Abstract
Renal masses identified on CT or MRI scans may raise suspicion of malignancy, leading to radical or partial nephrectomy; however, pathology reports may reveal benign conditions. This study aimed to identify clinical and radiological factors associated with discrepancies between imaging and pathology reports, which may inform strategies to enhance diagnostic accuracy. We retrospectively reviewed 132 patients who underwent partial or radical nephrectomy between April 2022 and October 2024. All patients underwent preoperative CT, and MRI was conducted when deemed necessary at the physician’s discretion. The discrepancy was defined as cases where imaging findings suspicious of malignancy were reported on CT or MRI, but the pathology report later confirmed benign findings. Predictive factors for discrepancies were identified using a multivariate logistic regression model. Of the 132 patients, 98 (74.2%) were diagnosed with renal cell carcinoma (RCC). Among the 31 patients who underwent MRI, 24 (77.4%) were diagnosed with RCC. The discrepancy rate between CT imaging and pathology was 20.6% (27/131). Of these, 7 patients underwent MRI, and in 1 case, the MRI report aligned with the pathology. The overall discrepancy between imaging and pathology was 19.8% (26/131). Multivariable regression analysis revealed that female sex (OR = 5.219, p = 0.001) and a tumor size < 2 cm (OR = 4.826, p = 0.002) were significant factors influencing the discrepancy between imaging and pathology reports. Smaller renal masses (< 2 cm) and female sex were associated with a higher likelihood of diagnostic discrepancy. Considering these factors during imaging interpretation may help reduce overdiagnosis and improve accuracy.
Similar content being viewed by others
Introduction
In the United States, kidney cancer ranks as the sixth most prevalent cancer in men, and the ninth most prevalent in women1. Global cancer statistics indicate that the incidence of kidney cancer is the fourteenth-most common cancer type, with its mortality rate positioned sixteenth2. The early identification and screening of kidney cancer have been recognized as key focus areas in research3. If kidney cancer that is confined to the organ is identified at an early stage, surgical removal could be a potential curative option. At present, a large percentage (50–61%) of renal tumors are detected incidentally, which is a significant rise from 13% that was noted in the 1970 s4,5.
Small renal masses (SRMs) refer to kidney lesions that show enhancement with contrast and have a maximum size of 4 cm or smaller4. Computed tomography (CT) play an essential role in diagnosing kidney cancer. Contrast-enhanced CT scan is a frequently used for early detection of SRMs. Magnetic resonance imaging (MRI) is performed to evaluate renal masses more precisely. CT and MRI can offer valuable insights regarding the histological subtypes of small renal masses6. Despite advancements in imaging technologies, it has been reported that between 10% and 30% of SRMs, which are typically presumed to be RCC based on preoperative imaging assessments, are ultimately found to be benign following surgical excision7.
Several studies have reported differences in the interpretation agreement rates between CT and MRI in the evaluation of renal mass characteristics8,9,10. When discrepancies occur in the interpretation of renal masses on CT or MRI, the most conclusive method for assessing characteristics is the histopathologic examination through renal biopsies. Nonetheless, it is important to note that not all renal masses are amenable to biopsy, and certain masses may be situated in locations that pose significant risks for such procedures. Owing to these associated risks, there are numerous instances in which tissue biopsy is not feasible11,12.
While numerous studies have assessed imaging accuracy in renal mass characterization, few have focused on identifying patient- or tumor-related factors that are predictive of diagnostic discrepancy. Moreover, there is a paucity of data examining the concordance between CT/MRI and final pathology in real-world surgical cohorts. Our study addresses this gap by analyzing factors associated with radiologic-pathologic discordance in a contemporary patient population.
Therefore, the aim of this study was to evaluate clinical and radiological factors that are associated with discrepancies between imaging findings and final pathology in patients undergoing surgery for renal masses. These factors, if recognized preoperatively, may support radiologists and urologists in minimizing diagnostic uncertainty.
Methods
This study was approved by the Institutional Review Board (IRB) of Chung-Ang University Gwangmyeoung Hospital (IRB No.2503-229-047), which waived the requirement for informed consent due to the retrospective nature of the study. All study procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki.
We retrospectively reviewed the medical records of 132 patients who underwent partial or radical nephrectomy at Chung-Ang University Gwangmyeoung Hospital between April 2022 and October 2024. Inclusion criteria required that patients had preoperative imaging, either CT or MRI. All patients initially underwent a CT scan, with MRI performed at the clinician’s discretion when additional information was deemed necessary. The mean size of the renal masses was measured using CT images.
Contrast-enhanced CT or contrast-enhanced MRI scans were performed to evaluate each renal mass. All imaging studies were interpreted by a single board-certified uroradiologist with over 10 years of experience. Imaging reports were classified into two main categories: benign and malignant. The benign category included diagnoses such as angiomyolipoma (AML), oncocytoma, hemorrhagic cyst, nodules, cystic lesions (Bosniak I, II, and IIF), and other benign findings. The malignant category included renal cell carcinoma (RCC), atypical RCC, Bosniak III, Bosniak IV, and cystic RCC. If the imaging report suggested malignancy, it was classified as malignant, even if benign conditions were also noted within the report.
Discrepancy between the imaging reports and pathology results was evaluated. If malignancy was suggested in either the CT or MRI report, a discrepancy was defined as the pathological report indicating benignity. When both CT and MRI were available, MRI findings were prioritized as the final imaging impression due to its superior soft-tissue contrast and higher reported accuracy in cystic lesion classification and small renal mass characterization9,17.
Descriptive statistics were used to summarize the frequencies and proportions of categorical variables. Continuous variables are presented as mean ± standard deviation. Comparisons of continuous variables were performed using the independent t-test, and categorical variables were compared using Fisher’s exact test. Predictive factors for discrepancies between imaging and pathology reports were assessed using a multivariate logistic regression model, with odds ratios (OR) and 95% confidence intervals (CI) calculated. Only significant factors identified through univariate analysis were included in the multivariate model. All statistical analyses were conducted using IBM SPSS® (version 27.0; SPSS Inc., Chicago, IL, USA). A p-value of < 0.05 was considered statistically significant.
Results
Among the 132 patients included in the study, the mean age was 59 years, with 81 male patients (61.4%) and 51 female patients (38.6%). The mean tumor size was 4.6 cm. Most patients (84, 63.6%) underwent robot-assisted partial nephrectomy (Table 1).
A total of 31 patients underwent MRI, and of these, 24 were diagnosed with RCC, with 18 of them having the clear cell subtype, the most common histological type. In contrast, 101 patients did not undergo MRI, and among them, 78 were diagnosed with RCC, with 68 patients (87.2%) having the clear cell subtype (Fig. 1).
In the group of 101 patients who only underwent preoperative CT, 88 patients (87.1%) were reported as RCC on the CT imaging report. Of these, 74 (84.1%) were confirmed as RCC on the pathological report. In 13 patients (12.9%) where the CT report indicated a benign condition, RCC was confirmed in 4 patients (30.8%) based on pathological report. Among them, six patients had cystic lesions, for which the Bosniak classification and corresponding pathological results are summarized in Supplementary Table 1. Among the 31 patients who underwent MRI, 30 patients (96.8%) were diagnosed with RCC on the MRI report, and 24 (80%) were confirmed to have RCC on the pathological report. One patient had a benign condition on both the MRI and pathological reports (Table 2).
In the subset of 31 patients who underwent both CT and MRI, 29 patients (80.6%) had RCC reported on both the CT and MRI imaging reports. The median tumor size in this group was 3 cm. Of these, 6 patients (20.7%) had benign tumors, with a median tumor size of 2 cm. In two patients where the CT report suggested benign findings, one was confirmed as RCC by both MRI and pathology, while the other was confirmed as benign by both MRI and pathology report (Table 3).
The discrepancy between the CT imaging report and the pathological report was 20.6% (27/131), while the discrepancy between the MRI imaging report and the pathological report was 19.4% (6/31). Among the 27 patients with discrepancies between the CT imaging report and pathological findings, 7 patients underwent MRI, and in 1 case, the MRI report was consistent with the pathological report.
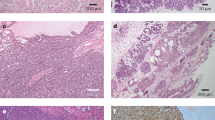
The overall discrepancy between imaging reports and pathological findings was 19.8% (26/131). Among the 26 discrepant cases confirmed as benign by pathology, the histologic diagnoses included AML (n = 21), oncocytoma (n = 2), simple cysts (n = 1), papillary renal neoplasm with reverse polarity (n = 1) and multilocular cystic renal neoplasm of low malignant potential (n = 1). The representative CT and MRI images showed a discrepancy between the imaging reports and the pathological findings, as shown in Fig. 2. Multivariable regression analysis revealed that being female (OR = 5.219, 95% CI = 1.891–14.405, p = 0.001) and having a tumor size of less than 2 cm (OR = 4.826, 95% CI = 1.785–13.051, p = 0.002) were significant factors influencing the discrepancy between imaging and pathological diagnoses (Table 4). Further detailed results regarding tumor size are provided in Supplementary Table 2.
Discussion
Our study provides important insights into the discrepancies between imaging and pathological findings in the renal masses, specifically focusing on predictive factors. The results revealed that smaller tumor size (< 2 cm) and female sex were significantly associated with discrepancies between preoperative imaging and pathological diagnoses. These findings emphasize the challenges in accurately predicting malignancy based on imaging alone, particularly for small renal masses9. Unlike previous studies, which primarily emphasized imaging accuracy, our research highlights patient-specific factors such as tumor size and sex contributing to misclassification13. By identifying these factors, we offer a novel perspective on preoperative imaging limitations and the need for personalized diagnostic approaches.
CT and MRI are widely used for renal mass evaluation, each offering distinct advantages. Contrast-enhanced CT remains the primary imaging modality due to its accessibility and high sensitivity for detecting renal tumors. MRI, on the other hand, has demonstrated superiority in characterizing renal mass histology, cystic lesions, and tumor thrombus in vena cava14,15,16. Previous studies have shown that MRI outperforms CT in differentiating Bosniak IIF and III lesions and small RCC subtypes potentially reducing unnecessary surgeries, which could impact patient’s decline in renal function17,18. The discrepancy rate in our study was 19.8%, which aligns with previous literature reporting a 10–30% rate of benign diagnoses in renal masses initially suspected to be malignant based on imaging findings. Our findings highlight the limitations of current imaging modalities, including CT and MRI, in distinguishing benign such as lipid-poor AMLs from malignant lesions with absolute certainty. While MRI is generally considered superior to CT in differentiating renal mass characteristics and various additional analytical techniques have been introduced19,20,21, our study demonstrated that discrepancies persisted even when MRI was utilized, suggesting the need for improved diagnostic techniques22. Further, the development of reporting systems with reproducible and reliable algorithms, such as PI-RADS for prostate cancer and VI-RADS for bladder cancer, is also necessary23,24.
One of the key findings was the significant association between tumor size and diagnostic discrepancies. Small renal masses (< 2 cm) were 4.8 times more likely to be misclassified compared to larger tumors. This is likely due to the increased difficulty in accurately characterizing small lesions, as well as their higher probability of being benign entities such as oncocytomas or AML. These findings support the argument that active surveillance may be a viable option for select patients with small renal masses, potentially reducing unnecessary surgeries and associated morbidity. Additionally, female sex was found to be a strong predictor of imaging-pathology discrepancy, with women exhibiting a 5.2 times higher likelihood of misclassification. The reason for this gender disparity remains unclear but could be related to differences in tumor biology or referral patterns. Further research is needed to elucidate these potential contributing factors.
Despite the strengths of our study, including a well-defined patient cohort and the use of both CT and MRI for analysis, have several limitations. First, this was a retrospective study conducted at a single institution, which may limit the generalizability of our findings. Second, the relatively small sample size of patients who underwent MRI may have influenced statistical power, limiting our ability to draw definitive conclusions. Third, the selective use of MRI based on physician discretion may have introduced selection bias, as patients with more complex lesions or unclear CT findings were more likely to undergo MRI. Furthermore, our study did not incorporate advanced imaging techniques such as radiomics or AI-based analysis.
Future studies should explore the integration of radiomics and AI algorithms into renal mass imaging to improve diagnostic accuracy25. Several studies are reporting the usefulness of radiomics for the diagnosis of RCC26,27. Machine learning models trained on large datasets may provide enhanced risk stratification, minimizing discrepancies between imaging and pathology28,29. Furthermore, since imaging reproducibility is a critical prerequisite for the successful application of radiomics and AI, future studies should also consider data normalization across different imaging platforms, particularly in MRI. Variability in image acquisition protocols and scanner types may influence feature extraction and model generalizability, underscoring the need for standardized imaging pipelines. Prospective multicenter trials are also needed to validate our findings in broader populations. Additionally, investigations into sex-specific tumor characteristics may further elucidate the observed gender-related differences in imaging discrepancies. Lastly, assessing the impact of imaging-pathology discrepancies on clinical decision-making will be essential in refining management strategies for renal masses. Our study underscores the importance of considering tumor size and patient sex when interpreting preoperative imaging. Despite advances in imaging technology, a significant rate of discrepancy remains, necessitating caution in treatment decision-making. Further research incorporating advanced imaging modalities and AI-driven approaches will be crucial in optimizing diagnostic accuracy and patient management.
Conclusion
In this study, factors contributing to the discrepancy between the image reports and pathology reports for renal masses after undergoing CT and MRI were gender and the size of the renal mass. Specifically, the risk of discrepancy increased for renal masses less than 2 cm, suggesting that this factor as well as female sex should be considered when making clinical decisions.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AML:
-
angiomyolipoma
- CT:
-
computed tomography
- CI:
-
confidence intervals
- IRB:
-
Institutional Review Board
- MRI:
-
magnetic resonance imaging
- OR:
-
odds ratio
- RCC:
-
renal cell carcinoma
- SRM:
-
small renal mass
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72 (1), 7–33 (2022).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263 (2024).
Harrison, H. et al. Risk prediction models for kidney cancer: A systematic review. Eur. Urol. Focus. 7 (6), 1380–1390 (2021).
Gill, I. S., Aron, M., Gervais, D. A. & Jewett, M. A. Clinical practice. Small renal mass. N Engl. J. Med. 362 (7), 624–634 (2010).
Sebastia, C. et al. Active surveillance of small renal masses. Insights Imaging. 11 (1), 63 (2020).
Wang, Z. J., Westphalen, A. C. & Zagoria, R. J. CT and MRI of small renal masses. Br. J. Radiol. 91 (1087), 20180131 (2018).
Jeon, H. G. et al. Benign lesions after partial nephrectomy for presumed renal cell carcinoma in masses 4 cm or less: prevalence and predictors in Korean patients. Urology 76 (3), 574–579 (2010).
Chan, J. et al. Comparison of Bosniak classification of cystic renal masses version 2019 assessed by CT and MRI. Abdom. Radiol. (NY). 46 (11), 5268–5276 (2021).
Kim, J., Lee, J. S., Jo, Y. & Han, W. K. Superiority of magnetic resonance imaging in small renal mass diagnosis where image reports mismatches between computed tomography and magnetic resonance imaging. Investig Clin. Urol. 64 (2), 148–153 (2023).
Kim, J. H. et al. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the united States from 2007 to 2014. JAMA Surg. 154 (3), 225–231 (2019).
Patel, H. D. et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J. Urol. 195 (5), 1340–1347 (2016).
Gao, B. et al. Avoiding needless nephrectomy: what is the role of small renal mass biopsy in 2024? Urol. Oncol. 42 (8), 236–244 (2024).
Papalia, R. et al. Accuracy of magnetic resonance imaging to identify pseudocapsule invasion in renal tumors. World J. Urol. 38 (2), 407–415 (2020).
Tshering Vogel, D. W. et al. Prospective comparison of Contrast-Enhanced ultrasound and magnetic resonance imaging to computer tomography for the evaluation of complex cystic renal lesions. Urology 154, 320–325 (2021).
Pei, X. et al. The value of enhanced multiparameteric MRI diagnostic model for preoperatively predicting surgical methods of inferior Vena Cava in patients with renal tumors and inferior Vena Cava tumor thrombus. BMC Med. Imaging. 23 (1), 86 (2023).
Smith, R. K., Navaratnam, A. & Vivian, P. Systematic review and meta-analysis of preoperative imaging prediction of renal cell carcinoma tumour thrombus inferior Vena Cava wall invasion. Clin. Radiol. 78 (7), 540–547 (2023).
Wang, Y. et al. MR texture analysis in differentiation of small and very small renal cell carcinoma subtypes. Abdom. Radiol. (NY). 48 (3), 1044–1050 (2023).
Rathi, N. et al. Predicting GFR after radical nephrectomy: the importance of split renal function. World J. Urol. 40 (4), 1011–1018 (2022).
Almalki, Y. E. et al. Bosniak classification version 2019: a prospective comparison of CT and MRI. Eur. Radiol. 33 (2), 1286–1296 (2023).
Blachura, T. et al. Diagnostic accuracy of the clear cell likelihood score and selected MRI parameters in the characterization of indeterminate renal masses - a single-institution study. Abdom. Radiol. (NY). 49 (11), 3893–3901 (2024).
Dunn, M. et al. Diagnostic performance and interreader agreement of the MRI clear cell likelihood score for characterization of cT1a and cT1b solid renal masses: an external validation study. AJR Am. J. Roentgenol. 219 (5), 793–803 (2022).
Wilson, M. P., Patel, D., Katlariwala, P. & Low, G. A review of clinical and MR imaging features of renal lipid-poor angiomyolipomas. Abdom. Radiol. (NY). 46 (5), 2072–2078 (2021).
de Silva, S. et al. Differentiation of renal masses with multi-parametric MRI: the de Silva St George classification scheme. BMC Urol. 22 (1), 141 (2022).
Xu, H. S. et al. Characterizing T2 iso- and hypo-intense renal masses on MRI: can templated algorithms improve accuracy? Clin. Imaging. 72, 47–54 (2021).
Potretzke, P. TA et al. Clinical implementation of an artificial intelligence algorithm for magnetic Resonance-Derived measurement of total kidney volume. Mayo Clin. Proc. 98 (5), 689–700 (2023).
Al-Mubarak, H. et al. Characterization of renal masses with MRI-based radiomics: assessment of inter-package and inter-observer reproducibility in a prospective pilot study. Abdom. Radiol. (NY). 49 (10), 3464–3475 (2024).
Anari, P. Y. et al. An MRI-based radiomics model to predict clear cell renal cell carcinoma growth rate classes in patients with von Hippel-Lindau syndrome. Abdom. Radiol. (NY). 47 (10), 3554–3562 (2022).
Kang, H. et al. Multiparametric MRI-Based machine learning models for the characterization of cystic renal masses compared to the Bosniak classification, version 2019: A multicenter study. Acad. Radiol. 31 (8), 3223–3234 (2024).
Suarez-Ibarrola, R., Hein, S., Reis, G., Gratzke, C. & Miernik, A. Current and future applications of machine and deep learning in urology: a review of the literature on urolithiasis, renal cell carcinoma, and bladder and prostate cancer. World J. Urol. 38 (10), 2329–2347 (2020).
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2024-00342763) and the Chung-Ang University Research Grants in 2025.
Author information
Authors and Affiliations
Contributions
CUL: data collection and management, data analysis, manuscript writing, JHK: data analysis, manuscript writing, GK: data collection and management, JJ: data collection and management, JWK: data collection and management, YSL: data collection and management, KM: data collection and management, JHT: data collection and management, SYC: data collection and management, IHC: data collection and management, JC: manuscript writing, project development, data collection and management, manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, C.U., Kim, J.H., Kim, G. et al. Impact of tumor size and sex on diagnostic discrepancies in renal mass imaging. Sci Rep 15, 19653 (2025). https://doi.org/10.1038/s41598-025-05266-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05266-9