis and contributes to the incidence of thromboembolic events by promoting a prothrombotic state14. Additionally, blood flow is reduced in the microcirculation, creating a chronic ischemic state or thrombotic occlusion due to microthrombus formation. Previous studies10,15 have reported that blood viscosity significantly increased in acute stroke patients with small vessel occlusion. Additional studies16,17 have showed that a correlation exists between a significant increase in blood viscosity and the effect of antiplatelet drugs in patients with acute coronary syndrome.
Although previous studies have investigated blood dilution therapy as a means to reduce blood viscosity, and the outcomes were not particularly effective, this does not necessarily indicate that blood viscosity plays no role in the pathophysiology of acute stroke18.
In this study, we confirmed that, in patients with acute ischemic stroke, high DBV was associated with a higher prevalence of old lacunar infarctions. In small vessel disease, the blood vessel diameter narrows due to arteriolar lipohyalinosis. Increased DBV in an environment with reduced contractibility function promotes vascular occlusion. Cerebral microbleeds were also associated with high DBV. While cerebral microbleeds are a type of small vessel disease, their pathophysiological mechanisms are influenced more by perforating vessel ruptures than by lacunar infarctions. Our study confirmed that cerebral microbleeds, as well as lacunar infarctions, were associated with DBV in patients with small vessel disease, which has not been reported previously. Old lacunar infarctions and cerebral microbleeds are among the most readily identifiable manifestations of small vessel disease on neuroimaging. In contrast, white matter hyperintensity is often more challenging to interpret, particularly in the presence of encephalomalacia due to prior large infarctions or traumatic brain injury. Moreover, white matter hyperintensity can also be observed in post-inflammatory states, making it difficult to attribute their presence solely to ischemic burden. This factor is likely to have significantly influenced the statistical significance observed between DBV and white matter hyperintensity. Additionally, sex and diabetes mellitus were also associated with DBV. Hypertension was independently associated with DBV in the multivariable analysis.
While this study is retrospective in nature, the timing, method, and types of tests for patients with acute ischemic stroke were systematically established. Blood sampling is performed immediately upon the patient’s arrival to the emergency department and is completed during the process of establishing intravenous (IV) access for the administration of fluids and medications. Therefore, in the emergency department, rapid blood viscosity testing is possible, ensuring that the effects of antiplatelet loading and hemodilution due to massive hydration were just as well controlled as they would be in prospective studies. Carotid IMT, which had not been analyzed in previous studies, was analyzed in this study. No significant association with common carotid IMT, a marker of systemic atherosclerosis, was observed. Another notable finding was the variation in blood viscosity testing according to age. While changes in SBV according to age were minimal, significant variation was observed in DBV with age. Additionally, DBV tended to decrease in older patients although not significantly. This trend may be explained by alterations in plasma components and fluctuations in blood pressure observed in older individuals. Further research is needed to examine the variation in blood viscosity testing according to age.
Conclusions
Among the patients with acute ischemic stroke, DBV was significantly higher in those with hypertension. DBV was associated with old lacunar infarctions and cerebral microbleeds, but not the subtype of the index stroke, large vessel atherosclerosis, and carotid IMT. Further research is needed to confirm the results of this study.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Data can be requested through the author (Sang-mi Noh) if necessary.
Abbreviations
- DBV:
-
Diastolic blood viscosity
- IMT:
-
Intima-media thickness
- SBV:
-
Systolic blood viscosity
References
Boehme, A. K., Esenwa, C. & Elkind, M. S. Stroke risk factors, genetics, and prevention. Circ. Res. 120, 472–495. https://doi.org/10.1161/CIRCRESAHA.116.308398 (2017).
Schneider, A. T. et al. Ischemic stroke subtypes: A population-based study of incidence rates among Blacks and Whites. Stroke 35, 1552–1556. https://doi.org/10.1161/01.STR.0000129335.28301.f5 (2004).
Gimbrone, M. A. Endothelial dysfunction, hemodynamic forces, and atherosclerosis. Thromb. Haemost. 82, 722–726. https://doi.org/10.1055/s-0037-1615903 (1999).
Silacci, P., Desgeorges, A., Mazzolai, L., Chambaz, C. & Hayoz, D. Flow pulsatility is a critical determinant of oxidative stress in endothelial cells. Hypertension 38, 1162–1166. https://doi.org/10.1161/hy1101.095993 (2001).
Cunningham, K. S. & Gotlieb, A. I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 85, 9–23. https://doi.org/10.1038/labinvest.3700215 (2005).
Lowe, G. D., Lee, A. J., Rumley, A., Price, J. F. & Fowkes, F. G. Blood viscosity and risk of cardiovascular events: The Edinburgh artery study. Br. J. Haematol. 96, 168–173. https://doi.org/10.1046/j.1365-2141.1997.8532481.x (1997).
Devereux, R. B. et al. Possible role of increased blood viscosity in the hemodynamics of systemic hypertension. Am. J. Cardiol. 85, 1265–1268. https://doi.org/10.1016/s0002-9149(00)00744-x (2000).
Gnasso, A. et al. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation 94, 3257–3262. https://doi.org/10.1161/01.cir.94.12.3257 (1996).
Lee, H., Heo, J., Lee, I. H., Kim, Y. D. & Nam, H. S. Association between blood viscosity and early neurological deterioration in lacunar infarction. Front. Neurol. 13, 979073. https://doi.org/10.3389/fneur.2022.979073 (2022).
Furukawa, K. et al. Increased blood viscosity in ischemic stroke patients with small artery occlusion measured by an electromagnetic spinning sphere viscometer. J. Stroke Cerebrovasc. Dis. 25, 2762–2769. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.07.031 (2016).
Adams, H. P. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 24, 35–41. https://doi.org/10.1161/01.str.24.1.35 (1993).
Touboul, P. J. et al. Mannheim intima-media thickness consensus. Cerebrovasc. Dis. 18, 346–349. https://doi.org/10.1159/000081812 (2004).
Cho, Y. I., Cho, D. J. & Rosenson, R. S. Endothelial shear stress and blood viscosity in peripheral arterial disease. Curr. Atheroscler Rep. 16, 404. https://doi.org/10.1007/s11883-014-0404-6 (2014).
Cowan, A. Q., Cho, D. J. & Rosenson, R. S. Importance of blood rheology in the pathophysiology of atherothrombosis. Cardiovasc. Drugs Ther. 26, 339–348. https://doi.org/10.1007/s10557-012-6402-4 (2012).
Song, S. H. et al. Elevated blood viscosity is associated with cerebral small vessel disease in patients with acute ischemic stroke. BMC Neurol. 17, 20. https://doi.org/10.1186/s12883-017-0808-3 (2017).
Jung, L. Y. et al. Rosuvastatin reduces blood viscosity in patients with acute coronary syndrome. Korean Circ. J. 46, 147–153. https://doi.org/10.4070/kcj.2016.46.2.147 (2016).
Li, X., Wang, Q., Xue, Y., Chen, J. & Lv, Q. Ticagrelor compared with clopidogrel increased adenosine and Cyclic adenosine monophosphate plasma concentration in acute coronary syndrome patients. Basic. Clin. Pharmacol. Toxicol. 120, 610–614. https://doi.org/10.1111/bcpt.12752 (2017).
Chang, T. S. & Jensen, M. B. Hemodilution for acute ischemic stroke. Stroke 46(1), e4–e5. https://doi.org/10.1161/STROKEAHA.114.007365 (2015).
Funding
The authors declare that this study received funding from the Catholic Medical Center Research Foundation (2022). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
S.M.N. contributed to the design and implementation of the research and to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Noh, SM. Clinical significance of blood viscosity in patients with acute ischemic stroke. Sci Rep 15, 22424 (2025). https://doi.org/10.1038/s41598-025-04998-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04998-y