Abstract
Sepsis significantly impacts the circulatory system and is associated with high mortality rates, particularly in patients with septic shock who require urgent treatment. Non-invasive cardiac output monitoring is a critical bedside tool for assessing fluid responsiveness. This study aimed to evaluate the agreement between cardiac output measurements obtained from the carotid artery and the left ventricular outflow tract (LVOT) in patients with septic shock in the emergency department (ED). A prospective observational study was conducted on adult patients diagnosed with septic shock and admitted to the ED between October 2023 and October 2024. Cardiac output was calculated using the standard formula (CO = VTI × cross-sectional area × heart rate) for both LVOT and carotid measurements. Agreement between LVOT-derived and carotid-derived cardiac output was assessed using Lin’s concordance correlation coefficient, intraclass correlation coefficient, Bland-Altman analysis, and percentage error. Forty patients with septic shock were included in the study. The mean carotid blood flow was 0.855 L/min, while the mean cardiac output measured by LVOT echocardiography was 5.329 L/min. Cardiac output measurements derived from carotid artery VTI and LVOT VTI showed a moderate agreement, as demonstrated by Lin’s Concordance Correlation Coefficient of 0.527 (p < 0.001) and an Intraclass Correlation Coefficient (absolute agreement) of 0.695 (p < 0.001). Bland–Altman analysis revealed a bias of − 0.47 (95% CI: −2.11 to 1.17), with a concordance interval ranging from − 10.51 (95% CI: −13.35 to − 7.67) to 9.58 (95% CI: 6.74 to 12.42). Non-invasive cardiac output measurements from the carotid artery exhibited only moderate agreement with those derived from the LVOT, accompanied by wide limits of agreement. This indicates that the two methods should not be utilized interchangeably for clinical decision-making in individual patients. Carotid artery measurements should not be regarded as a direct replacement for LVOT examinations.
Similar content being viewed by others
Introduction
Sepsis is characterized by an abnormal response of multiple organs, while septic shock represents a toxic state resulting in severe circulatory, cellular, and metabolic failure, substantially increasing mortality risk in the emergency department (ED)1,2,3.
Hemodynamic monitoring and cardiac output quantification are cornerstones in the management of septic shock patients. Recent evidence the appropriate administration of fluids in patients with septic shock significantly influences treatment outcomes4. Insufficient fluid administration can lead to renal failure and higher mortality rates, whereas excessive fluid delivery can result in organ edema, exacerbation of renal failure, cardiac failure, prolonged intensive care unit stays, extended ventilator usage, and increased mortality5,6. Therefore, determining the optimal fluid volume to improve cardiac output is critical in resuscitating patients with shock7.
The gold standard method for cardiac output assessment, pulmonary thermodilution, involves the insertion of a pulmonary artery catheter. However, this technique is complex, associated with a high risk of complications, and not feasible in the ED setting8.
Ultrasound-guided cardiac output assessment has transformed resuscitation strategies in septic shock. Ultrasound-based cardiac output monitoring has been shown to reduce fluid overload by 23% in septic patients, a critical advantage given the established link between fluid overload and increased mortality in this population4. The left ventricular outflow tract (LVOT) technique involves measuring the LVOT velocity time integral (VTI) and the LVOT diameter to estimate cardiac output. This method has demonstrated comparable results to conventional techniques9,10,11. However, it may not be practical for all patients, as accurate measurements can be challenging in individuals with narrow intercostal spaces. In such cases, assessing cardiac output using ultrasound of the carotid artery offers a more accessible option.
Previous studies have indicated that ultrasound-based cardiac output measurements from the carotid artery yield results comparable to standard methods in various settings. Carotid artery VTI offers several potential advantages over LVOT VTI for cardiac output assessment in the emergency setting. First, the superficial location of the carotid artery makes it more accessible for imaging, with reported feasibility rates of 92–96% compared to 78–85% for LVOT imaging in critically ill patients10. Moreover, carotid measurements require less technical expertise and can be performed more rapidly (mean time 45 s vs. 118 s for LVOT), a crucial consideration in time-sensitive resuscitation scenarios12. However, there is limited published data on non-invasive cardiac output monitoring in the ED. Additionally, while extensive research focuses on individuals with heterogeneous shock populations or healthy populations, septic shock patients remain a key area of study in critical care settings12,13,14,15,16,17,18. The primary aim of this study is to investigate the agreement between cardiac output measurements obtained using LVOT VTI and common carotid artery VTI in patients with septic shock, providing valuable insights for healthcare professionals in assessing fluid responsiveness in this population especially in resource limited country where sophisticated hemodynamic monitoring may be inaccessible, or equipment capabilities are restricted.
Methods
Design and setting
This single-center prospective observational study was conducted in the Department of Emergency Medicine at Khon Kaen University from October 2023 to October 2024. The hospital is a leading urban academic medical institution in northeastern Thailand, managing approximately 60,000 to 70,000 emergency patients annually.
Patients
Consecutive patients who met the eligibility criteria established by the attending physician in the emergency department during the study period were enrolled, which included patients aged 18 years or older with sepsis and hypotension necessitating vasopressors. Exclusion criteria included pregnancy, cardiac arrhythmias, aortic valve disease, aortic dissection, carotid stenosis greater than 50%, and mechanical ventilation.
Data collection
Demographic information was recorded for all enrolled patients. Cardiac output assessment was performed following vasopressor administration within 15 min of achieving a target systolic blood pressure of > 90 mmHg or a mean arterial pressure of > 65 mmHg. The assessments were conducted by experienced emergency physicians with over five years of practice in point-of-care ultrasonography.
All ultrasound measurements were carried out using the Mindray M9 ultrasonography apparatus (Mindray, Shenzhen, China). Patients were not sedated for the ultrasound procedure. For evaluating common carotid artery blood flow parameters, the Mindray L14-6Ns linear array transducer, operating at a frequency range of 6.0–14.0 MHz, was utilized. Cardiac ultrasonography was performed using the Mindray SP5-1s phased array transducer, operating at a frequency range of 1.0–5.0 MHz, and the pulse wave Doppler was applied to measure LVOT VTI in an apical five-chamber view. Cardiac output measurements using both the LVOT and carotid artery techniques were performed for all eligible patients.
In the LVOT technique, patients were set up in a supine position, and a parasternal long-axis view was acquired to assess the LVOT diameter. Utilize the zoom function and freeze the screen when the physician visualizes the aortic valve at mid-systole, then manually measure the LVOT diameter adjacent to the aortic annulus at the base of the leaflets. The apical five-chamber view was used to acquire VTI. Place the pulse wave Doppler gate at the LVOT in the apical five-chamber view at the aortic annulus, or the base of the aortic valve leaflets. Activate pulse wave Doppler. Our study measured LVOT VTI over 3–5 cardiac consecutive cardiac cycles and averaged it. Employ the cardiac calculation package to assess the LVOT VTI. Subsequently, define the contour of one of the systolic waveforms. The device will subsequently calculate the LVOT VTI. Cardiac output was calculated by the ultrasound machine based on the heart rate.
To assess the carotid arteries, patients stayed in the supine position. The carotid vascular preset was utilized. The common carotid artery was assessed in both transverse and longitudinal planes, beginning with the right carotid artery. Spectral Doppler tracings were acquired by positioning a 0.5 mm sample gate at the center of the vessel, 2–3 cm proximal to the carotid bulb in the longitudinal plane. The angle correction cursor was aligned parallel to the direction of blood flow. The VTI of the Doppler signal was assessed through manual tracings of a singular waveform. The waveform was recorded during end-expiration, exhibiting a distinct systolic upstroke with well outlined edges. The waveform is subsequently traced during the systolic phase, from the onset of the upstroke to the dicrotic notch, to define the interval for VTI calculation. The intimal-to-intimal carotid diameter was assessed at the cricoid cartilage level. The ultrasound machine computed cardiac output according to the heart rate. However, if the physician is unable to operate on the right carotid artery, the physician proceeded with the left side instead. All measurements, both carotid and LVOT were performed during end-expiration.
Analysis and statistics
The sample size for evaluating agreement between two measurement methods using the Bland-Altman method was calculated19. Based on previous studies18, a sample size of 40 was determined using a limit of agreement of 3.6, a standard deviation of 0.89, and a K2 value of 4.04.
Data were presented as mean ± standard deviation (SD), median with interquartile range (IQR), or frequency counts and percentages, as appropriate. The Bland-Altman method, Lin’s concordance correlation coefficient, and the intraclass correlation coefficient were used to assess the agreement between the velocity time integral (VTI) measurements obtained from the left ventricular outflow tract (LVOT) and those from the common carotid artery. Data analysis was performed using IBM SPSS for Windows, version 27.0, licensed to Khon Kaen University (SPSS Inc., Chicago, Illinois, USA). A p-value of less than 0.05 was considered statistically significant.
The Bland-Altman analysis was conducted to assess the agreement between carotid artery VTI and LVOT VTI measurements. This method calculates the mean difference (bias) between the two measurement techniques and the limits of agreement, defined as the bias ± 1.96 standard deviations of the differences. The limits of agreement represent the range within which 95% of the differences between measurements from the two methods are expected to lie. Percentage error was calculated using the formula20: Percentage Error = (1.96 × SD of the difference) / mean of the reference method × 100%.
Results
Baseline characteristics
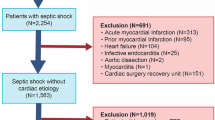
Over the course of one year, sixty-nine patients were screened; four patients exhibited inadequate visualization of the left ventricular outflow tract (5.7%), whereas carotid artery measurements were successfully obtained. Consequently, 40 patients were enrolled. This was presented in Fig. 1. The average age of participants was 67.79 years (SD 15.44). The cohort comprised 41.03% males and 58.97% females. For the LVOT technique, the median cardiac output was 5.329 L/min, with an interquartile range (IQR) from 4.110 to 6.793. For the carotid artery technique, the mean carotid blood flow was 0.855 L/min (SD 0.235). Demographic parameters are presented in Table 1.
Outcome
Lin’s Concordance Correlation Coefficient was 0.527 (p < 0.001), and the Intraclass Correlation Coefficient (absolute agreement) was 0.695 (p < 0.001). Bland–Altman analysis revealed a moderate level of agreement between the velocity time integral (VTI) measurements obtained from the left ventricular outflow tract (LVOT) and those from the common carotid artery, with a bias of − 0.47 (95% CI: −2.11 to 1.17), and a concordance interval ranging from − 10.51 (95% CI: −13.35 to − 7.67) to 9.58 (95% CI: 6.74 to 12.42). The percentage error using the formula: Percentage Error = (1.96 × SD of the difference) / mean of the reference method × 100%. Based on our data: 1.96 × SD of the difference between methods = 20.09 cm. Mean of the LVOT VTI (reference method) = 22.98 cm. The percentage error was 87.4%. These results are shown in Table 2; Fig. 2.
Discussion
Currently, point-of-care ultrasound serves as a bedside instrument for managing critically ill patients in the emergency department, significantly influencing patient outcomes21,22,23,24. This prospective observational study evaluated the agreement between cardiac output measurement techniques using left ventricular outflow tract (LVOT) velocity time integral (VTI) and common carotid artery parameters in patients with septic shock as possible indicators of cardiac function; however, carotid blood flow alone is not representative of total cardiac output.
The Bland-Altman analysis in our study revealed a bias of -0.47 cm with limits of agreement ranging from − 10.51 to 9.58 cm. These limits of agreement indicate that for an individual patient, the carotid artery VTI measurement could differ from the LVOT VTI measurement by as much as 10.51 cm below or 9.58 cm above. This results in a percentage error of 87.4%, significantly exceeding the advised threshold of 30% for clinical interchangeability as determined by Critchley and Critchley. The extensive limits of agreement observed in our study (-10.51 to 9.58 cm) indicate that carotid artery VTI measurements cannot be dependably utilized interchangeably with LVOT VTI measurements on an individual patient level. Such wide variability would be clinically significant and potentially concerning when making treatment decisions based on absolute VTI values, particularly in hemodynamically unstable patients where precise measurements may guide critical interventions. Moderate agreement was observed between cardiac output measurements obtained via LVOT VTI and carotid artery parameters in septic shock patients, as indicated by Lin’s Concordance Correlation Coefficient (0.527) and the Intraclass Correlation Coefficient (0.695 for absolute agreement). Previous studies have reported moderate to high correlations between these two techniques25,26,27,28. One study showed a moderate positive correlation between the absolute values of LVOT VTI and carotid artery blood flow, measured using time-averaged mean velocity (r = 0.60, p < 0.05), although a weaker correlation was observed during passive leg raising tests25. Another study highlighted that carotid flow assessment using handheld ultrasound is a viable method for evaluating fluid responsiveness, particularly in surgical settings where invasive monitoring (e.g., arterial line or pulmonary artery catheter) and other non-invasive methods (e.g., LVOT VTI) may not be feasible due to limited access to the chest29. Our study is distinct from others as it specifically targets septic shock patients in the emergency department, while other studies have examined varied populations (e.g., hemodynamically stable patients, surgical patients, or mixed shock cohorts). Moreover, our study used VTI measurements rather than other parameters (such as corrected flow time) that have been used in some previous studies26,27). However, contrasting evidence exists; one study found no significant correlation between carotid Doppler ultrasound and invasive cardiac output monitoring30.
Noteworthy finding of our study was the observed similarity between carotid VTI and LVOT VTI measurements (mean LVOT VTI 18.2 ± 5.3 cm vs. mean carotid VTI 17.7 ± 4.3 cm, with a bias of -0.47). Several physiological and technical factors may explain this observation: First, this similarity may reflect the fundamental hemodynamic principle that blood flow characteristics are relatively preserved across the arterial system in the absence of significant obstructive lesions. While absolute flow volume differs between the LVOT and carotid artery, the velocity-time profile as captured by VTI measurements may maintain more consistent characteristics between these sites. This supports the concept that flow patterns, rather than just absolute volumes, may be relatively preserved from central to peripheral arterial sites. Second, the comparable VTI values might be explained by compensatory mechanisms in vessel diameter and flow dynamics. The carotid artery, though smaller in diameter than the LVOT, may exhibit higher flow velocities due to its more peripheral location and narrower lumen, resulting in VTI values that numerically approximate LVOT measurements despite representing different absolute flow volumes. This phenomenon would be consistent with the conservation of energy principles in fluid dynamics. Third, in septic shock specifically, the characteristic vasodilation and altered vascular resistance may create more homogeneous flow patterns throughout the arterial tree compared to other hemodynamic states, potentially contributing to the similarity in VTI measurements between these anatomically distinct sites. Finally, it is important to note that while the VTI values are numerically similar, the Bland-Altman analysis revealed wide limits of agreement (-10.51 to 9.58), indicating substantial variability in individual measurements despite the small overall bias. This suggests that while population means are comparable, individual patient measurements still demonstrate meaningful differences between techniques.
The analysis of results utilizing the regression equation model (y = carotid artery blood flow * m + k) revealed the mean cardiac output calculated by the standard LVOT VTI method was 5.329 L/min, whereas the mean carotid artery blood flow was 0.855 L/min. When applying our derived regression equation (y = carotid artery blood flow * 1.92 + 0.57) to estimate cardiac output from carotid measurements, we observed moderate correlation with LVOT-derived values (r = 0.68, p < 0.001) with minimal bias (-0.04 L/min). However, wide variability of Bland-Altman and high percentage error (87.4%) between methods suggesting the regression approach may be unreliable for clinical decision-making in septic shock patients, where pathophysiologic factors like altered vascular compliance and blood flow redistribution influence measurements. Despite these limitations, carotid assessments offer non-invasive, rapid alternatives when LVOT measurements are unfeasible. The physiological relationship between carotid flow and CO exists, but significant individual variability limits acute application.
An important finding in our study relates to the feasibility of obtaining cardiac output measurements via the LVOT compared to the carotid artery. Out of the 69 patients screened for eligibility, four patients (5.7%) were excluded from the study solely because it was impossible to obtain an adequate cardiac output measurement via the LVOT, despite successful assessment through the carotid artery. The relatively small, yet clinically significant, proportion (5.7%) of patients in whom LVOT acquisition was unsuccessful, while carotid measurements were feasible, underscores the practical limitations of LVOT-based techniques in real-world emergency and critical care settings. These limitations are particularly relevant in patients with narrow intercostal spaces, obesity, pulmonary disease, or other factors that may obscure cardiac windows—conditions which are not uncommon in the septic shock or general critically ill population. In resource-limited settings, or in situations where obtaining high-quality cardiac echocardiographic images is challenging, carotid doppler measurement may serve as an alternative for at least partial hemodynamic assessment. While the main findings of our study caution against interchangeability between methods for precise cardiac output quantification, the ability to obtain any real-time hemodynamic information in these otherwise difficult cases may aid clinical decision-making, help monitor trends, or prompt further investigation.
The use of ultrasound for assessing patients in shock is increasingly recognized as the standard of care in emergency and critical care settings worldwide. This study reinforces the utility of ultrasound for emergency physicians who frequently manage septic shock patients. However, our findings suggest that carotid artery ultrasound, despite its potential advantages in terms of accessibility and ease of imaging, cannot reliably substitute for LVOT measurements in clinical decision-making for individual patients. Additionally, prior research has incorporated common carotid artery assessment into shock evaluation protocols, such as the Rapid Ultrasound in Shock (RUSH) velocity time integral protocol31. However, its adoption in clinical practice remains limited.
Limitations
This study has several limitations: (1) The research was conducted at a single center with a limited number of participants, which may restrict the generalizability of the findings to other healthcare settings with different patient demographics and clinical practices, (2) Our study evaluated both methods through isolated static measurements. The study design did not evaluate the ability of carotid VTI to track changes in cardiac output during fluid challenges or passive leg raising tests, which is the more clinically relevant application. The research did not evaluate the clinical implications of utilizing carotid artery measurements to direct fluid therapy. Future investigations should evaluate whether this approach improves patient outcomes, including morbidity and mortality, (3) We did not systematically test and compare measurements between both sides of common carotid arteries in each participant, which may affect the consistency of measurements. This raises questions about whether one side consistently yields measurements that correlate more effectively with LVOT VTI, as well as potential anatomical or pathological factors such as sedation, which may induce vasodilation and modify cerebral blood flow autoregulation. Additionally, seizures can significantly elevate cerebral perfusion demands, while sepsis may alter autonomic tone and vascular responsiveness, potentially affecting the accuracy of measurements on either side, (4) Our primary analysis concentrated on agreement metrics; future studies specifically designed to develop predictive models between these measurement techniques would be advantageous, (5) Our study collected data only after achieving target blood pressure parameters (MAP > 65 mmHg) which may not be generalizable to the assessment of cardiac output in septic shock patients during their most hypotensive state, which is precisely when such measurements might have the greatest clinical utility, (6) Cerebral autoregulation mechanisms maintain relatively constant cerebral blood flow despite changes in systemic hemodynamics. Various conditions including traumatic brain injury, stroke, intracranial hypertension, and certain medications can impair this autoregulation, potentially altering the relationship between carotid flow and cardiac output. We did not systematically assess for or control these variables, which may have contributed to measurement, and (7) We did not assess the inter-operator and intra-operator variability of carotid and LVOT measurements. Quantifying this variability would have provided important information about the reproducibility and reliability of both measurement techniques in the emergency department setting, particularly for the carotid measurements which are being evaluated as a potential alternative to LVOT assessments. Future studies should include systematic assessment of measurement reliability across different operators and within the same operator over repeated measurements, especially when evaluating novel techniques for hemodynamic monitoring in critically ill patients.
Conclusions
This study exhibited a moderate agreement between cardiac output measurements derived from LVOT VTI and the common carotid artery; however, the wide limits of agreement suggest caution in using these methods interchangeably in clinical decision-making for individual patients. This degree of consensus does not endorse carotid artery Doppler ultrasound as a direct substitute for LVOT echocardiography. Further research involving larger cohorts is necessary to ascertain whether this technique can be enhanced to attain greater agreement with established methods.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request”.
Change history
19 August 2025
The original online version of this Article was revised: References 21 and 25 were incorrectly cited in the Discussion section. The correct information now accompanies the original Article.
Abbreviations
- ED:
-
Emergency department
- VTI:
-
Velocity time integral
- LVOT:
-
Left ventricular outflow tract
- TDI:
-
Tissue doppler imaging
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
References
Singer, M. et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA 315, 801–810 (2016).
Phungoen, P. et al. Clinical factors associated with bloodstream infection at the emergency department. BMC Emerg. Med. 21, 30 (2021).
Bauer, M. et al. Mortality in sepsis and septic shock in europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care. 24, 239 (2020).
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247 (2021).
Kelm, D. J. et al. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 43, 68–73 (2015).
Claure-Del Granado, R. & Mehta, R. L. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 17, 109 (2016).
Hasanin, A. Fluid responsiveness in acute circulatory failure. J. Intensive Care. 3, 50 (2015).
Rajaram, S. S. et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst. Rev. 2013, CD003408 (2013).
Mercado, P. et al. Transthoracic echocardiography: an accurate and precise method for estimating cardiac output in the critically ill patient. Crit. Care. 21, 136 (2017).
Zhang, Y., Wang, Y., Shi, J., Hua, Z. & Xu, J. Cardiac output measurements via echocardiography versus thermodilution: A systematic review and meta-analysis. PLoS One. 14, e0222105 (2019).
Ienghong, K., Cheung, L. W., Tiamkao, S., Bhudhisawasdi, V. & Apiratwarakul, K. The diagnostic capabilities of the combined cardiac and lung point of care ultrasound in shocked patients at the emergency department - Resourced limited country. Eur. J. Radiol. Open. 9, 100446 (2022).
Peachey, T. et al. The assessment of Circulating volume using inferior Vena Cava collapse index and carotid doppler velocity time integral in healthy volunteers: a pilot study. Scand. J. Trauma. Resusc. Emerg. Med. 24, 108 (2016).
McGregor, D. et al. Emergency department non-invasive cardiac output study (EDNICO): a feasibility and repeatability study. Scand. J. Trauma. Resusc. Emerg. Med. 27, 30 (2019).
Porhomayon, J., Zadeii, G., Congello, S. & Nader, N. D. Applications of minimally invasive cardiac output monitors. Int. J. Emerg. Med. 5, 18 (2012).
Peng, Q. Y. et al. Common carotid artery sonography versus transthoracic echocardiography for cardiac output measurements in intensive care unit patients. J. Ultrasound Med. 36, 1793–1799 (2017).
Sidor, M., Premachandra, L., Hanna, B., Nair, N. & Misra, A. Carotid flow as a surrogate for cardiac output measurement in hemodynamically stable participants. J. Intensive Care Med. 35, 650–655 (2020).
Stolz, L. A. et al. Can emergency physicians perform common carotid doppler flow measurements to assess volume responsiveness? West. J. Emerg. Med. 16, 255–259 (2015).
Chowhan, G. et al. Efficacy of left ventricular outflow tract and carotid artery velocity time integral as predictors of fluid responsiveness in patients with Sepsis and septic shock. Indian J. Crit. Care Med. 25, 310–316 (2021).
Lu, M. J. et al. Sample size for assessing agreement between two methods of measurement by Bland-Altman method. Int. J. Biostat. 12 (2), (2016).
Critchley, L. A. & Critchley, J. A. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J. Clin. Monit. Comput. 15 (2), 85–91 (1999).
Ienghong, K., Cheung, L. W., Chanthawatthanarak, S. & Apiratwarakul, K. Automatic B-lines: a tool for minimizing time to diuretic administration in pulmonary edema patients in the emergency department of a developing country. Int. J. Emerg. Med. 17, 183 (2024).
Ienghong, K., Cheung, L. W., Tiamkao, S., Bhudhisawasdi, V. & Apiratwarakul, K. The impact of prehospital point of care ultrasounds on emergency patients length of stay in Thailand. J. Multidiscip Healthc. 16, 219–226 (2023).
Weile, J. et al. Point-of-care ultrasound induced changes in management of unselected patients in the emergency department - a prospective single-blinded observational trial. Scand. J. Trauma. Resusc. Emerg. Med. 28, 47 (2020).
Riishede, M. et al. Point-of-care ultrasound of the heart and lungs in patients with respiratory failure: a pragmatic randomized controlled multicenter trial. Scand. J. Trauma. Resusc. Emerg. Med. 29, 60 (2021).
Patnaik, R., Krishna, B. & Sampath, S. Correlation of common carotid artery blood flow parameters with transthoracic echocardiographic cardiac output for assessing fluid responsiveness after passive leg Raising (PLR) test in critically ill patients. Cureus 15, e40229 (2023).
van Houte, J. et al. Is the corrected carotid flow time a clinically acceptable surrogate hemodynamic parameter for the left ventricular ejection time? Ultrasound Med. Biol. 50, 528–535 (2024).
Cheong, I., Merlo, P. M. & Tamagnone, F. M. Correlation between corrected carotid flow time and left ventricular outflow tract velocity-time integral using a novel technique. J. Clin. Ultrasound (2024).
Bu, X. Y. et al. Comparison of carotid blood flow measured by ultrasound and cardiac output in patients undergoing cardiac surgery. Heart Surg. Forum. 26, E234–E239 (2023).
Gibson, L. E., Mitchell, J. E., Bittner, E. A. & Chang, M. G. An assessment of carotid flow time using a portable handheld ultrasound device: the ideal tool for guiding intraoperative fluid management? Micromachines (Basel) 14, 510 (2023).
Arango-Granados, M. C. et al. Correlation and concordance of carotid doppler ultrasound and echocardiography with invasive cardiac output measurement in critically ill patients. Intensive Care Med. Exp. 12, 69 (2024).
Blanco, P., Aguiar, F. M. & Blaivas, M. Rapid ultrasound in shock (RUSH) Velocity-Time integral: A proposal to expand the RUSH protocol. J. Ultrasound Med. 34, 1691–1700 (2015).
Acknowledgements
The authors sincerely thank Josh Macknick for serving as the English consultant.
Funding
It was supported by the Fundamental Fund of Khon Kaen University, which received funding from the National Science, Research and Innovation Fund (NSRF).
Author information
Authors and Affiliations
Contributions
S.C., K.B., K.I., and K.A. contributed to the planning of the study design. S.C., K.B., K.I., and K.A. were involved in data collection. S.C., K.B., and K.I. analyzed the data and drafted the initial version of the manuscript. L.W.C. and S.T. provided statistical support and supervision. S.C., K.I., and K.A. revised the manuscript. S.C. and K.I. finalized the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical considerations
This study adhered to the principles of the Helsinki Declaration and the guidelines of Good Clinical Practice. The Human Research Ethics Committee of Khon Kaen University approved the study (HE661063), with institutional ethics committee approval obtained on April 3, 2023. Informed consent was obtained from each participant prior to their inclusion in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chanthawatthanarak, S., Boonasa, K., Apiratwarakul, K. et al. Agreement between carotid and LVOT non-invasive cardiac output measurements in ED septic shock patients: a prospective observational study. Sci Rep 15, 19911 (2025). https://doi.org/10.1038/s41598-025-05077-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05077-y