Abstract
Autopsy personnel face substantial occupational risks from exposure to infectious agents, particularly during aerosol-generating procedures like bone sawing. The COVID-19 pandemic highlighted these dangers, underscoring the need for innovative safety solutions in resource-limited settings without negative-pressure autopsy suites. To address this, we developed a Low-Cost Infection Containment Chamber (LCICC)—a full-length, transparent, and impermeable structure designed to contain aerosols during high-risk autopsy procedures. This study evaluated the efficacy of LCICC using goat skulls to simulate aerosol generation during sawing. Aerosol densities were measured under both open-air conditions and with LCICC use, employing fluorescein dye for visualization and particle counter for quantification. Statistical analysis confirmed the chamber’s effectiveness in reducing occupational exposure. LCICC demonstrated a substantial reduction in aerosol levels, ranging from 85.96 to 88.38% across all particle sizes. Median aerosol densities were consistently recorded at 10.50 mg/m³ with LCICC use, compared to 74.80 to 90.40 mg/m³ in open-air conditions. LCICC’s affordability, ease of assembly, and reliable performance position it as a groundbreaking tool for protecting healthcare workers, particularly in low-resource settings. This innovation addresses critical gaps in autopsy safety protocols, offering a practical solution to mitigate risks associated with infectious aerosols while advancing occupational health standards in forensic pathology.
Similar content being viewed by others
Introduction
Autopsy personnel represent a high-risk occupational group, engaging in some of the most hazardous procedures within the medical field. Infectious agents can be transmitted during autopsies through several pathways: (i) percutaneous exposure, such as injuries caused by contaminated needles, scalpels, bone fragments, etc.; (ii) contact with mucous membrane or open wound, including contamination of the mouth, nose, conjunctivae, or fresh wounds with infectious blood, saliva, or other bodily fluids; and (iii) inhalation of aerosols generated during procedures like organ washing, tissue manipulation, or the use of high-speed oscillating saws for cutting bone1. While needlestick and sharp-object injuries are well-documented occupational hazards, the risks associated with aerosol inhalation during medical and forensic procedures are often neglected.
There was increased concern about health risks due to aerosol-generating procedures in autopsy during the COVID-19 pandemic, given the established transmission of SARS-CoV-2 via aerosols2,3,4. Studies confirm that aerosolized particles can remain suspended in the air for extended periods5,6,7. This is particularly relevant during autopsies, where significant aerosol generation occurs7.
During the COVID-19 pandemic, the Indian Council for Medical Research (ICMR) had recommended, wherever possible, avoiding autopsies on deceased individuals suspected or confirmed to be COVID-19 positive and to refrain from routine embalming due to the heightened risk of infection transmission8. However, autopsies were still required in medico-legal cases involving unnatural deaths. During the pandemic, even if the cause of death was unrelated to COVID-19, the deceased could still have harboured SARS-CoV-2. Consequently, it was assumed that every deceased individual was COVID-19 positive and to implement appropriate protective measures during autopsies4.
During the pandemic, numerous surgical studies focused on minimizing aerosolization of particles to enhance safety during procedures;9,10,11,12,13,14 however, there was a notable scarcity of research addressing similar measures from an autopsy perspective, despite the comparable risks15. Safeguarding healthcare workers (HCWs) involved in post-mortem examinations became imperative, particularly in resource-limited settings where negative pressure autopsy suite and/or adequate personal protective equipment is not available. In this context, we discuss our indigenously developed Low-Cost Infection Containment Chamber (LCICC) and its effectiveness, which represents a transformative approach to enhancing the safety of HCWs.
The inherent risk of autopsy
Autopsy rooms have always been recognized as one of the highest-risk working environment within healthcare facilities16,17,18. Autopsies are typically performed for medicolegal purposes or to ascertain the cause of death or underlying disease in deceased individuals. The availability of clinical information prior to the procedure can vary significantly, ranging from detailed diagnoses and medical reports to complete absence of data regarding the patient’s disease status or clinical history. As a result, often it may remain unclear at the outset or even at the end of the process whether autopsy personnel are exposed to infectious diseases19,20.
One of the autopsy procedures with a significant potential to generate high concentrations of infectious aerosols is removal of the skull vault. This process involves the use of an electric oscillating saw, operating typically at speeds between 20,000 and 30,000 rpm, to make a circumferential cut through the skull vault before brain extraction1,21,22. The resulting aerosol comprises a combination of dry bone particles, cerebrospinal fluid (CSF), and blood droplets1.
The oscillating blade of the autopsy saw exhibits characteristics akin to a spinning disc aerosol generator, albeit with the added complexity of variable speed21,22. This variability results in the production of aerosols of diverse sizes. These may range from larger droplets and fragments capable of directly striking the worker’s face to finer, highly respirable bone dust particles that pose an inhalation risk. In addition to the risks associated with the aerosolization of infectious agents, the inhalation of non-infectious bone dust also poses a significant concern for autopsy personnel, as it has been linked to hypersensitivity reactions1,6.
In this context, the development of a LCICC was essential for safely conducting high-risk autopsies on suspected or confirmed highly infectious cadavers when a negative pressure autopsy suite was not available23. The LCICC is a full-length, transparent, and impermeable chamber designed to accommodate a single cadaver. This innovative solution effectively contains the aerosols generated during procedures such as skull and brain removal, providing an additional layer of protection for the autopsy team while ensuring procedural efficiency and safety.
Methodology
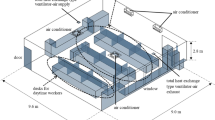
The authors began by creating both 2D and 3D models of the proposed LCICC. These models were presented to the Institute’s hospital infection control committee and bioengineers for feedback and refinement. The fabrication process involved modifying a standard straight mortuary table using locally available materials, ensuring cost-effectiveness. The mortuary table, measuring 8.8 ft in length and 3 ft in breadth, is fixed to the floor and features an exhaust vent positioned 4.2 ft above the table surface, running along its length.
The LCICC’s frame was built by installing four iron support pillars, one at each corner of the table, to connect it to the overhead exhaust vent. Transparent acrylic sheets were then used to form the walls of the chamber, providing visibility and impermeability. The design included strategically placed cut-outs in the walls to facilitate the attachment of long elbow length gloves, allowing for safe handling of the cadaver (Fig. 1).
Low-cost infection containment chamber prototype24.
To enable ease of access, the long wall on one side of the chamber was converted into a door that opens upwards, allowing the body to be slid in and out effortlessly. To ensure an airtight seal, all joints were secured with silicone sealant, effectively preventing air leaks and the door was lined with a rubber beading which acts as a seal once the door is closed. At the foot end of the chamber, a one-way valve was installed to permit air entry while preventing its exit, maintaining containment of aerosols. An additional opening was included at the same end to allow for the introduction of disinfectant into the sealed chamber when necessary.
The total cost of constructing the LCICC was ₹40,000 (470 USD approx.), making it an affordable and practical solution.
To replicate the conditions under which aerosols are generated during autopsies, goat skulls were used as models. The goat heads used in the study were procured from local butcher shops, where they are sold for human consumption, and no animal was euthanized specifically for the purposes of this study. They were then transported to the mortuary immediately to ensure freshness and anatomical integrity. Upon arrival, the skin was carefully removed, and the soft tissues were dissected to expose the underlying bone. In preparation for the experiment, meticulous steps were taken to create an optimal setup that would enhance the visualization of aerosolized particles.
A flat wood board was used as the base, over which a black cloth was carefully laid to provide a high-contrast background for detecting fluorescent droplets. Six scales (measuring 150 cm each) were placed along the horizontal, vertical and oblique axes to enable precise measurement of the aerosol spread in each direction and were secured in place with tape to prevent any movement during the procedure. To contain the spill and to prevent disruption of the scales, a transparent plastic sheet was placed over the scales covering the entire cloth, ensuring that these elements remained undisturbed throughout the experiment. Sponges were used to stabilize the goat head on the plastic sheet, effectively preventing slippage during sawing (Fig. 2).
The experiments were conducted under two controlled scenarios: in an open-air environment and within the LCICC.The primary aim was to compare aerosol levels, spread, and assess the containment capabilities of the LCICC. In this study, the term “open-air” refers to a non-enclosed environment where sawing procedures were conducted without any physical containment, allowing natural factors—such as ambient airflow—to freely influence the dispersion of aerosol particles generated during the process.
In total, 15 goat heads were sawn within the LCICC and 15 in an open-air setting using a BOSCH Professional GOP 30 − 28 oscillating saw. Specific anatomical sites on the goat skull, including the skull vault, nasal bone, mandible, occipital bone, and horns, were selected for sawing to replicate the various stages of an autopsy. To quantify aerosol dispersion during the procedure, a portable particle counter machine (Dust Trak TSI 8534 UK) was used (Fig. 3a).
In the open-air setting, the particle counter recorded aerosol levels directly in the vicinity of the operative site. During procedures conducted within the LCICC, the particle counter was strategically placed just outside the chamber at various locations, including the head end, water inlet, door, and hinge (Fig. 3b). This positioning aimed to assess aerosol containment across different points of the chamber and to monitor any variation in aerosol density during the autopsy process.
Fluorescent splatter and aerosol levels were meticulously measured and recorded in both scenarios. To enhance visualization of aerosol splatter, fluorescein dye mixed with saline was used to irrigate the mandible during sawing and the rate of flow was controlled using an IV set. The mandible was selected as the focal point for fluorescein dye application because our preliminary observations indicated it generated the greatest amount of aerosol (Figs. 4 and 5).
Upon completion of the bone sawing procedures, the dispersion of fluorescein-marked droplets around the operative site was meticulously assessed using ultraviolet (UV) illumination (Fig. 6). To enhance the visibility of the fluorescein splatter, all mortuary lights were turned off to convert the area into a dark room, and the operating area was illuminated using an Amici Vision 100 LED UV flashlight operating at a wavelength of 395 nm.
To capture the fluorescein spread an Aperlite YH-700n flash unit was modified by replacing the front lens with a UV bandpass filter, allowing only UV light to pass through, effectively converting it into a UV flash unit. This modified flash was paired with a Nikon D500 camera body mounted on a tripod for capturing high-resolution images. Once the focus on the operative site was locked, the camera triggered the UV flash to illuminate the fluorescein-marked droplets, enabling their visualization against the background.
The images were captured in Nikon’s RAW format (NEF) to preserve maximum detail and were subsequently imported into Adobe Lightroom Classic Version 8.4 for analysis. Each photograph was carefully examined for fluorescent droplets, which appeared as bright green spots in the images. When fluorescent droplets were detected, their spatial distribution were documented to assess the extent of aerosol dispersion. To determine the smallest detectable droplet size in the photographs, fluorescein dye was sprayed onto a sheet imprinted with graduated 1 cm square markings. This calibrated grid served as a reference to evaluate the resolution of the imaging setup and the smallest drop that could be measured was 10 μm in size.
Results
The results were based on the analysis of sawing the mandible bone, conducted both within the LCICC and in an open-air setting, as preliminary observations indicated that this procedure generated the highest amount of aerosol. For analysis, the average of all particle counter readings in both settings: for the LCIC, readings were taken near the hinge as it was regarded as the weakest structural point while in the open air, reading was taken an inch from the bone and the saw. For the LCIC, the hinge was deliberately chosen in order to assess whether aerosols could escape through any potential gaps or weak spots in the chamber’s design.
The data did not follow a normal distribution, as determined by the Shapiro-Wilk test, which yielded a p-value of < 0.05. Consequently, non-parametric tests were applied to analyse the data. The numerical analysis indicated significant differences in baseline values, with aerosol densities within the LCICC being higher than those in open-air conditions. The Mann-Whitney U Test revealed significant disparities across all particulate matter sizes, with chamber values consistently much lower than those observed in open-air settings.
Table 1 summarizes the aerosol density measurements during sawing procedures under two conditions: within the LCICC and in open-air environments, for different aerosol sizes. Median aerosol densities are reported in mg/m³, with interquartile ranges (25th to 75th percentiles) providing a comprehensive data overview.
Median aerosol densities for particle sizes of 1, 2.5, 4, and 10 μm in open-air conditions ranged from 74.80 [33.65, 100.50] mg/m³ to 90.40 [41.10, 115.00] mg/m³, indicating significant aerosol dispersal during the procedure. In contrast, the LCICC consistently recorded a median aerosol density of 10.50 [10.45, 10.50] mg/m³ across all particle sizes. The statistical significance of the data, with p-values well below 0.0001 across all particle sizes, reinforces the reliability of these results.
Analysis of the fluorescein droplet spread in the open-air environment revealed that most of the splatter was concentrated within 20 cm of the sawing site, with some droplets detectable up to 40 cm away (Fig. 7). Most of the droplets were distributed in the same plane as of the oscillating blade from the saw while the spread of droplets perpendicular to the plane of the saw blade was less. Notably, even with the particle counter positioned near the LCICC’s hinges—considered the weakest point—negligible aerosol levels were detected, further confirming the LCICC’s effectiveness in preventing aerosol leakage and minimizing potential exposure.
Discussion
Aerosols are defined as particles suspended in a gas, such as air, and play a significant role in the transmission of infectious agents during medical, surgical, and forensic procedures25,26,27,28,29. Depending on their size, aerosols exhibit distinct behaviours and associated risks. Small particles, measuring less than 5–10 μm, can follow airflow streamlines, enabling both short- and long-range transmission. These particles are particularly concerning as they can penetrate deep into the respiratory tract, reaching the alveoli26. In contrast, larger droplets with diameters exceeding 20 μm are influenced primarily by gravity, following ballistic trajectories and settling quickly. Intermediate-sized particles (10–20 μm) exhibit characteristics of both small and large droplets, settling faster than smaller particles but potentially carrying a lower infectious dose than larger droplets30.
Routine bone-sawing procedures during autopsies have been shown to generate aerosols within size ranges that pose significant health risks to personnel1,31,32. Particles smaller than 5 μm can be inhaled and are particularly concerning as they can travel deep into the respiratory tract, reaching the lungs and potentially causing disease. Research has highlighted the specific risks associated with aerosolized particles sized between 0.3 and 5 μm, which are of particular concern due to their ability to remain airborne for extended periods33.
The type of saw used and the bone’s size and density significantly influence the production and concentration of these particles. For instance, peak concentrations of aerosols within this size range have been observed to vary from 6.5 × 10³ particles/cfm when using a handsaw in an autopsy hall to 19.6 × 10⁶ particles/cfm with a band saw in a sawing cabin33. Considering that the average resting human inhales approximately 6 L of air per minute34, even the lowest concentrations of respirable particles could result in the inhalation of approximately 1.4 × 10³ particles per minute without protection33.
Although the majority of these particles consist of non-infectious bone dust, there is a risk that some may carry infectious agents. The unpredictable nature of aerosol generation, influenced by variations in bone sizes, densities, and autopsy conditions, complicates the assessment of exposure risks and highlights the importance of understanding aerosol classifications.
These classifications are critical for evaluating the risks associated with exposure and determining the efficacy of protective measures, such as personal protective equipment (PPE) and controlled environments. Protection against aerosols requires not only engineering controls but also the use of specialized and well-fitted PPE, such as filtering facepiece type 3 (FFP3) respirators or powered air-purifying respirators (PAPRs)35. However, such PPE can impair the work of autopsy personnel and therefore are generally used based on a risk assessment. Often in resource constrained setups adequate PPE’s may not be available thus putting the autopsy personnel at risk.
When performing aerosol-generating procedures in the mortuary, additional safety measures have been previously described for potentially infectious cases. For example, using a plastic bag over the head of the deceased, the saw, and the operator’s arms can significantly reduce the release of airborne particles into the mortuary environment36. More recently, transparent plastic craniotomy boxes have been utilized during post-mortems on COVID-19-positive individuals, offering an additional layer of containment and safety during calvarium removal15. Constructed from durable acrylic plastic, the box could be fully disinfected after use by cleaning its surfaces or immersing it in a disinfectant solution. Importantly, the COVID-19 rRT-PCR swab from the interior surface of the craniotomy box detected the presence of the virus after the autopsy, demonstrating its efficacy in confining bone dust and aerosols generated during skull opening15.
Similarly, the containment capabilities of the LCICC for varied aerosol sizes emphasize its efficacy as a protective measure. To quantify the effectiveness of the LCICC in minimizing aerosol dispersion, the percentage reduction in median aerosol density between open-air conditions and LCICC use was calculated for each particle size. The analysis revealed a reduction of 85.96% for 1 μm, 86.05% for 2.5 μm, 86.41% for 4 μm, and 88.38% for 10 μm particles. The total aerosol reduction, considering all particle sizes combined, was 87.10%. Once the cadaver is placed inside and the door is closed, the chamber effectively limits the spread of aerosolized particles. The chamber can also be connected to an overhead exhaust system, transforming it into a negative pressure environment to further reduce aerosol dispersal. Gloves attached to the sides of the chamber allow for safe sample collection or the conduct of autopsy procedures, minimizing direct contact between the autopsy team and potentially infectious fluids, tissues, or aerosols.
The use of such a containment strategy aligns with the National Institute for Occupational Safety and Health (NIOSH) Hierarchy of Controls, a framework used to identify and prioritize measures to protect workers fromoccupational hazards37. This hierarchy ranks interventions from most to least effective: elimination, substitution, engineering controls, administrative controls, and PPE. Engineering controls, such as the LCICC, are considered highly effective—if not ideal—because they are designed to block the hazard at the source before it comes into contact with the worker or enclosure of the process. They typically require minimal user action to function effectively and operate without interfering with the workflow or making the procedure more difficult.
Additionally, the transparent walls of the chamber allow for safe photographic and video documentation without the risk of contamination, further enhancing its utility. After completing the procedure, only the chamber requires decontamination, significantly reducing the cost, time, and effort typically associated with cleaning the entire autopsy facility. To mitigate exposure risks even further, the chamber allows the body to be decontaminated inside before being placed in a body bag. This step provides an additional layer of safety for personnel handling the body post-procedure. The authors have successfully utilized the LCICC not only in cases involving patients infected with COVID-19 but also in those with rabies, showcasing its effectiveness and versatility24. Beyond autopsies, the chamber could also be invaluable for the safe collection of specimens and samples from highly infectious cases.
Limitations of the study
The baseline aerosol density recorded within the LCICC was higher than that in the open-air setting. This difference is likely due to the natural dispersion of aerosols by ambient wind in the open environment—an effect that is inherently absent within the enclosed structure of the LCICC. However, environmental factors such as wind speed and direction were not measured in this study, which is a limitation that should be considered when interpreting these findings.
While fluorescein dye was used to visualize aerosol spread and serves as a useful proxy for dispersion patterns, it cannot accurately represent aerosols in the micron or sub-micron range.
Conclusion
Despite the risks and challenges posed by the COVID-19 pandemic, the medicolegal investigation of deaths remained essential as mandated by authorities and legislation. The pandemic highlighted the critical risks faced by HCWs during high-risk procedures, such as autopsies on potentially infected cadavers, underscoring the need for reliable biosafety measures. The authors believe that this innovation has the potential to revolutionize the management of infectious diseases by offering a practical solution for sampling and specimen collection in highly infectious cases such as Rabies, Ebola, Nipah Virus, COVID-19, and Tuberculosis. Additionally, it could serve as a crucial protective measure for autopsy teams, effectively addressing the risks posed by these infectious diseases.
The LCICC demonstrates a significant reduction in aerosol exposure during autopsies, offering a scalable and cost-effective solution to enhance safety for forensic personnel while maintaining the integrity of critical forensic procedures. While its design eliminates the need for negative pressure throughout the mortuary, its low construction cost and ease of use make it particularly suitable for resource-limited settings, simplifying operational requirements.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Green, F. H. Y. & Yoshida, K. Characteristics of aerosols generated during autopsy procedures and their potential role as carriers of infectious agents. Appl. Occup. Environ. Hyg. 5 (12), 853–858. https://doi.org/10.1080/1047322X.1990.10387806 (1990).
Jamieson, D. J. et al. Obstetricians on the coronavirus disease 2019 (COVID-19) front lines and the confusing world of personal protective equipment. Obstet. Gynecol. 135, 1257–1263. https://doi.org/10.1097/AOG.0000000000003919 (2020).
Evanoff, B. A. et al. Work-Related and personal factors associated with mental well-being during the COVID-19 response: survey of healthcare and other workers. J. Med. Internet Res. 22 (8), e21366. https://doi.org/10.2196/21366 (2020).
James, R. I. et al. Death in the time of corona: are we prepared? J. South. India Medicolegal Association. 12 (2), 108–117 (2020).
Jensen, P. A. et al. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Recomm. Rep. 54(RR–17), 1–141. (2005). https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5417a1.htm (2005).
Newsom, S. W., Rowlands, C., Matthews, J. & Elliot, C. J. Aerosols in the mortuary. J. Clin. Pathol. 36 (2), 127–132. https://doi.org/10.1136/jcp.36.2.127 (1983).
Burton, J. L. Health and safety at necropsy. J. Clin. Pathol. 56 (4), 254–260. https://doi.org/10.1136/jcp.56.4.254 (2003).
Indian Council of Medical Research. Standard guidelines for Medico-legal autopsy in COVID-19 deaths in India. (2020). https://eaaf.org/wp-content/uploads/covid19-PDFs/India/COVID19_AUTOPSY_GUIDELINES_2020_10052020.pdf.
Workman, A. D. et al. Airborne aerosol generation during endonasal procedures in the era of COVID-19: risks and recommendations. Otolaryngol. Head Neck Surg. 163 (3), 465–470. https://doi.org/10.1177/0194599820931805 (2020).
Dharmarajan, H. et al. Droplet and aerosol generation with endonasal surgery: methods to mitigate risk during the COVID-19 pandemic. Otolaryngol. Head Neck Surg. 164 (2), 285–293. https://doi.org/10.1177/0194599820949802 (2021).
Snyderman, C. H. & Gardner, P. A. Endonasal drilling May be employed safely in the COVID-19 era. Int. Forum Allergy Rhinol. 10 (9), 1118–1119. https://doi.org/10.1002/alr.22642 (2020).
Kim, M., Lee, M., Schwarz, J., Kacker, A. & Schwartz, T. H. A novel negative pressure, face-mounted antechamber to minimize aerosolization of particles during endoscopic skull base surgery. Oper. Neurosurg. (Hagerstown). 21 (3), 131–136. (2021). https://doi.org/10.1093/ons/opab173
Lee, M. et al. Development and validation of a patient face-mounted, negative-pressure antechamber for reducing exposure of healthcare workers to aerosolized particles during endonasal surgery. J. Neurosurg. 135 (6), 1825–1832. https://doi.org/10.3171/2020.10.JNS202745 (2021).
Gandham, E. J. et al. A negative-pressure face-mounted system reduces aerosol spread during endonasal endoscopic surgery. J. Neurol. Surg. B. Skull Base. 84 (3), 217–224. https://doi.org/10.1055/a-1774-6091 (2022).
Hasmi, A. H. et al. The craniotomy box: an innovative method of containing hazardous aerosols generated during skull saw use in autopsy on a COVID-19 body. Forensic Sci. Med. Pathol. 16 (3), 477–480. https://doi.org/10.1007/s12024-020-00270-z (2020).
Wilson, M. L. Infectious diseases and the autopsy. Clin. Infect. Dis. 43 (5), 602–603. https://doi.org/10.1086/506574 (2006).
Squier, W. & Ironside, J. Falling necropsy rates and risks to public health. Arch. Dis. Child. 91 (7), 551–553. https://doi.org/10.1136/adc.2005.087742 (2006).
Bonds, L. A., Gaido, L., Woods, J. E., Cohn, D. L. & Wilson, M. L. Infectious diseases detected at autopsy at an urban public hospital, 1996–2001. Am. J. Clin. Pathol. 119 (6), 866–872. https://doi.org/10.1309/MLUF-X0HR-5B96-GVAX (2003).
Templeton, G. L. et al. The risk for transmission of Mycobacterium tuberculosis at the bedside and during autopsy. Ann. Intern. Med. 122 (12), 922–925. https://doi.org/10.7326/0003-4819-122-12-199506150-00005 (1995).
Kantor, H. S., Poblete, R. & Pusateri, S. L. Nosocomial transmission of tuberculosis from unsuspected disease. Am. J. Med. 84 (5), 833–838. https://doi.org/10.1016/0002-9343(88)90060-5 (1988).
Dunskii, V. F. & Nikitin, N. V. Atomization of a liquid by a rotating disc and secondary break-up of droplets. J. Eng. Phys. 9, 41–45. https://doi.org/10.1007/BF00831832 (1965).
Lippmann, M. & Albert, R. E. A compact electric-motor driven spinning disc aerosol generator. Am. Ind. Hyg. Assoc. J. 28 (6), 501–506. https://doi.org/10.1080/00028896709342675 (1967).
Johnson, L. R. et al. Novel, safe, Indigenous cost-effective infection containment chamber for high-risk autopsies: the need of the hour. J. Forensic Med. Toxicol. 38 (1), 102–106. https://doi.org/10.5958/0974-4568.2021.00020.X (2021).
James, R. I. et al. A standardized protocol for the safe retrieval of infectious postmortem human brain for studying Whole-Brain pathology. Am. J. Forensic Med. Pathol. 44 (4), 303–310. https://doi.org/10.1097/PAF.0000000000000871 (2023).
Cole, E. C. & Cook, C. E. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am. J. Infect. Control. 126, 453–464. https://doi.org/10.1016/s0196-6553(98)70046-x (1998).
Infectious Diseases Society of America (ISDA). Preventing transmission of pandemic influenza and other viral respiratory diseases: personal protective equipment for healthcare personnel: update 2010. Chapter: 2 Understanding the Risk to Healthcare Personnel. (2010). https://www.nap.edu/read/13027/chapter/4#30.
Yan, J. et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. U S A. 115 (5), 1081–1086. https://doi.org/10.1073/pnas.1716561115 (2018).
Herfst, S. et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336 (6088), 1534–1541. https://doi.org/10.1126/science.1213362 (2012).
Centers for Disease Control and Prevention (CDC). Approaches to better understand human influenza transmission. (2010). https://www.cdc.gov/influenzatransmissionworkshop2010/
Tellier, R., Li, Y., Cowling, B. J. & Tang, J. W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 19 (1), 101. https://doi.org/10.1186/s12879-019-3707-y (2019).
Noble, W. C., Lidwell, O. M. & Kingston, D. The size distribution of airborne particles carrying micro-organisms. J. Hyg. (Lond). 61 (4), 385 –91.https://doi.org/10.1017/s0022172400020994 (1963).
Saternus, K. S. & Kernbach-Wighton, G. On the contamination of ambient air by preparations carried out with a band-saw. Forensic Sci. Int. 104 (2–3), 163– 71. https://doi.org/10.1016/s0379-0738(99)00108-5 (1999).
Wenner, L. et al. Aerosol generation during Bone-Sawing procedures in veterinary autopsies. Vet. Pathol. 54 (3), 425–436. https://doi.org/10.1177/0300985816688744 (2017).
Jardins, T. D. Cardiopulmonary Anatomy & Physiology: Essentials of Respiratory Care. ( Clifton Park, Cengage Learning, 2012).
Pauli, U., Karlen, S. & Summermatter, K. The importance of Fit-Testing particulate filtering facepiece respirators! Appl. Biosaf. 19 (4), 184–192. https://doi.org/10.1177/153567601401900402 (2014).
MacArthur, S., Jacobson, R., Marrero, H., Rahman, Z. & Schneiderman, H. Autopsy removal of the brain in AIDS: a new technique. Hum. Pathol. 17 (12), 1296–1297. https://doi.org/10.1016/s0046-8177(86)80578-0 (1986).
National Institute for Occupational Safety and Health (NIOSH). Hierarchy of Controls. (2021). https://www.cdc.gov/niosh/hierarchy-of-controls/about/index.html
Acknowledgements
The authors express their sincere gratitude to the Department of Neurosurgery for providing the particle counter for this study. They are also deeply grateful to Dr. Liaquat Roopesh Johnson for his invaluable assistance with statistical analysis and to Mr. Rajapandian for his dedicated support throughout the research. The authors also acknowledge Dr. Geeta Chacko for her guidance and support in developing this prototype.
Author information
Authors and Affiliations
Contributions
L.R.J. and R.I.J. conceptualised the study. L.R.J., D.M. and R.I.J. performed, collected, and analysed the data. All authors contributed to the original draft of the manuscript and critically reviewed and edited the manuscript. All authors had full access to all the data reported in the study and had final responsibility for the decision to submit for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from the Institutional Review Board and Ethics Committee of Christian Medical College Vellore, Tamil Nadu, India [IRB Min. No. 13486]. Goat heads used for the simulation of autopsy procedures were ethically procured using funds provided by the institutional fluid grant.
Consent for publication
The authors affirm that all personnel provided informed consent for the publication of the images in Figure Nos. 2, 3, 4, and 6.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Johnson, L.R., Manoj, D., Varughese, B.T. et al. Evaluating the effectiveness of a novel cost-effective aerosol containment chamber for high-risk autopsies: a pilot study. Sci Rep 15, 22858 (2025). https://doi.org/10.1038/s41598-025-05271-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05271-y