Abstract
Patients with recessive dystrophic epidermolysis bullosa (RDEB) frequently develop pseudosyndactyly due to recurrent hand blistering and subsequent scar contracture. Conventional release surgery typically employs allogeneic biological dressings or synthetic materials for wound isolation, yet existing approaches are associated with frequent dressing changes, prolonged healing cycles, and secondary trauma. This study pioneers the clinical application of intraoperatively shed epidermal tissue for fabricating autologous epidermal finger cuffs to cover post-surgical defects, evaluating its therapeutic efficacy. 13 RDEB patients with hand contractures were stratified into experimental (n = 6, autologous epidermal finger cuffs) and control (n = 7, traditional petrolatum gauze dressings) cohorts following standardized contracture release procedures. Outcome measures included wound healing time, dressing change frequency, hemorrhage during dressing changes (VAS scale), and 6-month functional assessments. The experimental cohort demonstrated significantly accelerated wound closure versus controls (19.0 ± 1.5 vs. 29.0 ± 2.5 days, U = 3.0, p = 0.002), with median healing time reduced by 34.5%. Experimental subjects required 50% fewer median dressing changes (W = 42, p = 0.004) and exhibited 55% lower hemorrhage VAS scores (U = 4.0, p = 0.001), with strong positive correlation between VAS scores and dressing frequency (r = 0.78, p < 0.01). Safety profiles showed no severe infections: experimental group reported 1 case (16.7%) of epidermal displacement requiring reinforcement, versus 2 control cases (28.6%) with mild inflammation. Six-month follow-up revealed 24% lower median finger webbing space depth loss in experimental group (1.9 mm vs. 2.5 mm), though statistically non-significant (U = 15, p = 0.35). Neither group exhibited contracture recurrence or significant functional improvement. This novel technique repurposes intraoperatively discarded epidermal tissue as biological dressings, effectively minimizing secondary trauma from adherent dressing removal. It presents a clinically superior alternative for RDEB management, characterized by reduced treatment burden and accelerated recovery.
Similar content being viewed by others
Introduction
Recessive Dystrophic Epidermolysis Bullosa (RDEB) is a rare genetic dermatosis caused by mutations in the COL7A1 gene, characterized by increased skin fragility, recurrent blistering, and progressive scar contracture1. The hands are frequently affected in RDEB, where pseudosyndactyly and scar contractures result in profound functional impairment and diminished quality of life2. While current therapeutic strategies—including surgical release, advanced dressings, and antifibrotic agents—partially alleviate symptoms, high recurrence rates (> 80%) and long-term management challenges remain critical clinical concerns3,4.
Conventional surgical approaches (e.g., pseudosyndactyly release) provide transient functional restoration but are often complicated by impaired wound healing and fibrotic recurrence5. Emerging evidence suggests that postoperative use of novel dressings (e.g., Integra dermal regeneration template gloves) may delay recurrence, yet fails to fully abrogate molecular pathways driving scar formation6. Pharmacologic interventions targeting RDEB fibrosis (e.g., Losartan and Decorin), which suppress TGF-β signaling to reduce collagen deposition, exhibit limited efficacy and require prolonged administration7,8. Recent advances in cellular therapies (e.g., autologous keratinocyte transplantation) and gene-editing technologies offer potential curative solutions; however, clinical translation remains constrained by safety concerns, cost, and procedural complexity9,10.
This study innovatively utilizes intraoperatively shed epidermal tissue to fabricate autologous finger cuffs for wound coverage after hand contracture release. The technique circumvents invasive harvesting, simplifies clinical workflows, and eliminates immunogenic risks. Notably, its application in RDEB-related hand contractures has not been systematically investigated. By comparing outcomes with conventional surgery combined with petrolatum-based dressings, this research evaluates finger cuffs’ efficacy in optimizing wound regeneration, enhancing aesthetic outcomes, and improving long-term prognoses. The findings aim to establish a novel, minimally invasive therapeutic paradigm for individualized RDEB management.
Materials and methods
Study design
This single-center, prospective cohort study adopted a non-randomized concurrent controlled design to evaluate the effects of autologous exfoliated epidermal finger cuffs on postoperative wound healing and web space depth maintenance in patients with RDEB following hand scar contracture release. This study adopted a non-randomized concurrent controlled design. Patients were allocated to the experimental group (autologous epidermal finger cuffs) or control group (petrolatum gauze dressings) based on surgical timeline and technical maturity: the first 7 patients enrolled between 2021 and 2023 received conventional treatment (control group), while the subsequent 6 patients (2023–2024) underwent the novel technique after standardizing epidermal cuff processing protocols (experimental group). Baseline characteristics were rigorously matched, with no significant differences in median preoperative finger web space depth (4.8 mm vs. 5.1 mm, p = 0.47) or age distribution (14.2 ± 3.8 years vs. 13.5 ± 4.1 years, p = 0.65) between groups. This design mitigated instability during the initial technical exploration phase while minimizing bias through blinded assessment (outcome measures were evaluated by research assistants unaware of group allocation). The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Zhengzhou University (Approval No.: 2023014), and written informed consent was obtained from all participants. All experiment were performed in accordance with relevant guidelines and regulations.
Inclusion and exclusion criteria
RDEB patients undergoing hand scar contracture release between 2021 and 2024 were enrolled, with diagnosis confirmed by genetic testing identifying biallelic pathogenic mutations in the COL7A1 gene. Inclusion criteria included age ≥ 6 years, grade III interdigital scar contractures (according to the Lucky classification), and absence of systemic antifibrotic therapy within 6 months preoperatively. Exclusion criteria comprised active wound infection, comorbid diabetes mellitus or immunosuppressant use, and insufficient follow-up compliance (defined as inability to complete ≥ 3 postoperative visits). A total of 13 patients were included, with 6 assigned to the experimental group (autologous exfoliated epidermal finger cuffs coverage) and 7 to the control group (traditional petrolatum gauze dressings).
Interventions and surgical procedures
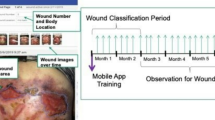
All patients underwent standardized scar contracture release. In the experimental group, intraoperatively shed epidermal tissue from released contractures was collected (Fig. 1A), processed into finger cuffs, and applied to cover surgical wounds (Fig. 1B), followed by fixation with silver ion-containing dressings under compression. In the control group, shed epidermal tissue was not reused. Postoperatively, both groups utilized soft brace11 to maintain interdigital angles ≥ 45°. Initial dressing changes were performed on postoperative day 7, with subsequent frequency adjusted based on exudate volume. Both pre- and post-operative pathological staining examinations were performed on hand epidermal tissues.
Surgical procedure and histological evaluation of scar contracture release with autologous exfoliated epidermal finger cuffs coverage. (A) Intraoperative view of scar contracture release. (B) Application of autologous exfoliated epidermal finger cuffs to cover the finger wound. (C–E) Postoperative appearance of the hand during the 1st, 2nd, and 3rd dressing changes, respectively. (F,G) Preoperative histology of the epidermal tissue: (F) Hematoxylin–eosin (HE) staining and (G) immunohistochemical (IHC) staining for cytokeratin 5/6 (CK5/6). (H,I) Histological analysis of the epidermal tissue at the 3rd dressing change: (H) HE staining and (I) IHC staining for CK5/6.
Outcome measures and data collection
Primary outcomes included postoperative wound healing parameters (healing time in days, frequency of dressing changes, hemorrhage during dressing changes assessed by Visual Analog Scale (VAS) score, and complications such as infection or graft detachment) and finger webbing space depth loss (mm) at 6 months postoperatively. Finger webbing space depth was measured preoperatively and monthly postoperatively using a vernier caliper (accuracy: 0.02 mm). The vertical distance from the apex of the II-III web space to the distal palmar crease was recorded, with triplicate measurements averaged for analysis. This study employed a VAS score to quantitatively evaluate intraoperative blood loss, with the following criteria: 0 points (completely dry gauze); 1 point (< 1 layer of gauze soaked); 3 points (1–2 layers soaked); 5 points (3–4 layers soaked); 8 points (≥ 5 layers soaked); and 10 points (requiring urgent intervention). This scoring system objectively reflects bleeding severity based on the number of soaked gauze layers in the surgical field, facilitating real-time intraoperative assessment and standardized documentation.
Statistical analysis
Normality of continuous variables was assessed using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation and compared via independent t-tests. Non-normally distributed data were reported as median (interquartile range) and analyzed using Mann–Whitney U or Wilcoxon signed-rank tests. Spearman’s rank correlation coefficient was used to evaluate correlations between variables. Effect sizes were calculated as Cohen’s d (for parametric tests) or Hedges’ g (for non-parametric tests), with 95% confidence intervals. A two-tailed p value < 0.05 was considered statistically significant.
Results
Wound healing time
Intraoperative photographs and postoperative dressing changes for the experimental group are shown in Fig. 1. Compared to the control group treated with traditional dressings (Fig. 2A,B), the majority of patients in the experimental group demonstrated significantly superior wound healing at the third dressing change (Fig. 1E). Patients in the experimental group (autologous exfoliated epidermal finger cuffs coverage) exhibited significantly shorter wound healing times compared to the control group (traditional petrolatum gauze dressings). The mean healing time in the experimental group was 19.0 ± 1.55 days (range: 17–21 days), while the control group required 29.0 ± 2.52 days (range: 26–32 days), with a statistically significant difference between groups (U = 3.0, p = 0.002) (Table 1). The median healing time in the experimental group was 19.0 days (IQR: 17.5–20.0), representing a 34.5% reduction compared to the control group.
Additionally, histopathological analysis of epidermal tissue from the surgical site was performed in the experimental group preoperatively (Fig. 1F,G) and during postoperative dressing change (Fig. 1H,I). The results revealed that the autologous exfoliated epidermal finger cuffs applied to the wounds did not survive (i.e., failed to engraft). However, fibroblast proliferation was observed beneath the epidermal cuffs in the majority of patients during the second or third dressing changes, with newly formed tissue adherent to the underlying dermis. By the second postoperative month, the epidermal finger cuffs gradually sloughed off, yet no new wounds formed at the sloughing sites. In Fig. 1A, intraoperative exfoliated finger epidermis is marked with red arrows, demonstrating epidermal shedding that frequently occurs following scar contracture release surgery, with these finger epidermis being repurposed. Figure 1B further illustrates the intraoperative epidermal collection site (red arrows) and the complete dissection of the web space to the metacarpophalangeal joint level, demarcated by blue arrows. During the third postoperative dressing change, epidermal specimens were harvested from the volar aspect of the little finger, as shown by red arrows in Fig. 1E.
Frequency of dressing changes and hemorrhage control
The median number of postoperative dressing changes in the experimental group was 2.5 (IQR: 2.0–3.0), significantly lower than the control group’s median of 5.0 (IQR: 4.0–5.0; W = 42, p = 0.004) (Table 2). The VAS score for hemorrhage during dressing changes was 1.8 ± 0.7 (median: 2.0, IQR: 1.0–2.0) in the experimental group, compared to 4.0 ± 0.9 (median: 4.0, IQR: 3.0–5.0) in the control group, reflecting a 55% reduction in the experimental group (U = 4.0, p = 0.001) (Table 3). Further analysis demonstrated a strong positive correlation between VAS scores and the frequency of dressing changes (r = 0.78, p < 0.01).
Safety and complications
No severe infections occurred in either group. One case in the experimental group experienced epidermal cuff mild displacement, requiring an additional dressing change, which resolved after fixation adjustment without affecting final healing. In the control group, two cases (28.6%) developed mild wound inflammation due to dressing adhesion, both resolved with local antimicrobial treatment.
Functional recovery and long-term outcomes
At 6 months postoperatively, the median finger webbing space depth loss was 1.9 mm (IQR: 1.7–2.1) in the experimental group and 2.5 mm (IQR: 2.0–2.7) in the control group; however, the difference did not reach statistical significance (U = 15, p = 0.35) (Table 4). No significant recurrence of scar contracture was observed in any patient during the 6-month follow-up. Hand function showed no substantial improvement in either group.
Discussion
This study is the first to propose the use of intraoperatively shed autologous epidermis from recessive dystrophic epidermolysis bullosa (RDEB) patients to fabricate biological finger sleeves for post-scar release wound coverage. The results demonstrated that the experimental group exhibited a 34.5% reduction in wound healing time (19.0 ± 1.5 days vs. 29.0 ± 2.5 days), a 50% decrease in dressing change frequency, and a significant 55% reduction in bleeding-related VAS scores compared to the traditional gauze group.
The core pathological basis of RDEB lies in COL7A1 gene mutations, which lead to deficiency of type VII collagen, disruption of the dermal–epidermal junction, and recurrent blistering upon minor friction. Aberrant activation of the TSP1-mediated TGF-β signaling pathway further exacerbates fibrosis and impairs wound healing12,13,14. Conventional petrolatum gauze dressings, requiring frequent changes, often induce mechanical epidermal stripping and aggravate wound damage. In contrast, autologous epidermal sleeves may directly promote re-epithelialization by preserving basement membrane components or residual extracellular matrix proteins (e.g., fibronectin, laminin). The significantly shortened healing time observed in the experimental group suggests that autologous epidermis may competitively bind microenvironmental TGF-β, thereby inhibiting TSP1-driven fibrotic pathways13,15, while providing a provisional scaffold for keratinocyte migration to accelerate wound closure16. Notably, the lower infection rate in the experimental group may reflect reduced bacterial colonization due to autologous epidermal coverage, consistent with prior reports highlighting Staphylococcus aureus susceptibility in RDEB wounds17.
Although gene therapy (e.g., CRISPR/Cas9-mediated COL7A1 correction18) and mesenchymal stem cell transplantation19 hold promise for curative RDEB treatment, their clinical translation remains limited by safety concerns, costs, and technical complexity. The autologous epidermal sleeve approach, utilizing intraoperative waste tissue without requiring ex vivo expansion or genetic modification, offers immediate applicability and cost-effectiveness, particularly in resource-limited settings. Future integration with tissue engineering strategies—such as combining epidermis with 3D-printed collagen scaffolds20 or incorporating TGF-β inhibitors (e.g., losartan21)—could enhance anti-fibrotic efficacy and improve long-term web space preservation (24% reduction in median web depth loss at 6 months in this study).
In this study, the histopathological staining and postoperative follow-up results failed to demonstrate successful engraftment of the epidermal finger cuffs at the wound beds, but the observed fibroblast proliferation beneath the non-viable cuffs suggests they may function as transient biological scaffolds, stimulating dermal repair and accelerating granulation tissue formation.
The absence of new wounds after cuff sloughing indicates that epidermal shedding occurred only after sufficient dermal remodeling, a critical advantage over traditional dressings that risk secondary trauma during removal. While long-term functional outcomes remained comparable between groups, the reduced dressing frequency, hemorrhage, and healing time support the clinical value of this technique as a cost-effective adjunct to surgical release. Further studies should explore optimizing scaffold retention time or combining this approach with antifibrotic therapies to enhance sustained benefits.
Limitations include the small sample size (experimental group: n = 6) and short follow-up duration, which may underestimate complications such as epidermal displacement (1 case in the experimental group). Furthermore, genetic mosaicism in RDEB fibroblasts22 may influence the biological activity of recycled epidermis, necessitating immunohistochemical validation of type VII collagen expression. Despite these constraints, the proposed "closed-loop trauma management" concept—repurposing surgical waste for wound repair—establishes a novel paradigm for pseudosyndactyly treatment. Implemented within a multidisciplinary framework (e.g., genetic evaluation combined with personalized rehabilitation23), this strategy could extend to other hereditary blistering disorders (e.g., junctional EB) or chronic ulcer management, ultimately reducing healthcare costs and improving patient quality of life.
Data availability
Further inquiries can be directed to the corresponding authors.
References
Zhou, X. et al. Surgical management of hand deformities in patients with recessive dystrophic epidermolysis bullosa. J. Plast. Surg. Hand Surg. 53(5), 1–7. https://doi.org/10.1080/2000656X.2019.1661846 (2019).
Lembo, F. et al. Release of pseudosyndactyly in recessive dystrophic epidermolysis bullosa using a dermal regeneration template glove: The Foggia experience. Orphanet. J. Rare Dis. 16(1), 45. https://doi.org/10.1186/s13023-021-01697-5 (2021).
Cianfarani, F. et al. Decorin counteracts disease progression in mice with recessive dystrophic epidermolysis bullosa. Matrix Biol. 75, 40–54. https://doi.org/10.1016/j.matbio.2018.12.001 (2019).
Naso, G. & Petrova, A. Cellular therapy options for genetic skin disorders with a focus on recessive dystrophic epidermolysis bullosa. Br. Med. Bull. 136(1), 83–95. https://doi.org/10.1093/bmb/ldaa029 (2020).
Condorelli, A. G. et al. Epidermolysis bullosa-associated squamous cell carcinoma: From pathogenesis to therapeutic perspectives. Int. J. Mol. Sci. 20(22), 5707. https://doi.org/10.3390/ijms20225707 (2019).
Graham, T. et al. Iterative codesign and testing of a novel dressing glove for epidermolysis bullosa. J. Wound Care. 28(1), 5–15. https://doi.org/10.12968/jowc.2019.28.1.5 (2019).
Bosch, R. J. et al. Squamous cell carcinoma secondary to recessive dystrophic epidermolysis bullosa: Report of eight tumours in four patients. J. Eur. Acad. Dermatol. Venereol. 13(3), 214–219 (1999).
Kim, M. et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm. Venereol. 98(2), 158–164. https://doi.org/10.2340/00015555-2781 (2018).
Feinstein, J. A. et al. Assessment of the timing of milestone clinical events in patients with epidermolysis bullosa from North America. JAMA Dermatol. 155(2), 196–203. https://doi.org/10.1001/jamadermatol.2018.4673 (2019).
Whitney, T. M., Ramasastry, S. & Futrell, J. W. Combined tissue expansion and free tissue transfer for reconstruction of the hand in epidermolysis bullosa-associated malignancy. Ann. Plast. Surg. 31(6), 563–567 (1993).
Wu, C. & Jiao, X.-H. Simple and affordable soft brace application in dystrophic epidermolysis bullosa patients. Front. Surg. 10, 1189962. https://doi.org/10.3389/fsurg.2023.1189962 (2024).
Neetu, B. & Prashant, B. Dystrophic calcinosis cutis in autosomal recessive dystrophic epidermolysis bullosa. BMJ Case Rep. 12(9), e231287 (2019).
Eijiro, A. et al. Diversity of mechanisms underlying latent TGF-β activation in recessive dystrophic epidermolysis bullosa. J. Invest. Dermatol. 141, 1450 (2020).
Von Bartenwerffer, W. et al. Mild recessive dystrophic epidermolysis bullosa associated with two compound heterozygous COL7A1 mutations. Eur. J. Dermatol. 21, 170 (2011).
Thomas, D. J. Engineering regenerative tissue systems using 3D bioprinting technology. A golden era for reconstructive surgery. Int. J. Surg. 90, 105982 (2021).
AtanasovaVelina, S. et al. Thrombospondin-1 Is a major activator of TGF-β signaling in recessive dystrophic epidermolysis bullosa fibroblasts. J. Invest. Dermatol. 139, 1497 (2019).
Filoni, A. et al. Morphological and morphometric analysis of cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa: A retrospective study. J. Eur. Acad. Dermatol. Venereol. 34, 1707 (2019).
Shota, T. et al. Efficient gene reframing therapy for recessive dystrophic epidermolysis bullosa with CRISPR/Cas9. J. Invest. Dermatol. 139, 1711 (2019).
Ellie, R. et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J. Am. Acad. Dermatol. 138, S125 (2019).
Nistor Manuela, T. et al. Characterization of the semi-interpenetrated network based on collagen and poly(N-isopropyl acrylamide-co-diethylene glycol diacrylate). Int. J. Pharm. 452, 92 (2013).
Reza, P. M. et al. Losartan treatment improves recessive dystrophic epidermolysis bullosa: A case series. Dermatol. Ther. https://doi.org/10.1111/dth.15515 (2022).
Rehman, A. U. et al. Whole-exome sequencing in a consanguineous Pakistani family identifies a mutational hotspot in the COL7A1 gene, causing recessive dystrophic epidermolysis bullosa. Clin. Dysmorphol. 29, 86 (2019).
Rokohl, A. C. et al. Importance of interdisciplinary collaboration for optimal treatment of orbital tumors. HNO 67, 528 (2019).
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
CW and TG conceived the study. TG and CW drafted the manuscript and performed the literature search and collected the data. XHJ and CW analyzed and visualized the data. XHJ and YLJ revised the manuscript and were the supporters of the study. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and consent to participate
This study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Zhengzhou University (Approval No.: 2023014), and written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, C., Guo, T., Jian, Y. et al. Reusing of intraoperative exfoliated finger epidermis in the treatment of hand scar contracture in patients with RDEB. Sci Rep 15, 19855 (2025). https://doi.org/10.1038/s41598-025-05278-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05278-5