Abstract
Topography can significantly influence the atmospheric deposition of heavy metals on the surface soil and further shape their spatial distribution. This study was carried out in Northwestern Guizhou Province, a key agricultural region contaminated with cadmium (Cd), lead (Pb), and zinc (Zn) in agricultural soil. In this study, geographical detectors were employed to quantitatively assess the impact of topography on the spatial distribution of Cd, Pb, and Zn in surface soils. Moreover, the influence of topography on the spatial distribution of Cd, Pb, and Zn in the surface soil was further ascertained using geographical detectors. The results demonstrated that the bulk atmospheric deposition fluxes of Cd in Dawan (DW), Dongfeng (DF), Jinzhong (JZ), Shuanglong (SL), and Heishitou (HST) were 1.538, 0.766, 0.802, 0.365, and 0.186 mg m− 2 a− 1, respectively; For Pb, the corresponding values were 47.13, 34.88, 38.25, 16.95 and 35.76 mg m− 2 a− 1, respectively; in the case of Zn, the fluxes were 579.26, 82.38, 83.20, 45.98, and 29.13 mg m− 2 a− 1, respectively. The atmospheric deposition fluxes of Cd, Pb, and Zn demonstrated a general downward trend from DW to SL because of rainfall reduction with increasing elevation. Industrial activities can significantly increase atmospheric deposition fluxes of Cd, Pb, and Zn. However, the atmospheric deposition fluxes of Cd, Pb, and Zn in the control area HST were significantly lower due to the interception effect of mountain. The results of the Pb isotopic analysis further supported the above conclusions. It also indicated that the atmospheric Pb in DW, DF, JZ, and SL were contaminated by multiple anthropogenic sources, whereas the atmospheric Pb in HST was primarily polluted by burning coal from residents. The geographical detector analysis results demonstrated that the spatial distribution of Cd, Pb, and Zn in the surface soil at JZ was affected by the interaction of topography with organic matter and particle size. In SL, the influence of the interactions of all topographies with physical and chemical characters on the spatial distribution of soil Cd and Pb was significant. The aspect was the only topographical factor influencing Zn. Furthermore, topography and pH were the leading factors affecting the spatial distribution of soil Cd, Pb, and Zn in HST. Therefore, this research provides a scientific basis for the identification of pollution sources and the development of effective soil heavy metal control strategies.
Similar content being viewed by others
Introduction
Soil is closely linked to human survival and production, because some heavy metals in soil and the atmosphere are deadly to production and life because of their hazardous effects, bioavailability, and non-degradability1,2. Though some trace quantities heavy metals were critical to life of human, animal, and plant, such as cadmium (Cd), lead (Pb), and zinc (Zn), they posed adverse impact on living organisms, even at low concentrations3. Furthermore, bioavailability of HMs can be concentrated in crops, which may threaten human health through the food chain4,5. Consequently, heavy metals has been recognized as one of most critical constituents of global environmental pollutants impacting human and biota6,7,8.
Heavy metal sources in the soil are mainly natural (rock weathering and pedogenesis), industrial, agricultural, traffic-related, and atmospheric deposition9,10,11,12,13,14. Natural sources connected to stratigraphy and lithology typically contribute less to the concentrations of heavy metals in soil. Industrial sources included metal smelting9,15,16,17, the phosphorus chemical industry18, thermal power plants19, and the electronics industry20. Agricultural sources largely comprise fertilizers, pesticides, green manure, and livestock manure21. Traffic sources are predominantly associated with the combustion of gasoline or diesel, tyre wear, brake, three-way catalytic converters, and road surfaces12,22,23. Atmospheric deposition has become increasingly prominent in recent years. Approximately 35% of the Cd in Chinese soil is attributed to atmospheric deposition24, with this proportion exceeding 40% in mining areas25. Particularly, this contribution rate can reach 50-93% for As, Cr, Hg, and Pb in some special mining area26. Typically, the contribution of wet deposition to the ground surface is great higher than that of dry deposition27. According to the summary and calculation, approximately 68-74% of Cd and Zn are derived from wet deposition and 25–33% from dry deposition28; some studies have that wet deposition controls the deposition of heavy metals in humid and semi-humid region, while dry deposition is the major contributor in arid and semi-arid regions29,30.
Furthermore, understanding the spatial distribution of heavy metals in soil, which is affected by topography and human activity, is crucial for source identification and pollution control. Topography, including elevation, slope, and aspect, plays a vital role in the distribution of heavy metals in the soil. The effect of topography on the distribution of Cd, Pb, and Zn is primarily related to hydrothermal conditions, air mass sources, and land cover. Some studies have demonstrated that the distribution of Pb in soil and mosses exhibits different characteristics at different altitudes and slopes, which have a redistribution effect on atmospheric deposition and human activities31,32. Some showed that altitude can influence the soil Pb distribution by controlling wet deposition. Additionally, different aspects can screen different sources of heavy metals for the interception effect of mountains30,33. However, previous studies have mainly focused on alpine ecosystems that are less affected by human activities, leading to some limitations for these research results.
Additionally, the geographical detector method, developed by Wang and Hu34, can quantitatively determine the effect of each factor on the spatial heterogeneity of heavy metals in soil and measure the contribution of each factor more intuitively, quickly, and effectively35. Some studies have used geographical detectors to investigated land use36,37,38, soil erosion39,40,41, water quality assessment42, and environmental quality assessment43,44,45. Recently, geographical detectors have been employed to investigate the factors driving heavy metal pollution in soil46,47,48. Researchers have categorized the factors influencing cultivated land change into natural and socioeconomic factors. Natural factors such as elevation and slope, are the primary influences on cultivated land change, whereas socioeconomic factors, including human activities, agricultural and industrial activities, and traffic, are the leading causes of heavy metal accumulation in the soil.
Northwest Guizhou Province is a typical geochemical anomaly region characterized by high enrichment of heavy metals, such as Cd, Pb, Zn, and Cr, in soil because of the wide distribution of carbonate rock and basalt21,49. Moreover, this region has been known for its indigenous Zn smelting and the presence of substantial Pb-Zn deposits50. Moreover, many industrial and mining enterprises are rich in mineral resources, which exacerbates local soil pollution. With the increase in car ownership and improvements in traffic conditions, traffic has become an important source of heavy metals. Consequently, this region faces severe soil heavy metal pollution from complex sources21,51,52.
Therefore, the objectives of this study were to investigate the effect of topography on the atmospheric deposition of Cd, Pb, and Zn under the impact of human activities, and to ascertain the influence of topography on the distribution of Cd, Pb, and Zn in surface soil by using geographical detectors.
Methods and materials
Study area
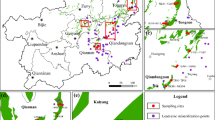
This study was conducted in Northwestern Guizhou Province, Southwest of China. To study the impact of topography on the atmospheric deposition of Cd, Pb, and Zn, four locations (Dawan Town (DW) (104.658601115, 26.758494302), Dongfeng Town (DF) (104.600916134, 26.820008754), Jinzhong Town (JZ) (104.384753434, 26.784036), and Shuanglong Town (SL) (104.134287581, 26.862175487) in Northwestern Guizhou Province were selected as the study sites according to the principle of gradual elevation increase. DW and JZ are industrial areas (including zinc smelting plant, thermal power plant, and cement plant), whereas DF and SL are non-industrial areas. Another site, Heishitou Town (HST) (104.005066137, 26.752846911) (a typical agricultural area), was set as the control site for the mountain barrier. Those locations were shown in Fig. 1.
Sample collection
Atmospheric deposition
At five strategically selected points stretching from east to west (Fig. 1), the samples of atmospheric deposition were collected. continuously throughout the year (from May 2022 to February 2023) according to the seasons (spring: March–May, summer: June–August, autumn: September–November, winter: December–February). Atmospheric deposition samples were collected via the setting vat (height: 30 cm, inner diameter: 15 cm), and five setting vats were placed at each sampling point. In order to prevent of proliferation of algae, methyl alcohol was added in the setting vat. Every atmospheric deposition sample was divided into two parts (dry deposition and wet deposition). The following treatments of atmospheric deposition samples referred to that of Zhang et al. (2022).
Soil samples
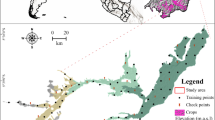
In order to study the effect of topography on spatial distribution of Cd, Pb, and Zn in surface soil, a total of 131 surface soil samples (0–20 cm depth) were collected from JZ, SL, and HST (Fig. 1). Each sample was a composite of 5 subsamples gathered from 5 spots within well-ventilated and dry outdoor locations, thoroughly mixed, and passed through a 0.149 mm sieve to remove sand, gravel, and plant debris. Subsequently, the samples were stored in glass bottles at room temperature for heavy metal analysis.
Sample analysis
Mass concentration of cd, pb, and Zn
The microwave digestion method was used to determine the concentrations of Cd, Pb, and Zn in atmospheric deposition and soil. All samples were weighed in a digester, and 1 mL of concentrated hydrochloric acid, 4 mL of nitric acid, 1 mL of hydrofluoric acid, and 1 mL of hydrogen peroxide were added in the digester. Samples were then digested at 175℃ for 20 min by using a microwave digestion device. Once cooled to room temperature, the digestion tank lid was carefully opened, and the acid was removed at 150℃ until the contents were nearly dry. The resulting solution was transferred to a 50 mL volumetric flask for constant volume filtration. Subsequently, 10 mL of the filtrate was taken to determine the heavy metal concentrations using inductively coupled plasma mass spectrometry (ICP-MS). All reagents were of analytical grade or higher. The glassware was cleaned by soaking in 10% HNO3 for at least two days, followed by rinsing with deionised water before use. The average blank levels for these elements were lower than the method detection limits. The recovery efficiencies of these elements ranged from approximately 90–110%. Three replicate samples were analyzed, and the relative standard deviations (RSD) for heavy metal concentrations did not exceeding 5%.
Soil physical and chemical characters
Physical and chemical properties of tested soil encompassed pH, soil organic matter (SOM), cation exchange capacity (CEC), and texture. The methodology employed to ascertain SOM content was drawn from Bao53. Soil particle size represented primary particle composition of soil, and both the size distribution and mean diameter of particles were measured by the laser particle size analyzer (Bettersize 2600, Dandong Bettersize Instruments Ltd.).
Pb isotope
In this study, five TSP samples from DW, DF, JZ, SL, and HST, respectively, collected in 2023, were analysed for Pb isotopic compositions. The Pb isotopic compositions were analysed using an Agilent 7900 quadrupole inductively coupled plasma mass spectrometer (Agilent Technologies). Moreover, 208Pb/206Pb and 206Pb/207Pb ratios were determined in this study. The precision and accuracy of the elemental analyses were verified using the NIST of Standards and Technology standard reference material (SRM 1648).
Influence of topography on cd, pb, and Zn in surface soil by using geographical detector
Geodetector is a collection of statistical methods designed to detect spatial heterogeneity and identify the driving forces behind it, implemented within the Geo Detector software42. Geodetector comprises 4 detectors: factor detector, interaction detector, risk detector, and ecological detector. In this study, the factor detector and interaction detector were employed to assess the spatial heterogeneity of variable Y and to determine the extent to which factor X accounts for the spatial variability of variable Y. The formula is as follows:
where q represents the explanatory power of the independent variable, with a range of [0,1]; L denotes the stratification of the dependent or independent variable; Nh and σh are the unit number and variance of layer h, respectively; N and σ are the unit number and variance of the entire study area, respectively. The interaction detector within the Geo Detector software is employed to examine the interactions between different influencing factors and whether the interaction of the impact factors X1 and X2 will weaken or enhance the explanatory power of the dependent variable Y, or whether the effects of these factors on Y are independent of each other41. The 5 types of interaction between two independent variables (X1, X2) on the dependent variable are shown in Table 1. The larger the q value is, the stronger the influence of a certain factor or the interaction effect between two factor is. Moreover, DEM data, used in this analysis, were acquired from the Geospatial Data Cloud website of the Computer Network Information Center, Chinese Academy of Sciences (http://www.gscloud.cn). Terrain factor data, encompassing slope, aspect, and elevation, were generated through operations such as splicing, clipping, and data extraction using ArcGIS 10.2 software.
Data processing
The data were pre-processed and transformed using Microsoft Excel 2007. IBM SPSS Statistic (version 25.0) was used to analyse variance (ANOVA). A T-test was used to check the outliers of all experimental data, and the Kolmogorov-Smirnov (K-S) test was used to test the normality of the data.
Results and discussion
Atmospheric deposition fluxes of cd, pb, and Zn
As shown in Fig. 2, the bulk atmospheric deposition fluxes for Cd in DW, DF, JZ, SL, and HST were 1.538, 0.766, 0.802, 0.365, and 0.186 mg m− 2 a− 1, respectively; 47.13, 34.88, 38.25, 16.95 and 35.76 mg m− 2 a− 1, respectively, for Pb; and 579.26, 82.38, 83.20, 45.98, and 29.13 mg m− 2 a− 1, respectively, for Zn. Thus, the bulk atmospheric deposition fluxes of Cd, Pb, and Zn gradually increased from DW to SL with the general improvement of elevation, expect for in JZ. Because JZ was a polluted area for existing of many smelting slags and a Zn smelter, the atmospheric deposition fluxes of Cd, Pb, and Zn increase to some extent. Furthermore, the wet deposition fluxes of Cd and Zn were significantly higher than those of the dry deposition at all 4 sites (Table 2), indicating that wet deposition was the primary contributor. Moreover, the wet deposition fluxes of Cd and Zn showed a downward trend from DW to SL because of rainfall reduction with increasing elevation30. On the one hand, because the study area is located in the ascent section of the Yunnan-Guizhou Plateau, the warm and humid air current from the Indian Ocean can not transport to the leeward side, leading to that it difficult to form rainfall in the high altitude area54; on the other hand, in the high altitude area, the low air density results in frustration of ascending motion of water vapor, which is difficult to form strong convective activity. Additionally, with increase of elevation, the effect of anthropogenic sources gradually decrease, leading to lack of condensation nucleus in the high altitude area. The dry deposition fluxes of Pb in these sites showed different characteristics that the contribution of dry deposition was higher than that of wet deposition (Table 2). Compared to DW, DF, JZ, and SL, HST was located on the other side of the mountain (Fig. 2). Thus, the bulk atmospheric deposition fluxes of Cd, Pb, and Zn were generally lower, and the contributions of dry and wet deposition were different, which was caused by the interception effect of the mountains for atmospheric particulates carrying many heavy metals30,33.
The influence of topography on atmospheric deposition by using a Pb isotope
To further analyze the impact of topography on atmospheric deposition, the Pb isotopic compositions of atmospheric deposition at the 5 locations were determined. Figure 3 showed that all the Pb isotopic compositions of atmospheric deposition in DW, DF, JZ, SL, and HST were near anthropogenic sources, including slag, smelter dust, dust from burning coal, gasoline dust, and diesel dust, indicating that Pb in atmospheric deposition was primarily derived from anthropogenic activities9,12,17,55(Cloquet et al., 2006; Wang et al., 2019; Xuan, 2019; Yu et al., 2022). Some studies have demonstrated that the ratio of 208Pb/206Pb was a good index for identifying the anthropogenic sources and natural sources because anthropogenic sources possessed higher values of 208Pb/206Pb than natural sources17,56,57. In this study, the ratio of 208Pb/206Pb from DW to SL showed a downward trend, indicating that atmospheric Pb pollution gradually decreased with increasing elevation. Because of the interception function of the mountain, contaminants from Eastern Weining cannot be transported to the HST, leading to a lower ratio of 208Pb/206Pb for atmospheric deposition in HST than in other regions. Furthermore, the distribution of Pb isotopic composition points exhibited the same characteristics.
The influence of topography and soil physical and chemical characteristics on the spatial distribution of Cd, Pb, and Zn
According to the geographical detector analysis method, the q value was employed to quantify the single-factor effects of the driving factors on the accumulation of Cd, Pb, and Zn in cultivated soil. A higher q value indicates stronger explanatory power of the influencing factor on the dependent variable, and vice versa. The topographic characteristics and spatial distribution of soil physical and chemical characters in JZ, SL, and HST were shown in Fig S1–S3.
Single factor analysis
Figure 4 displayed the explanatory power (q value) of each driving factor for different heavy metals in JZ, SL, and HST. The explanatory power of various factors for the three heavy metals varied, and the explanatory power of each factor for Cd, Pb, and Zn in the three study areas also differed. As illustrated in Fig. 4a, the primary influencing factors for Cd in the HST were pH (0.130) and slope (0.116), whereas the main influencing factors for Pb were sand (0.121) and clay (0.106). Regarding the distribution of Zn in HST, clay (0.109) was the principal influencing factor, whereas pH (0.056) and sand (0.071) served as secondary influencing factors. The factors influencing the accumulation of Cd, Pb, and Zn in the cultivated soil of the SL study area are shown in Fig. 4b. The leading factors influencing Cd were organic matter (0.521) and clay (0.497), followed by pH (0.355), silt (0.407), and sand (0.332). Sand (0.421), pH (0.258), organic matter (0.242), clay (0.208), and slope (0.223) were the primary factors influencing the distribution of Pb. The main factor affecting Zn distribution was powder (0.255), followed by organic matter (0.217) and cation exchange capacity (CEC; 0.189). Furthermore, in JZ (Fig. 4c), the primary factor influencing Cd was organic matter (0.468), followed by clay (0.344), and sand (0.348). The leading factor affecting Pb was clay (0.382), and the secondary factor was organic matter (0.300). The order of the factors influencing the spatial distribution of Zn was organic matter (0.431), clay (0.414), and sand (0.416). Thus, pH, organic matter, and particle size have strong explanatory power for the spatial distribution of Cd and Pb. Unlike Cd and Pb, the factors influencing the spatial distribution of Zn in the three study areas were different, and the leading factors varied, suggesting that the reasons for Zn accumulation in the different study areas are diverse35. The above results demonstrate that the soil physical and chemical characteristics had a significant impact on the distribution of Cd, Pb, and Zn, whereas the influence of topography was limited.
Interaction analysis
Because interactions between topography and soil physical and chemical characteristics probably existed, the interaction detection tool in the geographical detector was used to identify the interplay between the two factors and explain the enhancement or weakening of the interaction between the two variables. The interaction effects of these factors on the spatial distributions of Cd, Pb, and Zn were shown in Fig. 5. These findings revealed that the effect of the interaction of multiple factors on Cd, Pb, and Zn in the three areas was greater than that of a single factor. Most interactions were nonlinear enhancements, and a few were bilinear enhancement. This observation supported the idea that multiple factors govern the accumulation of Cd, Pb, and Zn in surface soil. Generally, the external environmental conditions in the three study areas were complex and diverse.
In the HST, the interactions between pH, slope, clay, and other factors significantly influenced the spatial distribution of Cd. Therefore, the CEC∩slope (0.685) had the greatest impact on Cd spatial distribution. The interactions between silt, sand, slope, aspect, and other factors exhibited a prominent effect on Pb spatial distribution, with the largest q value in sand∩aspect (0.570). The interactions between pH, clay, aspect, and other factors affected the distribution of Zn, and CEC∩slope (0.500) had the largest impact. Consequently, topography, pH, and particle size played leading roles in the spatial distribution of Cd, Pb, and Zn in the surface soil of the HST. Differences in topographic distribution caused a secondary distribution of soil particles such as high organic matter, low clay, and high water content in the lower parts, resulting in differences in the spatial distribution of Cd, Pb, and Zn15,21,25. Furthermore, some studies have shown that low-altitude areas were easily polluted by local pollution sources, whereas high-altitude areas were affected by pollution from long-range transport30. Thus, topography can affect the atmospheric deposition of heavy metals in the mountainous areas and further influence the their spatial distribution in surface soil. The spatial distributions of Cd, Pb, and Zn in the area were consistent with elevation (Fig. S4), indicating this region was affected by atmospheric deposition and natural sources21.
In SL, the interactions between pH, organic matter, clay, and other factors exhibited a strong enhancement effect on the spatial distribution of Cd, and the interaction between pH and clay (q = 0.815) had the greatest impact on Cd distribution. The elevation, slope, and aspect were significantly related to the physical and chemical characteristics of soil, suggesting that Cd in surface soil SL primarily originated from anthropogenic sources, such as coal combustion, traffic-related sources and extensive use of agricultural supplies21,58,59. Combined with the actual Cd distribution (Fig. S4), a high-content area appeared in the central and northern parts of this area (populated area), indicating the accumulation of anthropogenic sources. Furthermore, the interactions between pH, silt, slope aspect, and other factors demonstrated strong explanatory power for the spatial distribution of Pb and Zn. The factors affecting Pb were ranked as follows: silt∩pH (0.754) > silt∩clay (0.665) > clay∩pH (0.631) > silt∩sand (0.605). Therefore, aspect and slope had significantly effects on pH and silt content, indicating that human activities had less influence on soil Pb than on Cd. Combined with the real spatial distribution of Pb (Fig. S4), the vehicle exhaust was the main source of Pb accumulation and soil acidification in the area near the road. The interaction between organic matter and pH, with a q value of 0.526, was the most significant factor affecting Zn spatial distribution. Moreover, the aspect indeed influenced Zn distribution. Additionally, slope and elevation interacted less with other factors. Thus, Zn principally originated from natural sources and atmospheric deposition.
The interactions between all impact factors were had substantial explanatory power (> 0.640) for the spatial distribution differences in Cd, Pb, and Zn in JZ. The interactions of various influencing factors in JZ had similar effects on Cd, Pb, and Zn in the surface soil, indicating that the three metals in JZ had similar sources. Moreover, the aspect had significant interactions with organic matter, clay, and silt, demonstrating local pollution sources probably affected the spatial distribution of Cd, Pb, and Zn. Additionally, the slope showed an interaction with SOM for all three metals. Based on the actual distributions of the three trace metals (Fig. S4), they were mainly affected by the smelting plant and the surrounding slags.
Conclusion
The atmospheric deposition fluxes of Cd, Pb, and Zn showed a general downward trend from DW to SL due to rainfall reduction with increasing elevation. Industrial activities can significantly increase atmospheric deposition fluxes in industrial areas. However, the atmospheric deposition fluxes of Cd, Pb, and Zn in the control area (HST) were significantly lower because of mountain interception. The results of the Pb isotope analysis further demonstrated the above conclusions and indicated that the atmospheric Pb of DW, DF, JZ, and SL were contaminated by multiple anthropogenic sources, whereas the atmospheric Pb of HST was primarily affected by burning coal from residents.
The geographical detector analysis results indicated that the spatial distribution of Cd, Pb, and Zn in the surface soil at JZ was affected by the interaction of topography (elevation and aspect) with organic matter and particle size, with the main sources being smelting plants and surrounding smelting slags. In SL, the influence of interactions of all topographic factors with physical and chemical characteristics on the spatial distribution of soil Cd and Pb was significant, indicating contributions from multiple anthropogenic sources. The aspect was the only influencing topographic factor for Zn, and further analysis showed that atmospheric deposition was the primary external source of Zn in the surface soil of the SL. Furthermore, topography and pH were the leading factors affecting the spatial distribution of soil Cd, Pb, and Zn in the HST, and further analyses demonstrated that they were mainly influenced by atmospheric deposition.
Therefore, this study provides a scientific basis for the identification of pollution sources and the development of effective soil heavy metal control strategies.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Somayaji, A., Sarkar, S., Balasubramaniam, S. & Raval, R. Synthetic biology techniques to tackle heavy metal pollution and poisoning. Synth. Syst. Biotechnol. 7(3), 841–846. https://doi.org/10.1016/j.synbio.2022.04.007 (2022).
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. & Sutton, D. J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum (ed Luch, A.) vol 101 (Springer, Basel, 2012). https://doi.org/10.1007/978-3-7643-8340-4_6.
Khan, S., Cao, Q., Zheng, Y. M., Huang, Y. Z. & Zhu, Y. G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in beijing, China. Environ. Pollut. 152(3), 686–692. https://doi.org/10.1016/j.envpol.2007.06.056 (2008).
Imseng, M. et al. Fate of CD in agricultural soils: A stable isotope approach to anthropogenic impact, soil formation, and soil-plant cycling. Environ. Sci. Technol. 52(4), 1919–1928. https://doi.org/10.1021/acs.est.7b05439 (2018).
Imseng, M. et al. The fate of Zn in agricultural soils: A stable isotope approach to anthropogenic impact, soil formation, and soil–plant cycling. Environ. Sci. Technol. 53(8), 4140–4149. https://doi.org/10.1021/acs.est.8b03675 (2019).
Ahmed, M. K. et al. Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river buriganga, Bangladesh. Environ. Sci. Pollut. Res. 22(20), 15880–15890. https://doi.org/10.1007/s11356-015-4813-z (2015).
Carolin, C. F., Kumar, P. S., Saravanan, A., Joshiba, G. J. & Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 5(3), 2782–2799. https://doi.org/10.1016/j.jece.2017.05.029 (2017).
Islam, M. S., Ahmed, M. K., Habibullah-Al-Mamun, M. & Masunaga, S. Trace metals in soil and vegetables and associated health risk assessment. Environ. Monit. Assess. 186(12), 8727–8739. https://doi.org/10.1007/s10661-014-4040-y (2014).
Cloquet, C., Carignan, J., Libourel, G., Sterckeman, T. & Perdrix, E. Tracing source pollution in soils using cadmium and lead isotopes. Environ. Sci. Technol. 40(8), 2525–2530. https://doi.org/10.1021/es052232+ (2006).
Huang, M., Ivey, C., Hu, Y., Holmes, H. A. & Strickland, M. J. Source apportionment of primary and secondary PM2.5: Associations with pediatric respiratory disease emergency department visits in the U.S. State of Georgia. Environ. Int. 133, 105167. https://doi.org/10.1016/j.envint.2019.105167 (2019).
Wang, L. et al. Possible application of stable isotope compositions for the identification of metal sources in soil. J. Hazard. Mater. 407, 124812. https://doi.org/10.1016/j.jhazmat.2020.124812 (2021).
Wang, J. et al. Source apportionment of heavy metal and their health risks in soil-dustfall-plant system nearby a typical non-ferrous metal mining area of Tongling, Eastern China. Environ. Pollut. 254, 113089. https://doi.org/10.1016/j.envpol.2019.113089 (2019).
Wu, J., Li, J., Teng, Y., Chen, H. & Wang, Y. A partition computing-based positive matrix factorization (PC-PMF) approach for the source apportionment of agricultural soil heavy metal contents and associated health risks. J. Hazard. Mater. 388, 121766. https://doi.org/10.1016/j.jhazmat.2019.121766 (2020).
Zhuo, H., Fu, S., Liu, H., Song, H. & Ren, L. Soil heavy metal contamination and health risk assessment associated with development zones in Shandong, China. Environ. Sci. Pollut. Res. 26(29), 30016–30028. https://doi.org/10.1007/s11356-019-05979-1 (2019).
Kang, M. J. et al. Mobility of metal(loid)s in roof dusts and agricultural soils surrounding a Zn smelter: Focused on the impacts of smelter-derived fugitive dusts. Sci. Total Environ. 757, 143884. https://doi.org/10.1016/j.scitotenv.2020.143884 (2021).
Peng, J. et al. Soil heavy metal pollution of industrial legacies in China and health risk assessment. Sci. Total Environ. 816, 151632. https://doi.org/10.1016/j.scitotenv.2021.151632 (2022).
Yu, E. et al. Superposition effects of zinc smelting atmospheric deposition on soil heavy metal pollution under geochemical anomaly. Front. Environ. Sci. 10 https://doi.org/10.3389/fenvs.2022.777894 (2022).
Jiao, W., Chen, W., Chang, A. C. & Page, A. L. Environmental risks of trace elements associated with long-term phosphate fertilizers applications: A review. Environ. Pollut. 168, 44–53. https://doi.org/10.1016/j.envpol.2012.03.052 (2012).
Raja, R. et al. Impairment of soil health due to fly ash-fugitive dust deposition from coal-fired thermal power plants. Environ. Monit. Assess. 187(11). https://doi.org/10.1007/s10661-015-4902-y (2015).
Yang, S. et al. An integrated analysis on source-exposure risk of heavy metals in agricultural soils near intense electronic waste recycling activities. Environ. Int. 133, 105239. https://doi.org/10.1016/j.envint.2019.105239 (2019).
Zhang, Q. et al. Source identification and superposition effect of heavy metals (HMS) in agricultural soils at a high geological background area of karst: A case study in a typical watershed. Int. J. Environ. Res. Public Health. 19(18), 11374. https://doi.org/10.3390/ijerph191811374 (2022).
Taghvaee, S. et al. Source apportionment of ambient PM2.5 in two locations in central Tehran using the positive matrix factorization (PMF) model. Sci. Total Environ. 628–629, 672–686. https://doi.org/10.1016/j.scitotenv.2018.02.096 (2018).
Gérardin, F. & Midoux, N. Attenuation of road dust emissions caused by industrial vehicle traffic. Atmos. Environ. 127, 46–54. https://doi.org/10.1016/j.atmosenv.2015.12.006 (2016).
Luo, L., Ma, Y., Zhang, S., Wei, D. & Zhu, Y. G. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 90(8), 2524–2530. https://doi.org/10.1016/j.jenvman.2009.01.011 (2009).
Liu, B. et al. Dominant roles of torrential floods and atmospheric deposition revealed by quantitative source apportionment of potentially toxic elements in agricultural soils around a historical mercury mine, Southwest China. Ecotoxicol. Environ. Saf. 242, 113854. https://doi.org/10.1016/j.ecoenv.2022.113854 (2022).
Feng, W. et al. Atmospheric bulk deposition of heavy metal(loid)s in central South china: fluxes, influencing factors and implication for paddy soils. J. Hazard. Mater. 371, 634–642. https://doi.org/10.1016/j.jhazmat.2019.02.090 (2019).
Wu, Y. et al. Atmospheric deposition of trace elements to Daya bay, South China sea: Fluxes and sources. Mar. Pollut. Bull. 127, 672–683. https://doi.org/10.1016/j.marpolbul.2017.12.046 (2018).
Vithanage, M. et al. Deposition of trace metals associated with atmospheric particulate matter: Environmental fate and health risk assessment. Chemosphere 303, 135051. https://doi.org/10.1016/j.chemosphere.2022.135051 (2022).
Liu, Y. et al. Environmental effect of heavy metals deposition in Arid city. Global NEST J. 21(3), 405–409. https://doi.org/10.30955/gnj.003143 (2019).
Zhong, Z. et al. Terrain-modulated deposition of atmospheric lead in the soils of alpine forest, central China. Sci. Total Environ. 790, 148106. https://doi.org/10.1016/j.scitotenv.2021.148106 (2021).
Bing, H. et al. Barrier effects of remote high mountain on atmospheric metal transport in the Eastern Tibetan plateau. Sci. Total Environ. 628–629. https://doi.org/10.1016/j.scitotenv.2018.02.035 (2018).
Lee, C. S., Li, X., Zhang, G., Peng, X. & Zhang, L. Biomonitoring of trace metals in the atmosphere using moss (Hypnum Plumaeforme) in the Nanling mountains and the Pearl river delta, Southern China. Atmos. Environ. 39(3), 397–407. https://doi.org/10.1016/j.atmosenv.2004.09.067 (2005).
Tositti, L. et al. Deposition processes over complex topographies: Experimental data meets atmospheric modeling. Sci. Total Environ. 744, 140974. https://doi.org/10.1016/j.scitotenv.2020.140974 (2020).
Wang, J. F. & Hu, Y. Environmental health risk detection with geogdetector. Environ. Model Softw. 33, 114–115. https://doi.org/10.1016/j.envsoft.2012.01.015 (2012).
Zeng, J. et al. A practical method for identifying key factors in the distribution and formation of heavy metal pollution at a smelting site. J. Environ. Sci. 127, 552–563. https://doi.org/10.1016/j.jes.2022.06.026 (2023).
Fan, Z., Duan, J., Lu, Y., Zou, W. & Lan, W. A geographical detector study on factors influencing urban park use in Nanjing, China. Urban Fore. Urban Green. 59(October 2020). https://doi.org/10.1016/j.ufug.2021.126996 (2021).
Liu, S. et al. Evaluation of heavy metal distribution characteristics of agricultural soil–rice system in a high geological background area according to the influence index of comprehensive quality (IICQ). Environ. Sci. Pollut. Res. 27(17), 20920–20933. https://doi.org/10.1007/s11356-020-08453-5 (2020).
Luo, Y., Wang, Z., Zhou, X., Hu, C. & Li, J. Spatial-temporal driving factors of urban landscape changes in the karst mountainous regions of Southwest china: A case study in central urban area of Guiyang City. Sustainability 14(14). https://doi.org/10.3390/su14148274 (2022).
Chu, L., Sun, T., Wang, T., Li, Z. & Cai, C. Temporal and spatial heterogeneity of soil erosion and a quantitative analysis of its determinants in the three Gorges reservoir area, China. Int. J. Environ. Res. Public Health. 17 (22), 1–21. https://doi.org/10.3390/ijerph17228486 (2020).
Du, Z., Xu, X., Zhang, H., Wu, Z. & Liu, Y. Geographical Detector-based identification of the impact of major determinants on aeolian desertification risk. PLOS ONE 11(3). https://doi.org/10.1371/journal.pone.0151331 (2016).
Liao, K. et al. Quantitative analysis of the factors influencing the Spatial distribution of Benggang landforms based on a geographical detector. ISPRS Int. J. Geo-Inf. 11(6). https://doi.org/10.3390/ijgi11060337 (2022).
Wang, J., Hu, M., Zhang, F. & Gao, B. Influential factors detection for surface water quality with geographical detectors in China. Stoch. Env. Res. Risk Assess. 32(9), 2633–2645. https://doi.org/10.1007/s00477-018-1532-2 (2018).
Bai, L., Jiang, L., Yang, D. & Liu, Y. Quantifying the Spatial heterogeneity influences of natural and socioeconomic factors and their interactions on air pollution using the geographical detector method: A case study of the Yangtze river economic belt, China. J. Clean. Prod. 232, 692–704. https://doi.org/10.1016/j.jclepro.2019.05.342 (2019).
Zhou, D., Lin, Z., Liu, L. & Qi, J. Spatial-temporal characteristics of urban air pollution in 337 Chinese cities and their influencing factors. Environ. Sci. Pollut. Res. 28 (27), 36234–36258. https://doi.org/10.1007/s11356-021-12825-w (2021).
Zou, B. et al. An integrated H-G scheme identifying areas for soil remediation and primary heavy metal contributors: A risk perspective. Sci. Rep. 7(1), 341. https://doi.org/10.1038/s41598-017-00468-2 (2017).
Qiao, P. et al. Quantitative analysis of the factors influencing soil heavy metal lateral migration in rainfalls based on geographical detector software: A case study in Huanjiang county, China. Sustainability 9(7). https://doi.org/10.3390/su9071227 (2017).
Qiao, P., Yang, S., Lei, M., Chen, T. & Dong, N. Quantitative analysis of the factors influencing Spatial distribution of soil heavy metals based on geographical detector. Sci. Total Environ. 664, 392–413. https://doi.org/10.1016/j.scitotenv.2019.01.310 (2019).
Zhao, Y., Deng, Q., Lin, Q., Zeng, C. & Zhong, C. Cadmium source identification in soils and high-risk regions predicted by geographical detector method. Environ. Pollut. 263, 114338. https://doi.org/10.1016/j.envpol.2020.114338 (2020).
Luo, K. et al. Spatial distribution and migration of cadmiumin contaminated soils associated witha geochemical anomaly: A case studyin Southwestern China. Pol. J. Environ. Stud. 28(5), 3799–3807. https://doi.org/10.15244/pjoes/94847 (2019).
Wen, H. et al. Zn/Cd ratios and cadmium isotope evidence for the classification of lead-zinc deposits. Sci. Rep. 6(April), 1–8. https://doi.org/10.1038/srep25273 (2016).
Yang, W. et al. Spatial distribution, food chain translocation, human health risks, and environmental thresholds of heavy metals in a maize cultivation field in the heart of china’s karst region. J. Soils Sedim. 22(10), 2654–2670. https://doi.org/10.1007/s11368-022-03256-2 (2022).
Zhou, Y. et al. Legacy of multiple heavy metal(loid)s contamination and ecological risks in farmland soils from a historical artisanal zinc smelting area. Sci. Total Environ. 720, 137541. https://doi.org/10.1016/j.scitotenv.2020.137541 (2020).
Bao, S. Soil Agrochemistrical Analysis 3rd edn (China Agriculture, 2007).
Beckford, H. O., Chu, H., Song, C., Chang, C. & Ji, H. Geochemical characteristics and behaviour of elements during weathering and pedogenesis over karst area in Yunnan–Guizhou plateau, Southwestern China. Environ. Earth Sci. 80(2). https://doi.org/10.1007/s12665-020-09343-2 (2021).
Xuan, B. Characteristics of heavy metal pollution and Pb isotope tracing in soil vertical profiles of suburban vegetable fields in Guiyang city. https://link.cnki.net/doi/10.27048/d.cnki (2019). https://doi.org/10.27048/d.cnki.ggzsu.2019.000228.
Peng, Y. Distribution and Migration of Heavy Metals among System of the Slag, Soil and Plant in the Indigenous Zinc Smelting Area of Northwestern Guizhou Province, China. (2019). https://kns.cnki.net/kcms2/article/abstract?v=BQVG6Ge829ZwdvqeOHVpjOresO6sxsVoInIHaRQGx9xTEPjHVY4u5z44XPazokGzuN-JlMlkVBmsJ4zQXeRTuEQnPQUbPWdq3_Fl45xjA0W7vQmRhZ3cxkmYEge-8mJKOPGOcOhxkEI=&uniplatform=NZKPT&language=CHS.
Kong, X. et al. Heavy metal bioaccumulation in rice from a high geological background area in Guizhou province, China. Int. J. Environ. Res. Public Health. 15 (10), 2281. https://doi.org/10.3390/ijerph15102281 (2018).
Guan, D. X., Sun, F. S., Yu, G. H., Polizzotto, M. L. & Liu, Y. G. Total and available metal concentrations in soils from six long-term fertilization sites across China. Environ. Sci. Pollut. Res. 25(31), 31666–31678. https://doi.org/10.1007/s11356-018-3143-3 (2018).
Shao, D., Zhan, Y., Zhou, W. & Zhu, L. Current status and temporal trend of heavy metals in farmland soil of the Yangtze river Delta region: Field survey and meta-analysis. Environ. Pollut. 219, 329–336. https://doi.org/10.1016/j.envpol.2016.10.023 (2016).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization was performed by Q.Z. and H.L.; Methodology, Visualization and Investigation were performed by Q.Z., X.M. and Z.G. Writing—Review & Editing was performed by H.L. and X.L. The first draft of the manuscript was written by Q.Z. and all authors commented on previous versions of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Q., Liu, H., Mei, X. et al. Topography-driven variability in atmospheric deposition and soil distribution of cadmium, lead and zinc in a mountainous agricultural area. Sci Rep 15, 20894 (2025). https://doi.org/10.1038/s41598-025-05258-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05258-9