Abstract
Optimal nutrition and physical activity are vital for enhancing physical performance and preventing metabolic diseases like obesity. Recently, interest has grown in long-term fasting, particularly water-only fasting, which involves no food intake and unlimited water consumption. Our study investigated the effects of 8 days of water-only fasting combined with aerobic exercise in 13 middle-aged men, focusing on metabolic, hormonal, and immune changes. Results showed that fasting had a more significant impact than exercise, leading to changes in glucose, uric acid, IGF-1, IGF-2, GH, leptin, and cortisol, improved total antioxidant status (TAS), and reduced lipid peroxidation. While exercise enhanced the effects of fasting on triglycerides, insulin, GH, TAS, PerOX, and IL-6, changes in total protein and lactate were solely due to exercise. Overall, combining fasting and exercise led to a metabolic shift from carbohydrates to fatty acids and hormonal adaptations to stress. These results, although derived from a small group of patients, offer a promising outlook for further research into the effects of fasting combined with physical activity on health and weight maintenance.
Similar content being viewed by others
Introduction
Complete fasting, called a zero-calorie diet, involves completely stopping food intake while consuming any amount of water. The existing literature indicates both positive and negative aspects of prolonged fasting. However, it has not yet been clearly established how long a person should fast in order to secure optimal health benefits safely. In general, fasting unfolds through three distinct phases, each playing a vital role in the body’s adaptation: the post-absorptive phase (0–24 h), the gluconeogenic phase (24 h to 10 days), and the conservation of protein phase (beyond 10 days). Among them, the gluconeogenic phase stands out, during which the body changes the way it produces energy in the absence of external food sources1. To determine the optimal duration of fasting for health, the concept of hormetic adaptation was introduced. This concept aims to determine the moment during fasting when beneficial changes in the body cease and the possibility of deterioration of health begins2. The main feature of the hormetic concept is that low levels of biological, chemical, physical, or mental stress enhance adaptive responses that not only condition, repair, and restore normal functions of damaged tissues/organs, but moderately compensate, reducing ongoing underlying damage3.
It has been shown that a beneficial adaptation to fasting is, among others, reduced insulin concentration, which is a strong inhibitor of lipolysis4,5. Under these conditions, it was possible to release increased amounts of fatty acids and activate ketogenesis. An important point to note is that ketone bodies are used for energy production in most tissues of the body, except for erythrocytes and certain types of cancer cells. This is significant because it increases the survival rate of those tissues which are able to use ketone bodies during periods of fasting. It is worth mentioning that, after a few weeks of fasting, β-hydroxybutyrate and acetoacetate provide up to two thirds of the energy required by the brain6. During the initial stages of fasting, ketone bodies are associated with an increased release of free radical oxygen species (ROS) and elevated ratios of NAD+/NADH and AMP/ATP in mitochondria7,8. This initiates a temporary stress to the mitochondria, which activates a protective cellular response that subsequently leads to an improvement in mitochondrial function and the body’s ability to combat oxidative stress8. During fasting, the consumption of ketone bodies increases also in the heart9 and in skeletal muscles10. According to a study by Evans et al.11 the use of ketone bodies during intense exercise may increase by up to five times. However, higher concentrations of β-hydroxybutyrate can induce oxidative stress, typically by causing inflammation that damages lipids, proteins, and DNA in cells, as a result of the production of pro-inflammatory cytokines, tumor necrosis factor-α (TNFα), interleukins IL-1β and IL-6, or the chemokine CCL212,13. Apart from this particular situation, different fasting models demonstrate that caloric restriction can positively regulate immune response and speed up regeneration processes such as angiogenesis or wound healing14,15. Furthermore, during physical activity, skeletal muscle plays a significant role as a secretory organ by releasing various myokines, including IL-6, which helps regulate energy metabolism16. Similarly, TNF-α has widespread metabolic effects throughout the body, and both cytokine concentrations can vary during certain conditions such as fasting and exercise17,18,19. It should be added that physical exercise, just like fasting, can be a very powerful agent that changes the body’s metabolism in many directions, depending on its intensity and duration.
It has been shown that controlled physical exercise included in the training framework resulted in, among others, positive regulation of the concentrations of adipokines (lipocain 2, omentin 1 or neuralgin 4) and many myokines (decortin, follistatin, myostatin, activin A, transforming growth factor beta-1—TGF-B-1) and beneficial cardiometabolic and somatic changes in obese men20,21,22.
The regulation of carbohydrate and lipid metabolism is critically influenced by hormones, cytokines, and myokines. Moreover, physical activity plays a pivotal role in enhancing metabolic processes, leading to substantial health benefits. Despite this, our understanding of how fasting, when paired with physical activity, affects metabolic regulation remains limited. It is essential to explore whether this combination produces beneficial or adverse effects. To address this important question, we will conduct a examination of the impacts of fasting and physical activity on metabolic parameters, pro-inflammatory markers, and oxidative stress. This study is a first step in uncovering the mechanisms by which water-based fasting, when paired with exercise, can enhance human health and overall well-being.
Purpose of the study
The paper aims to demonstrate the positive and negative effects of the simultaneous impact of 8-days of water-only fasting and two maximum intensity exercise tests on the bodies of healthy, middle-aged men. This assessment has been made by recording the metabolic, hormonal, and immune changes in the body modified by both those factors.
Material and methods
Participants
Thirteen healthy, middle-aged men (57.25 ± 9.93 years; 81.72 ± 9.19 kg; 178.54 ± 4.77 cm) volunteered to undergo 8-days of water-only fasting, during which each individual consumed ad libitum moderately mineralized water of an identical composition. The research participants were selected from an advertisement in the press. The conditions for initial qualification were male gender, age between 30 and 70 years, good health, previous personal experience in fasting for several days, average physical activity, following a mixed diet, not using alcohol, nicotine, psychoactive substances and excess medications. The body weight of the participants was 60–95 kg, body mass index 20–29.9 kg/m2, systolic blood pressure 100–140 mmHg and diastolic blood pressure 60–90 mmHg. Medical supervision was made available prior to the study, during it, and for 3 days afterwards. During medical examinations, no health contraindications to participation in the experiment were found. Additionally, participants were required to make a declaration that, for at least one month before the period of the study, they had not taken any medications or dietary supplements, and had not engaged in any form of smoking or the drinking of alcohol. Moreover, participants declared that they had not participated in fasting for longer than 3 days in the last 6 months. Before commencing the study, the participants were fully informed about the study’s procedures, and possible risks. Prior written consent to participate in the study was obtained from each participant. The experimental protocol was approved by the Research Ethics Committee of the Jan Długosz University in Częstochowa, Poland (KE-0/1/2019). All procedures performed in studies was conducted in accordance with the Declaration of Helsinki.
Study protocol
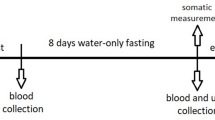
The subjects of the study came to the laboratory in the morning of the first day, between 8:00 a.m. and 9:30 a.m., after having fasted for 12 h. Age was noted, and somatic data (body height—BH, body weight—BM, fat tissue content—BF, fat-free mass—FFM, total body water—TBW, and body mass index—BMI) were recorded, using the Tanita TBF 300A body composition analyzer (Tanita, Amsterdam, the Netherlands). Then, with the subject sitting down, blood samples were taken from the antecubital vein. The blood was allowed to clot at room temperature and then centrifuged at 1500×g for 15 min. The serum so obtained was divided into aliquots and stored at − 80 °C for later analysis. Additional blood samples were collected in heparinized tubes. Plasma was also obtained from the blood by treating it with EDTA. Then, the concentrations of: glucose (G), triglycerides (TG), free fatty acids (FFA), glycerol (GLY), β-hydroxybutyrate (β-HB), total protein (TP), insulin-like growth factor binding protein (IGF-BP3), urea (U) and uric acid (UA) were determined.
Concentrations of hormones such as insulin (IRI), insulin-like growth factor 1 (IGF-1), insulin-like growth factor 2 (IGF-2), growth hormone (GH), cortisol (C), leptin (L), ghrelin (GHRL), orexin A (ORE-A), orexin B (ORE-B) were also determined. Additionally, the concentrations of total antioxidant status/capacity (TAS/TAC), total oxidative status/capacity (TOS/TOC), interleukin IL-6, and human tumor necrosis factor alpha (TNF-α) were determined in the serum. A capillary blood sample was also collected to determine the lactate concentration (LA). The homeostatic model assessment of insulin resistance (HOMA-IR) was defined as fasting serum insulin (milliunit per liter) multiplied by fasting glucose (millimole per liter) divided by 22.5.
Then, a cycloergometric examination of the lower limbs was performed. This test started at 60 W and the load was increased by 30 W every 3 min until the individual maximum exercise capacity was achieved. The work rate was constant and amounted to 60 rpm. Immediately after the end of the exercise, venous blood was collected again and the concentrations of the above-mentioned biochemical variables were determined according to the procedure described above. On the ninth morning, the subjects returned to the laboratory, where the research procedure used before the 8-days of water-only fasting was repeated.
Study procedure
Spinreact (Girona, Spain) tests were used to determine the concentration of the following metabolites: glucose (GOD-POD, liquid test), triglycerides (GPO-POD, liquid test), total protein (Biuret Colorimetric), urea (Urease-GLDH, liquid test), uric acid (Uricase-POD, liquid test). Moreover, β-hydroxybutyrate concentration was determined enzymatically using the RANBUD diagnostic kit (Randox Laboratories Ltd., Crumlin, County Antrim, UK). Among the metabolites analysed, serum FFA and glycerol concentrations were determined using colorimetric/fluorimetric assay kits (EFFA-100 and cat. no. EGLY-200 respectively) (BioAssay Systems, Hayward, California). Capillary blood lactate concentration was determined using the Epoc Blood Analysis System (Ottawa, Canada). Serum insulin concentration was determined by the immunoradiometric method using Immunotech SA kits (France). Human ELISA kits (Bioassay Technology Laboratory, China) were used to determine the levels of: GHRL (Cat. No. E3091Hu), ORE-A (Cat. No. E1296Hu) and ORE-B (Cat. No. E1282Hu). The CLIA method was used to determine the levels of GH, IGF-1, IGF-BP3, IL-6, and cortisol in serum using the MAGLUMI -1000 apparatus with appropriate kits (Snibe Diagnostic, Shanghai International Holding Corp. GmbH, Europe, Hamburg, Germany; Cortisol-CLIA, Shenzhen New Industries Biomedical Engineering Co. Ltd., Shenzhen, China). An immunological kit from DRG International, Inc., USA was used for the quantitative determination of leptin concentration in serum—DRG Leptin (Sandwich) ELISA (EIA-2395). The concentration of IGF-2 in serum was determined enzymatically using an ELISA immunoassay kit (Mediagnost GmbH, Tübingen-Reutlingen, Germany). Serum total antioxidant status/capacity (TAS/TAC) was determined using the Immundiagnostik test (Immundiagnostik AG, Bensheim, Germany). The PerOx (TOS/TOC) photometric test kit was used to determine the total oxidative state/capacity (TOS/TOC) in serum (Immundiagnostik AG, Bensheim, Germany). TNF-α concentration was determined using DRG immunoenzymetric assay (EIA-4641), (DRG International, Inc., Springfield, New Jersey, USA).
Statistical analysis
Mean (M) and standard deviation (± SD) values were calculated for each parameter. The Shapiro–Wilk test was used to check the normality of distribution. A paired t-test was used to determine the effect of 8-days of water-only fasting on somatic variables. Nonparametric Friedmann repeated-measures ANOVA by rank was also used, utilising the Dunn-Bonferroni post hoc test to determine the effects of fasting and exercise testing. Partial eta-squared (ηp2) was calculated to determine effect size. Values at p < 0.05 were considered statistically significant. The values obtained were subjected to statistical analysis using SPSS Statistics 20. Sample size analysis was performed using Statistica v 13.3 (TIBCO) based on the results of the pilot study. We estimated the sample size necessary to demonstrate statistical significance between the study groups p = 0.05 and a test power of 0.8, which was 12 patients.
Results
In terms of somatic changes, a significant reduction in BW, relative (%) and absolute (kg) BF values, absolute (kg) FFM and TBW values as well as BMI (p < 0.001) were observed under the influence of 8-days of water-only fasting. Moreover fasting intervention increased relative (%) values of FFM (p < 0.01) and TBW (p < 0.001).
The results presented in Table 1 show that carbohydrate metabolism was significantly modified under the influence of 8-days of water-only fasting and exercise tests (p < 0.001).
Fasting significantly reduced G concentrations and HOMA-IR values both at rest and after exercise. However, only the combination of fasting and physical activity significantly lowered insulin levels. Fasting did not affect lactate concentration, which significantly increased (p < 0.001) after physical exercise, regardless of the fasting intervention.
The results in Table 2 show that variables related to fat and protein metabolism (FFA, GLY, TG, β-HB, TP, and UA) also changed significantly under the influence of the fasting and exercise tests. The fasting caused a significant increase in the concentration of β-HB and UA, both at rest and after exercise. The fasting also increased the concentration of FFA (p < 0.01) and GLY (p < 0.05) in serum under resting conditions. It should also be noted that the exercise test increased GLY (p < 0.01) and TP (p < 0.001) levels before fasting, as well as TG (p < 0.05) and TP (p < 0.001) concentrations after fasting. Fasting did not appear to impact the TP, which was significantly influenced by exercise.
As shown in Table 3, in terms of hormonal changes, the study showed that 8-days of water-only fasting and exercise tests caused significant changes in the concentrations of IGF-1, IGF-BP3, GH, L, and C, ORE-B and IGF-2. Post hoc analysis showed that fasting changed the concentrations of IGF-1, IGF-BP3, L and C at rest and under post-exercise conditions, as well as IGF-2 only at rest. Physical exercise resulted in a significant increase in IGF-BP3 concentration (p < 0.01) before fasting intervention. Additionally, exercise significantly enhanced GH concentration (p < 0.01). In summary fasting alone produces a statistically significant reduction in IGF-1, IGF-2, IGF-BP3, and L, while simultaneously elevating levels of GH and C. Notably, incorporating physical activity amplifies the positive effects of fasting on GH.
As shown in Table 4, variables determining the oxidative status and body immunity, i.e. TAS, PerOX, and IL-6, also changed significantly as a result of the combined effect of fasting and physical exercise. Trends were observed showing increased TAS concentration and reduced PerOx after fasting. Additionally, exercise significantly enhanced the effects of fasting for both parameters. However, the exercise test induced an increase in IL-6 concentration (p < 0.001) after 8-days of water-only fasting. In summary, fasting alone does not lead to statistically significant changes in oxidative stress and IL-6, whereas the combination of fasting and physical activity does introduce changes in these markers.
Discussion
In the current study the combined effect of 8-days of water-only fasting and an exercise test performed twice resulted in a significant reduction in glucose, insulin and HOMA-IR. Moreover, the fasting element induced a greater share of these changes than the two sets of exercise. Numerous studies indicate that reducing the level of glucose in the bloodstream leads to a decline in the concentration of circulating insulin while increasing the concentration of glucagon, even on the first day of fasting23. This reduced amount of glucose in the blood is nonetheless sufficient to meet the glycolytic needs of the brain and other tissues during the initial days of fasting24. However, after 8-days of water- only fasting, the body’s metabolism undergoes significant changes. These changes are intense and extended over time, and already after 24 h of fasting glycogen stores are depleted and the body begins to produce glucose through the process of gluconeogenesis in the liver. Despite reduced levels, insulin continues to moderate the rate of gluconeogenesis by promoting lipolysis and providing the liver with fatty acids25. At the same time, the increased concentration of glucagon in this phase of fasting causes a decrease in the level of malonyl-CoA. This leads to the formation of ketone bodies, which the brain uses instead of glucose. This helps to conserve glucose, which is used by many tissues, including red blood cells, bone marrow and kidney medulla. Then prolonged fasting shifts the gluconeogenesis process from the liver to the kidneys26. Low glucose and insulin concentrations caused by fasting are believed to decrease the risk of cardiovascular disease (CVD)27 and to help the body adapt to fasting conditions28. In the current study, prior to the 8-day water-only fast, normal levels of glucose and insulin concentrations, as well as correct values of the HOMA-IR index, were observed. However, a significant decrease in these values after 8 days of water-only fasting suggests that it was this factor that controlled glycemia at that time29.
In the current study, as well as in previous works7,27, there was no significant effect of single maximum intensity physical exercises on glycemic control. After cycloergometric exercise test it was observed that lactate concentration had significantly increased, compared to resting conditions, as measured both before the 8-days of water-only fasting and at its completion. These results suggest that glycolysis plays an important role in providing energy protection to the body, even when blood glucose concentrations are reduced.
Moreover, under these conditions, lactate inhibits lipolysis and limits the mitochondrial uptake of fatty acids by muscles30. Despite the moderate impact of a single exercise on metabolism, it should be borne in mind that repeated physical exercise, included in the training framework, is important in the control of glycemia and insulin resistance31 and in health prevention32.
The combined effect of fasting and physical exercise caused significant changes in body weight, including a reduction in body fat. Such a change can be associated with the fact that from the second day of fasting there is a significant increase in the concentration of FFA and glycerol in the blood, which indicates the stimulation of lipolysis1,33. In the current study, 8-days of water—only fasting resulted in further intensification of lipolysis, as both serum FFA and glycerol concentrations remained elevated, although triglyceride concentrations did not change at rest. It was also noticed that already in the initial days of fasting, increased concentrations of FFA and glycerol in plasma were accompanied by an increase in the concentration of gluco- and ketogenic amino acids23. Together, these changes activate the process of gluconeogenesis and ketogenesis in the liver. The plasma concentration of β-HB, the most important ketone body, increased many times in our study and slightly exceeded the upper limit of ketonemia34. This condition can cause ketoacidosis and may be dangerous to a person’s health. It has also been noted that increased concentrations of ketone bodies during fasting may be beneficial for the body because these substrates are used by the brain, skeletal muscles, kidneys and heart23.
Therefore, because of possible ketoacidosis, it should be acknowledged that an 8-days of water-only fasting, despite its advantages, may pose a health risk, especially to individuals with liver disease of any kind35.
However, these changes were not reflected in the concentration of total protein in the serum of the men we examined, because its concentration did not change, which may also be related to the described effect of saving body proteins in the later days of fasting1. It also appears that the increased release of glucogenic and ketogenic amino acids described above23 did not affect changes in serum total protein concentrations.
The lack of change in the concentration of total protein in serum is confirmed by the unchanged concentration of urea after the fasting intervention. Moreover, a significant increase in total protein concentration was observed after exercise tests, both before and after the fasting intervention17,36. However, the lack of changes in post-exercise urea concentration indicates that this increase was not caused by metabolic changes in total protein and was probably related to, among others, a transient decrease in plasma volume in the post-exercise period37.
The 8-days of water-only fasting resulted in a more than two-fold increase in serum uric acid concentration, and the physical exercise did not modify this change. On the one hand, an increase in this concentration can be considered beneficial because uric acid acts as a strong antioxidant and accounts for approximately 60% of the antioxidant potential of human plasma38. However, it is known that in the cellular environment this compound increases oxidative stress, which can cause systemic inflammation39. It has also been shown that hyperuricemia causes significant changes in systemic and renal hemodynamics, which may result in loss of autoregulatory renal function40 or lead to hypertension, insulin resistance, obesity, hypertriglyceridemia and metabolic syndrome41. Observation of the volunteers we studied several days after the end of the famine did not reveal any of these negative effects.
Observations of volunteers participating in our experiment, which we conducted for several days after the end of the starvation, did not show any of these negative effects.
The described experiment, which included fasting intervention and two exercise tests, took place under conditions of severe stress, which, among other things, activates the hypothalamic–pituitary–adrenal (HPA) axis. To monitor HPA activity, the study measured serum cortisol concentrations, which increased significantly after 8 days of water-only fasting, both at rest and after exercise. Also, the study by Steinhauser et al.33 showed a significant increase in cortisol levels already on the first day of fasting. Moreover, these studies showed a negative correlation between serum cortisol levels and the duration of caloric restriction. This means that the concentration of cortisol in the serum, as an important stimulator of lipolysis, increases in the initial days of caloric restriction, then decreases significantly and returns to the initial level after a few weeks of fasting. The 8-days of water-only fasting undertaken in the current study was long enough to maintain cortisol concentration at a much higher level than the baseline. Our previously unpublished data showed that 14-day and 21-day fasting resulted in further increases in serum cortisol concentrations. It appears, therefore, that elevated serum cortisol concentrations persist for longer than 3 weeks in cases of complete fasting. Physical exercise is a factor that increases plasma cortisol concentration42. However, in the current study, exercises testing did not increase serum cortisol concentrations either before or after fasting. Therefore, the physical exercise used was too weak a factor in stimulating cortisol secretion, while 8-days of water only-fasting showed such an effect.
An important aspect of various forms of caloric restriction is their impact on the feeling of hunger and activation of hypothalamic circuits regulating appetite. While leptin inhibits appetite43, orexigenic peptides such as GHRL and orexins enhance this feeling and activate the hunger center44. The current study shows that 8-days of water-only fasting definitely led to hypoleptinemia, while the concentrations of GHRL, ORE-A, and ORE-B did not change significantly under these conditions, and only increasing trends of these hormones appeared. The occurrence of hypoleptinemia and no or slight increases in GHRL concentration had previously been observed after 72 h of fasting in people with diabetes45, after 4 days of caloric restriction in adult men43, or after 1 week of intermittent Ramadan fasting46. The study by Steinhauser et al.33 suggested a possible correlation between decreased leptin levels and increased cortisol concentrations during fasting, which also seems likely in the current study. Other authors also indicated that acute fasting-induced hypoleptinemia occurs already on the first day of fasting and may stimulate the HPA axis and thus cause a switch from carbohydrate metabolism to lipid metabolism in humans. Hypoleptinemia and nosignificant changes in GHRL and orexins concentrations occurring in the current study indicate that after 8-days of water-only fasting there is no acute activation of the hunger center and no significant increase in appetite. This observation is confirmed by the statements of people who have fasted, indicating that the strongest feeling of food craving occurs on the first day of fasting, after which it gradually decreases. Scientific reports also indicate the excellent mental well-being of volunteers subjected to 8-days of water-only fasting47. The physical exercise tests used in the current study did not cause any changes in the concentrations of leptin, GHRL, ORE-A, and ORE-B.
The study of Hollstein et al.48 indicated that an important issue in energy adaptation to fasting seems to be the activation of the GHRL/GH/IGF-1 axis. In the current study, the combined effects of fasting and physical exercise altered the concentrations of GH and IGF-1, with no impact on GHRL. Notably, there was only an upward trend observed in GH levels during resting conditions after the fasting intervention. This lack of change in GH concentration during rest contrasts with findings from other studies, which indicated an increase in this hormone following short-term fasting49,50. All these suggest that the separate effect of fasting or physical exercise is too weak a stimulus for changes in GH concentration. GH is known to achieve most of its anabolic effects by stimulating the release of IGF-1 by the liver. The study of Hollstein et al.48 showed a large increase in GH concentration during short-term fasting and no changes in IGF-1 concentration. In the current study, as already mentioned, there was an increase in GH concentration caused by fasting only under conditions of physical exercise, while the concentration of IGF-1 decreased significantly during fasting, both at rest and during physical exercise. The described negative correlations between GH and IGF-148 suggest that IGF-1 may influence the secretion of GH by the hypothalamus using negative feedback51. The results of our study seem to confirm this relationship.
In the present study, IGF-BP3 and IGF-2 concentrations changed under fasting and exercise conditions52, and the exclusive effect of fasting reduced IGF-BP3 concentrations both at rest and during exercise. However, in another study, which included a 36-h fast, a decrease in IGF-1 concentration was observed in a group of healthy women without changes in IGF-BP3 concentration50. The parallel decrease in IGF-1 and IGF-BP3 concentrations during fasting in our experiment suggests that the intensity of the IGF-1 binding process by IGF-BP3 was limited compared to the state before fasting and that physical exercise had no effect on these relationships. IGF-1, like IGF-2, is structurally similar to insulin53. IGF-2 has a hypoglycemic effect, which is strongly manifested in some diseases54. In healthy men participating in our study, the glycemic effect of IGF-2 did not occur under the fasting intervention, because the concentration of this hormone decreased similarly to the concentration of glucose.
It is unclear whether and how some forms of fasting affect the immune system, despite their growing popularity due to their potential immunomodulatory effects55. For this reason, a serum concentration of IL-6 and TNF-α was examined in this study as a markers of host immune response. Although IL-6 is generally considered a pro-inflammatory molecule, numerous studies have shown that IL-6 can act as an anti-inflammatory cytokine56,57. Moreover, contracting skeletal muscle produces IL-6, which acts as a myokine58 and regulates multiple processes such as energy metabolism16, muscle regeneration59,bone remodeling60,61, and hormone secretion of hormones such as insulin62 and cortisol63. Furthermore, physical activity boosts IL-6 secretion, which is dependent on duration, intensity, and the size of muscle mass engagement16. In the current study, an increase in IL-6 levels was observed after the combined impact of fasting and exercise, as well as an increase in circulating FFA concentrations after fasting. It is likely that, in both conditions, the body switches from using glucose to utilizing fat as an energy source. All that leads to an upregulation of lipolysis, and an increased level of IL-6 in the blood may have a significant impact on this particular process18. In a separate study, it was demonstrated that monocytes were not a primary source of increased IL-6 following physical activity64. These results suggest that changes in the level of IL-6 were related more to its metabolic role as a myokine than to its immune function. Regardless of that possibility, further studies are necessary to comprehend how IL-6 signaling maintains homeostasis during fasting accompanied by physical activity.
It is currently unclear whether different types of fasting can substantially reduce the levels of key-proinflammatory cytokines such as TNF-α19. The fluctuation of TNF-α concentration during fasting depends both on the participants’ physiological condition, e.g. whether they are healthy, obese, physically active or not, and on other factors, such as the length of the fast, and the types of food restriction protocol in use65. While studies have demonstrated the benefits of intermittent fasting (IF) in reducing basal levels of TNF-α66, very little is known about the impact of zero-calorie fasting on immune defense. Surprisingly, in contrast to IF interventions, a 10-day zero-calorie diet increased systemic inflammation biomarkers such as IL-6 and TNF-α in circulation and subcutaneous adipose tissue. It also led to an increase in adipose tissue macrophages and enhanced systemic pro-inflammatory pathways. According to the authors of those papers, severe calorie restriction led to inflammation signals, which caused a metabolic shift towards lipid utilization. In this context, activated immune cells could help to clear up lipids, lipid by-products, and dying cells67. Results from the current study revealed that 8-days of water-only fasting and physical activity have no statistically significant effect on serum levels of pro-inflammatory TNF-α. However, there was an upward trend in the concentration of this cytokine after the fasting period. It is thus not excluded that prolonged calorie restriction could drive inflammation. Taken together, the results from the current study complement the existing data on the impact of water-only fasting on systemic inflammatory mediators. Nonetheless, further research is needed to determine the mechanisms underlying the immunomodulatory effects of a water-only diet.
Total antioxidant status (TAS) in serum represents the body’s ability to defend itself against oxidation. The current study found that an 8-days of water-only fasting improved blood TAS and reduced lipid peroxidation. This result is consistent with previous research that involved a 10-day fasting period, which resulted in decreased lipid peroxidation and increased total antioxidant capacity in the plasma68. Moreover, multiple studies have demonstrated that IF can reduce oxidative stress69,70. Also, regular physical exercise training is known as one of the most powerful ways to boost antioxidant response71,72. In the current study it was shown that fasting enhances the positive impact of physical activity on the body’s antioxidant status. Additionally, uric acid functions as a powerful scavenger of free radicals in plasma73 and its concentration correlates with increased antioxidant capacity during fasting69,74. Our study also shows that calorie restriction leads to elevated levels of uric acid, thus confirming the beneficial effects of water-only fasting on the body’s antioxidant status.
This paper highlights the positive aspects of water-only fasting, such as increased lipolysis and enhanced antioxidant reserves. However, it is important to summarize several potential risks associated with fasting. As previously noted, fasting raises the concentration of ketone bodies, which can result in ketoacidosis—a condition that poses significant risks for individuals with liver damage32. Additionally, dietary interventions that elevate uric acid levels may lead to hyperuricemia, negatively affecting hemodynamics, hypertension, and renal function37,38. During fasting, the body breaks down proteins, converting the released amino acids into substrates for gluconeogenesis1,72. This process has raised concerns about potential weakening of the muscular system. However, recent evidence compellingly demonstrates that after seven days of fasting, muscle strength and oxidative enzyme levels in skeletal muscle remain remarkably preserved. It is important to note, though, that this extended fasting period does lead to a decline in high-intensity endurance capacity73. This insight challenges the traditional view of fasting’s impact on muscle health and highlights the body’s remarkable adaptability. Recent research has revealed a fascinating phenomenon: during periods of fasting, monocytes are known to re-enter the bone marrow. This process has significant implications, as refeeding leads to increase in monocyte counts in the bloodstream, but also alters the body’s immune responses to bacterial infections74. Our research has demonstrated that the key pro-inflammatory cytokine IL-6 functions more like myokine and is linked to physical activity during fasting. Changes in myokines that occur during physical activity play a significant role in non-pharmacological therapies aimed at reducing obesity-related disorders75. We hypothesize that incorporating physical activity into fasting may influence the immune system, although we do not yet have direct evidence to support this. Understanding these interactions could revolutionize our approach to nutrition, fasting, and immune health.
Limitations and future prospects
The presented study has several limitations. The main one is the relatively small group of 13 volunteers participating in them. It should be mentioned here that a group considered by some critics to be small in such a difficult experience as 8 days of complete fasting and the accompanying physical exercise of maximum intensity is extremely difficult to assemble and has not been the subject of research so far, perhaps due to such obstacles. Another limiting factor in this work is the discrepancy between the research protocols used by other authors and ours. Various forms of intermittent fasting or caloric restriction excluding physical exercise are the most frequently used in experiments. Another factor limiting drawing general conclusions is the daily variability of the concentrations of some hormones and the lack of examination of the long-term effects caused by 8-day fasting in the body. Although we avoided the first restriction by conducting research at a similar time of the day, in order to draw far-reaching conclusions, biochemical determinations would have to be made at different times of the day and several days and weeks after the end of the research. It also seems important to examine the impact of different types of physical exercise of varying intensity combined with the fasting intervention. Finally, the experimental protocol can also be improved by testing whether lifestyle influences the results, for example by dividing subjects into physically active and inactive groups or by consuming different types of diets before the experiment. We also failed to find similar studies conducted on women, both premenopausal women, characterized by high hormonal variability, and menopausal women.
Therefore, the presented research should be treated as preliminary, because diversifying the protocols of future research with the limiting factors described above would provide a broader picture of the impact of fasting combined with physical exercise on the human body.
Conclusions
The results of this study showed that 8-days of water-only fasting had a broader impact on metabolic, hormonal, and immune changes than two sets of intense physical exercise in healthy middle-aged men. Significant metabolic changes involving reduction of carbohydrate metabolism and activation of fat metabolism, along with stimulation of ketogenesis, supported by improvement of antioxidant status and IL-6 signalling, appear to be beneficial because they lead to improved metabolic control of the body. The findings regarding the combination of fasting with physical activity, although preliminary, show promise as a useful tool for everyday life, such as during training or for weight maintenance. The results indicate potential health benefits related to changes in carbohydrate and lipid metabolism, which could be significant in the treatment of obesity and other metabolic diseases. Future research should aim to increase the study group size, evaluate the long-term metabolic and hormonal effects of prolonged fasting in comparison to a ketogenic diet, and implement the diet and exercise regimen in individuals with metabolic diseases.
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Palmer, B. F. & Clegg, D. J. Starvation ketosis and the kidney. Am. J. Nephrol. 52, 467–478. https://doi.org/10.1159/000517305 (2021).
Horne, B. D., Muhlestein, J. B. & Anderson, J. L. Health effects of intermittent fasting: Hormesis or harm? A systematic review. Am. J. Clin. Nutr. 102, 464–470. https://doi.org/10.3945/ajcn.115.109553 (2015).
Calabrese, E. J. et al. Hormesis defines the limits of lifespan. Ageing Res. Rev. 91, 102074. https://doi.org/10.1016/j.arr.2023.102074 (2023).
Pilis, K. et al. Effect of 8-days of water-only fasting and vigorous exercise on anthropometric parameters, lipid profile and HOMA-IR in middle-aged men. Biomed. Hum. Kinet. 15, 289–297. https://doi.org/10.2478/bhk-2023-0035 (2023).
Kolb, H., Stumvoll, M., Kramer, W., Kempf, K. & Martin, S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 16, 232. https://doi.org/10.1186/s12916-018-1225-1 (2018).
Cahill, G. F. Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 26, 1–22. https://doi.org/10.1146/annurev.nutr.26.061505.111258 (2006).
Anderson, E. J., Yamazaki, H. & Neufer, P. D. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J. Biol. Chem. 282, 31257–31266. https://doi.org/10.1074/jbc.M706129200 (2007).
Miller, V. J., Villamena, F. A. & Volek, J. S. Nutritional ketosis and mitohormesis: Potential implications for mitochondrial function and human health. J. Nutr. Metab. 2018, 5157645. https://doi.org/10.1155/2018/5157645 (2018).
Puchalska, P. & Crawford, P. A. Multi-dimensional roles of Ketone bodies in fuel metabolism, signaling and therapeutics. Cell Metab. 25, 262–284. https://doi.org/10.1016/j.cmet.2016.12.022 (2017).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368. https://doi.org/10.1126/science.abc8861 (2020).
Evans, M., Cogan, K. E. & Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 595, 2857–2871. https://doi.org/10.1113/JP273185 (2017).
Shi, X. et al. β-Hydroxybutyrate activates the NF-kappaB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell Physiol. Biochem. 33, 920–932. https://doi.org/10.1159/000358664 (2014).
Chriett, S. et al. Prominent action of butyrate over beta-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 9, 742. https://doi.org/10.1038/s41598-018-36941-9 (2019).
Luo, M. J. et al. Fasting before or after wound injury accelerates wound healing through the activation of pro-angiogenic SMOC1 and SCG2. Theranostics 10, 3779–3792. https://doi.org/10.7150/thno.44115 (2020).
Rostami, E. et al. Effect of intermittent fasting on saving zone of stasis in burn wounds in rats. Burns 49, 901–913. https://doi.org/10.1016/j.burns.2022.06.010 (2023).
Kistner, T. M., Pedersen, B. K. & Lieberman, D. E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 4, 170–179. https://doi.org/10.1038/s42255-022-00538-4 (2022).
Górecka, M., Krzemiński, K., Mikulski, T. & Ziemba, A. W. ANGPTL4, IL-6 and TNF-α as regulators of lipid metabolism during a marathon run. Sci. Rep. 12, 19940. https://doi.org/10.1038/s41598-022-17439-x (2022).
Wueest, S. et al. Interleukin-6 contributes to early fasting-induced free fatty acid mobilization in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R861-867. https://doi.org/10.1152/ajpregu.00533.2013 (2014).
He, Z., Xu, H., Li, C., Yang, H. & Mao, Y. Intermittent fasting and immunomodulatory effects: A systematic review. Front. Nutr. 10, 1048230. https://doi.org/10.3389/fnut.2023.1048230 (2023).
Atashak, S. et al. High-intensity interval training improves Lipocalin-2 and Omentin-1 levels in men with obesity. Int. J. Sports Med. 43, 328–335. https://doi.org/10.1055/a-1560-5401 (2022).
Saeidi, A. et al. Differential effects of exercise programs on neuregulin 4, body composition and cardiometabolic risk factors in men with obesity. Front Physiol. 7, 797574. https://doi.org/10.3389/fphys.2021.797574 (2022).
Ataeinosrat, A. et al. Intensity dependent effects of interval resistance training on myokines and cardiovascular risk factors in males with obesity. Front Endocrinol. (Lausanne) 10, 895512. https://doi.org/10.3389/fendo.2022.895512 (2022).
Ghimire, P. & Dhamoon, A. S. Ketoacidosis. StatPearls. https://www.statpearls.com/point-of-care/23877 (2023).
Ramnanan, C. J., Edgerton, D. S., Kraft, G. & Cherrington, A. D. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes. Metab. 13, 118–125. https://doi.org/10.1111/j.1463-1326.2011.01454.x (2011).
Qian, K. et al. Hepatic ALT isoenzymes are elevated in gluconeogenic conditions including diabetes and suppressed by insulin at the protein level. Diabetes Metab. Res. Rev. 31, 562–571. https://doi.org/10.1002/dmrr.2655 (2015).
Sahoo, B., Srivastava, M., Katiyar, A., Ecelbarger, C. & Tiwari, S. Liver or kidney: Who has the oar in the gluconeogenesis boat and when?. World J. Diabetes 14, 1049–1056. https://doi.org/10.4239/wjd.v14.i7.1049 (2023).
Volek, J. S. et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 44, 297–309. https://doi.org/10.1007/s11745-008-3274-2 (2009).
Vink, R. G. et al. Diet-induced weight loss decreases adipose tissue oxygen tension with parallel changes in adipose tissue phenotype and insulin sensitivity in overweight humans. Int. J. Obes. 41, 722–728. https://doi.org/10.1038/ijo.2017.38 (2017).
Sadowska-Krępa, E., Gdańska, A., Pridalova, M., Rozpara, M. & Grabara, M. The effect of calorie restriction on the anthropometric parameters, HOMA-IR index, and lipid profile of female office workers with overweight and obesity: a preliminary study. Int. J. Occup. Med. Environ. Health 35, 693–706. https://doi.org/10.13075/ijomeh.1896.01963 (2022).
Brooks, G. A. Lactate as a fulcrum of metabolism. Redox Biol. 35, 101454. https://doi.org/10.1016/j.redox.2020.101454 (2020).
Frączek, B., Pięta, A., Burda, A., Mazur-Kurach, P. & Tyrała, F. Paleolithic diet—Effect on the health status and performance of athletes?. Nutrients 13, 1019. https://doi.org/10.3390/nu13031019 (2021).
Ashcroft, S. P., Stocks, B., Egan, B. & Zierath, J. R. Exercise induces tissue-specific adaptations to enhance cardiometabolic health. Cell Metab. 36, 278–300. https://doi.org/10.1016/j.cmet.2023.12.008 (2024).
Steinhauser, M. L. et al. The circulating metabolome of human starvation. JCI Insight 3, e121434. https://doi.org/10.1172/jci.insight.121434 (2018).
Vinay, P. et al. Acetate metabolism during hemodialysis: metabolic considerations. Am. J. Nephrol. 7, 337–354. https://doi.org/10.1159/000167500 (1987).
Rosen, E., Bakshi, N., Watters, A., Rosen, H. R. & Mehler, P. S. Hepatic complications of anorexia nervosa. Dig. Dis. Sci. 62, 2977–2981. https://doi.org/10.1007/s10620-017-4766-9 (2017).
Wadley, A. J., Turner, J. E. & Aldred, S. Factors influencing post-exercise plasma protein carbonyl concentration. Free Radic. Res. 50, 375–384. https://doi.org/10.3109/10715762.2015.1131824 (2016).
Bejder, J., Andersen, A. B., Goetze, J. P., Aachmann-Andersen, N. J. & Nordsborg, N. B. Plasma volume reduction and hematological fluctuations in high-level athletes after an increased training load. Scand. J. Med. Sci. Sports 27, 1605–1615. https://doi.org/10.1111/sms.12825 (2017).
Schumacher, H. R. Jr. et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 59, 1540–1548. https://doi.org/10.1002/art.24209 (2008).
La Russa, D. et al. Oxidative balance and inflammation in hemodialysis patients: biomarkers of cardiovascular risk?. Oxid. Med. Cell Longev. 2019, 8567275. https://doi.org/10.1155/2019/8567275 (2019).
Sánchez-Lozada, L. G., Tapia, E., Rodríguez-Iturbe, B., Johnson, R. J. & Herrera-Acosta, J. Hemodynamics of hyperuricemia. Semin. Nephrol. 25, 19–24. https://doi.org/10.1016/j.semnephrol.2004.09.004 (2005).
Battelli, M. G., Bortolotti, M., Polito, L. & Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 2557–2565. https://doi.org/10.1016/j.bbadis.2018.05.003 (2018).
Whittaker, J. & Harris, M. Low-carbohydrate diets and men’s cortisol and testosterone: Systematic review and meta-analysis. Nutr. Health 28, 543–554. https://doi.org/10.1177/02601060221083079 (2022).
Doucet, E., Pomerleau, M. & Harper, M. E. Fasting and postprandial total ghrelin remain unchanged after short-term energy restriction. J. Clin. Endocrinol. Metab. 89, 1727–1732. https://doi.org/10.1210/jc.2003-031459 (2004).
Dardzińska, J. A., Wernio, E. & Małgorzewicz, S. Fasting and postprandial ghrelin changes in older and younger volunteers. Minerva Endocrinol. 47, 413–420. https://doi.org/10.23736/S2724-6507.21.03407-6 (2022).
Nuttall, F. Q., Almokayyad, R. M. & Gannon, M. C. The ghrelin and leptin responses to short-term starvation vs a carbohydrate-free diet in men with type 2 diabetes; a controlled, cross-over design study. Nutr. Metab. 13, 47. https://doi.org/10.1186/s12986-016-0106-x (2016).
Alzoghaibi, M. A., Pandi-Perumal, S. R., Sharif, M. M. & BaHamman, A. S. Diurnal intermittent fasting during Ramadan: The effects on leptin and ghrelin levels. PLoS ONE 9, e92214. https://doi.org/10.1371/journal.pone.0092214 (2014).
Stec, K. et al. Effects of fasting on the physiological and psychological responses in middle-aged men. Nutrients 15, 3444. https://doi.org/10.3390/nu15153444 (2023).
Hollstein, T. et al. Effects of short-term fasting on Ghrelin/GH/IGF-1 axis in healthy humans: The role of Ghrelin in the thrifty phenotype. J. Clin. Endocrinol. Metab. 107, e3769–e3780. https://doi.org/10.1210/clinem/dgac353 (2022).
Veldhuis, J. D. Neuroendocrine control of pulsatile growth hormone release in the human: relationship with gender. Growth Horm. IGF Res. 8, 49–59. https://doi.org/10.1016/s1096-6374(98)80024-5 (1998).
Grottoli, S. et al. Growth hormone/insulin-like growth factor I axis, glucose metabolism, and lypolisis but not leptin show some degree of refractoriness to short-term fasting in acromegaly. J. Endocrinol. Invest. 31, 1103–1109. https://doi.org/10.1007/BF03345660 (2008).
Hartman, M. L., Veldhuis, J. D. & Thorner, M. O. Normal control of growth hormone secretion. Horm. Res. 40, 37–47. https://doi.org/10.1159/000183766 (1993).
Rosenfeld, R. G., Pham, H., Oh, Y., Lamson, G. & Giudice, L. C. Identification of insulin-like Growth factor binding protein-2 (IGF-BP-2) and a low molecular weight IGF-BP in human seminal plasma. J. Clin. Endocrinol. Metab. 70, 551–553. https://doi.org/10.1210/jcem-70-2-551 (1990).
Rinderknecht, E. & Humbel, R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J. Biol. Chem. 253, 2769–2776. https://doi.org/10.1016/S0021-9258(17)40889-1 (1978).
Balduyck, B. et al. Solitary fibrous tumor of the pleura with associated hypoglycemia: Doege-Potter syndrome: A case report. J. Thorac. Oncol. 1, 588–590. https://doi.org/10.1097/01243894-200607000-00016 (2006).
Buono, R. & Longo, V. D. When fasting gets tough, the tough immune cells get going-or die. Cell 178, 1038–1040. https://doi.org/10.1016/j.cell.2019.07.052 (2019).
Aliyu, M. et al. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 111, 109130. https://doi.org/10.1016/j.intimp.2022.109130 (2022).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888. https://doi.org/10.1016/j.bbamcr.2011.01.034 (2011).
Pedersen, B. K. & Febbraio, M. A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406. https://doi.org/10.1152/physrev.90100.2007 (2008).
Zhang, C. et al. Interleukin-6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J. Biol. Chem. 288, 1489–1499. https://doi.org/10.1074/jbc.M112.419788 (2013).
Blanchard, F., Duplomb, L., Baud’huin, M. & Brounais, B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 20, 19–28. https://doi.org/10.1016/j.cytogfr.2008.11.004 (2009).
Sims, N. A. Influences of the IL-6 cytokine family on bone structure and function. Cytokine 146, 155655. https://doi.org/10.1016/j.cyto.2021.155655 (2021).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 17, 1481–1489. https://doi.org/10.1038/nm.2513 (2011).
Steensberg, A., Fischer, C. P., Keller, C., Møller, K. & Pedersen, B. K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 285, E433-437. https://doi.org/10.1152/ajpendo.00074.2003 (2003).
Starkie, R. L., Rolland, J. & Febbraio, M. A. Effect of adrenergic blockade on lymphocyte cytokine production at rest and during exercise. Am. J. Physiol. Cell. Physiol. 281, C1233-1240. https://doi.org/10.1152/ajpcell.2001.281.4.C1233 (2001).
Liu, B., Hutchison, A. T., Thompson, C. H., Lange, K. & Heilbronn, L. K. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes. Res. Clin. Pract. 13, 408–415. https://doi.org/10.1016/j.orcp.2019.07.001 (2019).
Moro, T. et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 14, 290. https://doi.org/10.1186/s12967-016-1044-0 (2016).
Fazeli, P. K. et al. Prolonged fasting drives a program of metabolic inflammation in human adipose tissue. Mol. Metab. 42, 101082. https://doi.org/10.1016/j.molmet.2020.101082 (2020).
Wilhelmi de Toledo, F. et al. Influence of long-term fasting on blood redox status in humans. Antioxidants 9, 496. https://doi.org/10.3390/antiox9060496 (2020).
Albrahim, T., Alangry, R., Alotaibi, R., Almandil, L. & Alburikan, S. Effects of regular exercise and intermittent fasting on neurotransmitters, inflammation, oxidative stress, and brain-derived neurotrophic factor in cortex of ovariectomized rats. Nutrients 15, 4270. https://doi.org/10.3390/nu15194270 (2023).
Mohr, A. E., McEvoy, C., Sears, D. D., Arciero, P. J. & Sweazea, K. L. Impact of intermittent fasting regimens on circulating markers of oxidative stress in overweight and obese humans: A systematic review of randomized controlled trials. Adv. Redox Res. 3, 100026. https://doi.org/10.1016/j.arres.2021.100026 (2021).
El Assar, M., Álvarez-Bustos, A., Sosa, P., Angulo, J. & Rodriguez-Manas, L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int. J. Mol. Sci. 23, 8713. https://doi.org/10.3390/ijms23158713 (2022).
Sołtysik, B. K. Contribution of physical activity to the oxidative and antioxidant potential in 60–65-year-old seniors. Antioxidants 12, 1200. https://doi.org/10.3390/antiox12061200 (2023).
Roumeliotis, S., Roumeliotis, A., Dounousi, E., Eleftheriadis, T. & Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: A review. Nutrients 11, 1911. https://doi.org/10.3390/nu11081911 (2019).
Grundler, F. et al. Interplay between oxidative damage, the redox status, and metabolic biomarkers during long-term fasting. Food Chem. Toxicol. 145, 111701. https://doi.org/10.1016/j.fct.2020.111701 (2020).
Wolfe, R. R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 84, 475–482. https://doi.org/10.1093/ajcn/84.3.475 (2006).
Kolnes, K. J. et al. Effects of seven days’ fasting on physical performance and metabolic adaptation during exercise in humans. Nat. Commun. 16, 122. https://doi.org/10.1038/s41467-024-55418-0 (2025).
Janssen, H. et al. Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity 56, 783–96.e7. https://doi.org/10.1016/j.immuni.2023.01.024 (2023).
Acknowledgements
The authors would like to thank all study participants who contributed their time to this project.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
K.P. conceived the idea, designed the study, was responsible for data collection, participated in writing the manuscript and in the literature review, and also critically revised the manuscript. U.G. participated in the preparation of the manuscript, verified the data and critically revised the manuscript. A.P. reviewed the literature, collected and prepared the data. K.S. collected data and critically revised the manuscript. P.D. supervised and performed statistical analyses. M.K. collected data and critically revised the manuscript. A.K.L. participated in data collection. S.L. initially verified the data, analyzed the data. R.K. verified the data. W.P. analyzed the data, reviewed the literature. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pilis, K., Godlewska, U., Pilis, A. et al. Metabolic and hormonal effects of an 8 days water only fasting combined with exercise in middle aged men. Sci Rep 15, 22805 (2025). https://doi.org/10.1038/s41598-025-05164-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05164-0