Abstract
Azvudine is recommended for patients with coronavirus disease 19 (COVID-19); however, its optimum therapeutic time window and its impact on mortality of patients are unclear. This single-centre, retrospective study from 1 November 2022 to 27 February 2023 conducted at the Peking Union Medical College Hospital was to discuss the dosing window of azvudine and compare the prognostic impact on COVID-19 patients of azvudine use within and after the defined time window. Therapeutic time window referred to the time interval between the onset of the disease and the drug administration. 28-day all-cause mortality and the incidence of 28-day disease progression were assessed using univariate logistic regression and adjusted for covariates through multivariate logistic regression analysis. A total of 421 COVID-19 patients using azvudine and 720 patients not using any anti-SARS-CoV-2 drugs were enrolled. After propensity score matching, 302 patients treated with azvudine and 302 patients without antiviral drugs were included. Multivariate logistic regression analysis showed that the use of azvudine was significantly protective until 8 days of symptom onset for COVID-19 patients. Compared with the latter, treatment with azvudine reduced the all-cause mortality rate (OR 0.55, 95% CI 0.30–1.00) and disease progression rate (OR 0.52, 95% CI 0.29–0.93) to 28 days. The study indicated that the benefit of azvudine seemed more significant within 8 days of symptoms onset and the administration of azvudine reduced the risk of death in adult COVID-19 patients. In the future, large randomized controlled trials (RCT) studies are needed to confirm our conclusions because of the inherent limitation of single-centre, retrospective study.
Similar content being viewed by others
Introduction
Since the outbreak of coronavirus disease 2019 (COVID-19), more than 700 million people have been infected, and nearly 7 million have died worldwide by May 2023, causing a huge global health and economic burden and widespread concern1. As the strains continue to mutate, the Omicron strain has become more transmissible; however, the rate of hospitalisation has decreased significantly compared with previous strains2,3. In late 2022, China saw the novel coronavirus Omicron strain outbreak’s peak as the epidemic policy was adjusted1,4. Several antiviral drugs were administered during the period5,6.
Antiviral drugs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including nirmatrelvir-ritonavir, remdesivir, and molnupiravir, are recommended for the treatment of patients with COVID-19 who have a high risk of disease progression7. Three real-world studies in Hong Kong have shown that both molnupiravir and nirmatrelvir-ritonavir reduced the risk of death and disease progression in COVID-19 patients8,9,10. In addition, azvudine, an antiviral drug developed in China, was approved for the China Formula of Diagnosis and Treatment for COVID-19 (trial version 10)11,12. Several studies have indicated that azvudine treatment facilitated negative conversion in COVID-19 patients13,14. Two real-world studies conducted at Xiangya Hospital showed that treatment with azvudine reduced the incidence of composite endpoint, which was composed of mechanical ventilation, admission to ICU and all-cause death, compared with no antiviral drug treatment or treatment with nirmatrelvir-ritonavir15,16; however, azvudine has not been found to definitively reduce all-cause mortality in patients.
Additionally, extending the therapeutic window for antiviral therapy remains a challenge. Recent studies showed that nirmatrelvir-ritonavir and molnupiravir should be used within 5 days of symptom onset17,18,19,20. Although remdesivir can be administered within 7 days, it is administered intravenously, which limits its use in outpatients21. Azvudine functioned as an inhibitor of RNA-dependent RNA polymerase (RdRp), which was also the target of remdesivir, and it was still unknown whether azvudine administered five days after onset of illness will be effective, potentially providing more antiviral options for the general population.
Although some real-world studies on azvudine have been published, whether azvudine reduces mortality and the therapeutic window for azvudine remains unknown. This study aimed to retrospectively discuss the dosing time window for azvudine and compare the prognostic impact of using azvudine within and after the defined time window in a large academic hospital.
Results
Patients’ demographics and comorbidities
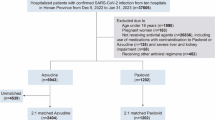
From 1 November 2022 to 27 February 2023, a total of 421 COVID-19 patients were recorded receiving azvudine and 720 hospitalised patients with confirmed COVID-19 who had not used antiviral drugs at Peking Union Medical College Hospital (PUMCH), all with 28 days of follow-up. A total of 310 patients receiving azvudine were propensity-score-matched to the inpatients according to the inclusion and exclusion criteria (Fig. 1). Among 310 patients received azvudine, four (1.29%) patients had an unclear clinical category at baseline. In both groups, the most common comorbidity was hypertension (50.6% vs. 47.1%, p = 0.34), followed by cardiovascular disease (27.5% vs. 21.9%, p = 0.07) and diabetes (31.5% vs. 28.7%, p = 0.41); however, a higher proportion of patients not treated with antivirals had chronic kidney disease (CKD) (15.1% vs. 8.06%, p = 0.003) (Supplementary Table S1). Figure 2 showed the baseline characteristics after Propensity score matching (PSM) between patients treated with azvudine and those not treated with antivirals. After PSM, we included 302 patients treated with azvudine and 302 patients who were not treated with antivirals. Age, sex, and comorbidities were balanced between the two groups.
Patients’ clinical characteristics and outcomes during SARS-CoV-2 infection
Before PSM, patients without antiviral therapy had a higher clinical category at baseline and proportions were balanced after PSM. After PSM, a higher proportion of patients not treated with antivirals received corticosteroids (72.2% vs. 58.9%, p = 0.001) and tocilizumab (18.5% vs. 12.3%, p = 0.04) than patients treated with azvudine. In addition, a higher proportion of patients not receiving antivirals required advanced oxygen therapy than patients receiving azvudine during SARS-CoV-2 infection. Among patients, 6 (1.99%) patients treated with azvudine and 29 (9.60%) controls received invasive mechanical ventilation or ECMO (Supplementary Table S2, Supplementary Fig. S1). At day 28, A total of 20 (6.45%) of the 310 patients treated with azvudine died.
Therapeutic time window of azvudine
In the univariate and multivariate logistic analysis, the administration of azvudine after 5 days of disease onset reduced all-cause mortality at 28 days. To further investigate the time window for azvudine administration, multivariate logistic regression analysis with regrouping according to the time from onset to administration revealed that azvudine administration after 8 days of onset no longer reduced the risk of death (Table 1, Supplementary Table S3). However, there was no significant change in odds ratio (OR) when changing the time window of treatment with azvudine to 6, 7, and 8 days compared to the time window of 5 days, and the use of azvudine within the time window of treatment significantly reduced the risk of death in all cases (Supplementary Table S3).
Primary outcome: 28-day all-cause mortality
Before PSM, the administration of azvudine after 8 days slightly increased the risk of death compared to treatment with azvudine within 8 days of disease onset (Supplementary Table S4). Treatment with azvudine reduced all-cause mortality by 51% at 28 days compared with patients not receiving antivirals (OR 0.49, 95% CI 0.28–0.87), and the risk of death was reduced with azvudine only within 8 days of disease onset (OR 0.23, 95% CI 0.08–0.65).
In multivariate logistic regression, treatment with azvudine within 8 days of disease onset significantly reduced the risk of death at 28 days after adjusting for age, sex, comorbidities, and concomitant medications, and the OR was not influenced by different covariates (Table 2).
Secondary outcome: 28-day composite endpoint
At day 28, a total of 22 (7.10%) of the 310 patients treated with azvudine achieved the composite endpoint, which included invasive mechanical ventilation, extracorporeal membrane oxygenation (ECMO), or death. Before PSM, the use of azvudine after 8 days also slightly increased the risk of the composite endpoint compared to azvudine treatment within 8 days of disease onset (Supplementary Table S4). After PSM, 22 (7.26%) patients treated with azvudine and 44 (14.2%) controls showed disease progression. Univariate logistic regression analysis showed that azvudine administration within 8 days of disease onset reduced the incidence of the composite endpoint. After adjusting for all covariates, only the use of azvudine within 8 days significantly reduced the incidence of the composite outcome by 66% (Table 3).
Subgroup analysis
Subgroup analysis of baseline information showed that the use of azvudine in patients who were < 65 years reduced the risk of death. Additionally, the use of azvudine in patients treated with corticosteroids during infection reduced mortality. (Supplementary Fig. S2).
A subgroup analysis of patients with moderate or greater disease severity at baseline revealed that the effectiveness of azvudine administered within 8 days of disease onset in reducing the risk of death was not affected by age, sex, comorbidities, or concomitant medications. In contrast, comorbidities including CKD and malignant tumour increased the risk of death. Additionally, the patients who died were more likely to have used anti-inflammatory drugs (Supplementary Fig. S3).
Safety
Of the 310 patients treated with azvudine, 45 (14.5%) experienced drug-related adverse reactions, 41 (91.1%) of whom had at least one comorbidity, 43 (93.5%) had a CTCAE grade ≤ 2, and 2 (4.44% in patients with adverse reactions, 0.65% in all patients using azvudine) had grade 322. Liver enzyme and serum creatinine levels were monitored in 153 patients (49.4%). Among the patients who experienced adverse reactions, 26 (57.8%) had elevated liver enzyme levels, 15 (57.7%) returned to normal levels after drug discontinuation, and 8 (30.8%) showed a decreasing trend. Ten (22.2%) patients had elevated serum creatinine levels, including 1 (10.0%) patient with chronic kidney disease and 7 (70.0%) patients with normalised serum creatinine levels after drug discontinuation. In addition, three (6.67%) patients developed dizziness and nausea after taking the drug, and three (6.67%) patients developed diarrhoea. Moreover, two (4.44%) patients had elevated blood pressure, one of whom had hypertension, and one (2.22%) patient experienced hallucinations (Supplementary Table S5).
Discussion
More than four years have passed since the outbreak of COVID-19 in late 2019, and the spread of the disease remains a major threat to human health worldwide1. Effective, safe, and affordable antiviral regimens are yet to be explored. This study reviewed the clinical data of adult patients with COVID-19 at PUMCH during the epidemic in China between late 2022 and early 2023. The results suggested that the therapeutic window of azvudine was expected to be extended to eight days after onset and the oral administration of azvudine might reduce mortality of patients.
Antiviral drugs against COVID-19 are divided into two main classes: protease inhibitors and competitive substrates of viral RNA-dependent RNA polymerase (RdRp)23. Azvudine is a nucleoside analogue that inhibits viral RdRp and can block the viral replication process. In addition, azvudine was enriched in the thymus in vivo and could activate T cells, and its phosphorylation activation in the thymus is also conducive to the body’s immune protection response14,24. Nirmatrelvir-ritonavir, a protease inhibitor combined with a pharmacological enhancer, has been shown to be effective in reducing the risk of hospitalisation or death in high-risk adults in both phase III clinical trials and real-world studies25. Nevertheless, nirmatrelvir-ritonavir, as a potent cytochrome P450 3A4 (CYP3A4) inhibitor, presents clinically significant drug-drug interaction risks by elevating plasma concentrations of CYP3A4-metabolized medications. This pharmacokinetic limitation restricts its use in patients receiving polypharmacy for comorbidities, as many patients are on multiple drugs for long periods of time due to comorbidities. Molnupiravir is a competitive substrate for viral RdRp with fewer drug interactions26. However, both drugs are expensive for patients in many developing countries, currently costing approximately $200–300 for a course of treatment in China. Azvudine, as a new antiviral drug, which costs one-tenth of the price of nirmatrelvir-ritonavir in China, has the advantages of a lower price and fewer drug interactions; however, more clinical evidence on its efficacy and safety is needed. In fact, our preliminary study found that the effectiveness and safety of azvudine were comparable to those of nirmatrelvir-ritonavir27.
The therapeutic time window is critical for antiviral therapy. Current anti-SARS-CoV-2 therapies, such as nirmatelvir-ritonavir, require the administration of drugs within 5 days after infection, which is often impractical. In addition, many patients still require prolonged antiviral therapy post 5 days of infection. Pneumonia caused by SARS-CoV-2 tends to appear 7–14 days after the onset of the disease, according to the previous experience of COVID-19 treatment. Severe and critical patients have impaired viral clearance due to the administration of glucocorticoids, tocilizumab, and baricitinib. Our retrospective study suggested that azvudine could extend the window for treating SARS-CoV-2 in adult patients. The protective efficacy of azvudine was observed when the drug was delayed to within 8 days of symptom onset and the finding was stable when regrouping patients according to the time from symptom onset to administration. The extended therapeutic window of azvudine might be attributed to its pharmacological mechanism, such as remdesivir, another RdRp inhibitor, which has a therapeutic window of seven days21. They all have a longer therapeutic time window than nirmatelvir-ritonavir, a 3-chymotrypsin-like protease inhibitor. However, the underlying mechanisms require further investigation. In summary, azvudine is an effective antiviral option, even in the late stages of infection.
In this retrospective study, 20 of the 302 patients in the azvudine treatment group included in the analysis died within 28 days, while 38 of the 302 patients who did not receive antiviral therapy died. Azvudine treatment reduced all-cause mortality by 28 days. The crude all-cause mortality rate, according to another retrospective study from Xiangya Hospital, was 1.934/1000 person-days in the Azvudine group and 4.128/1000 person-days in the non-antiviral group16. The differences in all-cause mortality between the two studies may be due to the different populations included in the analyses. The severity in this study ranged from mild to critical, whereas the subjects of the Xiangya study excluded patients who were severely or critically ill at admission. To reduce bias due to patient demography, comorbidities, and combined drug use, we additionally performed a multivariate logistic regression28,29. After adjusting for all covariates, treatment with azvudine within the therapeutic time still reduced the risk of 28-day mortality, as well as the incidence of invasive mechanical ventilation, or ECMO, within 28 days of patient admission.
The clinical spectrum of COVID-19 ranges from asymptomatic upper respiratory tract infections to critical pneumonia30. Preexisting comorbidities such as hypertension, diabetes, cardiovascular disease, chronic kidney disease, and cancer are associated with a poor prognosis31. Our study reported similar findings that CKD and malignant tumour increased the risk of death in COVID-19 patients.
The study had some limitations. First, as a single-centre retrospective study, the presence of bias can affect the reliability of the results, but cannot be eliminated, even though we adopted statistical methods such as PSM, multivariate regression, and subgroup analysis to adjust and minimise bias due to differences in baseline between the two groups. Patients taking azvudine were matched 1:1 by PSM with patients not taking antiviral drugs who were hospitalised at the same time according to age, sex, comorbidities, and clinical categories; whether the baseline information was matched was assessed by standardised mean difference (SMD). However, the administration time was not unified, which also contributed to selection bias. With the exception of 4 (1.29%) azvudine-treated patients whose clinical classification was unclear and some patients could only be judged as moderate or severe category, there was a difference in the number of patients with the critical subtype between the treatment and control groups. In addition, we used a big data query and analysis system of our hospital to collect information on all COVID-19 patients who had taken azvudine and included different disease severity to reduce selection bias, which made it generalizable to different healthcare settings. Second, vaccination was not collected in this study, which has been proved to reduce mortality32. In addition, secondary infection was associated with worse prognosis of COVID-19 patients33. This retrospective study did not evaluate the impact of vaccination and secondary infection on the effectiveness of azvudine administration, which should be of concern in future studies. Third, prolonged therapeutic window was purely analysed from statistical trends. Therefore, pharmacological mechanisms need to be explored in future studies. Given these limitations, large-scale randomised controlled trials (RCT) studies are needed in the future to confirm our results and generalize these findings to larger populations34.
Conclusions
This retrospective study suggested that the therapeutic window of azvudine could be extended to 8 days and the use of azvudine as a safe and economical anti-viral drug could reduce mortality of adult patients with COVID-19. The extended therapeutic window provided a new reference point for future clinical practice. However, considering the limitations of retrospective studies, future large-scale real-world RCTs are needed. Azvudine was granted conditional approval for COVID-19 treatment in China, but more data from RCTs and real-world studies are needed to determine whether it should be recommended as a first-line treatment.
Methods
Study design and population
This retrospective, single-centre, real-world study was conducted at PUMCH from 1 November 2022 to 27 February 2023 to assess the therapeutic time window of azvudine in treating COVID-19 patients and the efficacy of azvudine in improving clinical outcomes. We included all patients who visited our hospital who (1) were ≥ 18 years old, (2) infected with SARS-CoV-2 confirmed by nucleic acid test or antigen test, and (3) were received azvudine because of SARS-CoV-2 infection. However, patients who (1) used antiviral drugs other than azvudine during SARS-CoV-2 infection, (2) had missing endpoints, or (3) had unknown interval between administration of azvudine and symptom onset were excluded. Hospitalised patients at PUMCH who were ≥ 18 years old and did not receive antiviral therapy were also enrolled. Antiviral drugs included nirmatrelvir/ritonavir, molnupiravir and azvudine (duplicated in treated group). All patients received standard medical care according to the China Formula of Diagnosis and Treatment for COVID-19 (version 9) before 5 January 2023 and (version 10) after 5 January 2023. Patients received azvudine were administered 5 mg day− 1 azvudine tablets orally (5 tablets once a day) for 7 days. This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (I-23PJ946). As this was a retrospective study, the requirement for informed consent was waived by Institutional Review Board of Peking Union Medical College Hospital. All procedures followed the Declaration of Helsinki and its amendments. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplementary Table S6)35.
Data collection
All patients were reviewed and queried through the Big Data Query and Analysis System (http://cdr.pumch.local/) of PUMCH. Clinical data were obtained from the electronic medical record system, including demographics (age and sex), clinical information (comorbidities, oxygen therapy), therapeutic time window (time interval from symptom onset to azvudine use), and concomitant medication (corticosteroids, anti-inflammatory drugs), as well as admission and discharge time or time of death. Comorbidities included hypertension, cardiovascular disease, cerebrovascular disease, diabetes, CKD, chronic obstructive pulmonary disease (COPD), and interstitial lung disease (ILD). Advanced oxygen therapy included high-flow nasal cannula oxygen therapy (HFNC), facemask with reservoir, mechanical ventilation or ECMO. Anti-inflammatory drugs including tocilizumab and baricitinib. According to the China Formula for the Diagnosis and Treatment of COVID-19 (version 10), the severity of SARS-CoV-2 infection is classified into four categories: mild, moderate, severe, and critical (Supplementary Table S7).
Outcomes
The primary outcome was the 28-day all-cause mortality. The secondary outcome was the 28-day composite endpoint of disease progression, which included invasive mechanical ventilation, ECMO, and death. Each patient was followed up on day 28 after administration to determine their survival status. The adverse effects related to azvudine were also assessed. Data on possible clinical manifestations, such as diarrhoea and nausea, were collected at follow-up, and relevant laboratory tests, including liver transaminases and serum creatinine, were also collected from the start of azvudine to 3 days after the end of the drug. Adverse events were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE version 5.0)22.
Statistical method
PSM was performed based on baseline characteristics, including age, sex, comorbidities (hypertension, cardiovascular disease, diabetes, CKD, cerebrovascular disease, COPD, autoimmune disease), and severity, to match patients treated with azvudine with those hospitalised at PUMCH due to SARS-CoV-2 infection. A 1:1 nearest-neighbor matching method without replacement was implemented with a caliper width of 0.05. The SMD was calculated to assess whether the baseline characteristics were balanced, and the cutoff was defined as 0.136,37. Because not every patient had an oxygen saturation level recorded and the severity of patients treated with azvudine had missing values, we only classified patients as mild or non-mild, including moderate, severe and critical. The X2 test, ANOVA, and Kruskal–Wallis rank sum test were used to compare baseline characteristics of patients treated with azvudine and controls. The effectiveness of azvudine was assessed using logistic regression, and OR and 95% confidence intervals (CI) were calculated. Age, sex, comorbidities (including CKD and malignancy) and concomitant medications (including corticosteroid, baricitinib and tocilizumab) were adjusted using multivariate logistic regression.
All statistical analyses were conducted using R (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-tailed, and the significance level was p < 0.05. Bar plots were generated using GraphPad Prism version 8.0.2. for windows, GraphPad Software, San Diego, California USA, https://www.graphpad.com.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020 (2023). https://covid19.who.int/
Tian, D., Sun, Y., Xu, H. & Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 94, 2376–2383. https://doi.org/10.1002/jmv.27643 (2022).
Zhou, Y., Zhi, H. & Teng, Y. The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity. J. Med. Virol. 95, e28138. https://doi.org/10.1002/jmv.28138 (2023).
Joint prevention and control Mechnism of The State Council for the Novel Coronavirus Pneumonia. Notice on further optimizing and implementing the prevention and control measures of the novel coronavirus (2022). http://www.gov.cn/xinwen/2022-12/07/content_5730443.htm
Barghash, R. F. et al. Navigating the COVID-19 therapeutic landscape: unveiling novel perspectives on FDA-Approved medications, vaccination targets, and emerging novel strategies. Molecules. 29 https://doi.org/10.3390/molecules29235564 (2024).
Barghash, R. F. et al. In Silico modeling as a perspective in developing potential vaccine candidates and therapeutics for COVID-19. Coatings. 11, 1273 (2021).
Lamontagne, F. et al. A living WHO guideline on drugs for covid-19. BMJ. 370, m3379. https://doi.org/10.1136/bmj.m3379 (2020).
Wai, A. K. et al. Association of molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg. Health West. Pac. 30, 100602. https://doi.org/10.1016/j.lanwpc.2022.100602 (2023).
Wong, C. K. H. et al. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong kong’s Omicron BA.2 wave: a retrospective cohort study. Lancet Infect. Dis. 22, 1681–1693. https://doi.org/10.1016/s1473-3099(22)00507-2 (2022).
Wong, C. K. H. et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus Ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong kong: an observational study. Lancet 400, 1213–1222. https://doi.org/10.1016/s0140-6736(22)01586-0 (2022).
Yu, B. & Chang, J. The first Chinese oral anti-COVID-19 drug azvudine launched. Innov. (Camb). 3, 100321. https://doi.org/10.1016/j.xinn.2022.100321 (2022).
Diagnosis and treatment protocol for COVID-19 in China. (trial version 10). (2023). https://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm
Ren, Z. et al. Open-Label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. (Weinh). 7, e2001435. https://doi.org/10.1002/advs.202001435 (2020). A Randomized.
Zhang, J. L. et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal. Transduct. Target. Ther. 6, 414. https://doi.org/10.1038/s41392-021-00835-6 (2021).
Deng, G. et al. Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J. Med. Virol. 95, e28756. https://doi.org/10.1002/jmv.28756 (2023).
Sun, Y. et al. Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine. 59, 101981. https://doi.org/10.1016/j.eclinm.2023.101981 (2023).
Arbel, R. et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N. Engl. J. Med. 387, 790–798. https://doi.org/10.1056/NEJMoa2204919 (2022).
Hammond, J. et al. Oral nirmatrelvir for High-Risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408. https://doi.org/10.1056/NEJMoa2118542 (2022).
Li, H. et al. Association of nirmatrelvir/ritonavir treatment on upper respiratory severe acute respiratory syndrome coronavirus 2 reverse Transcription-Polymerase chain reaction (SARS-Cov-2 RT-PCR) negative conversion rates among High-Risk patients with coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 76, e148–e154. https://doi.org/10.1093/cid/ciac600 (2023).
Saravolatz, L. D., Depcinski, S. & Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin. Infect. Dis. 76, 165–171. https://doi.org/10.1093/cid/ciac180 (2023).
Gottlieb, R. L. et al. Early Remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 386, 305–315. https://doi.org/10.1056/NEJMoa2116846 (2022).
Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0, < (2017). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
Poduri, R., Joshi, G. & Jagadeesh, G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell. Signal. 74, 109721. https://doi.org/10.1016/j.cellsig.2020.109721 (2020).
Sheng, N. et al. Selectively T cell phosphorylation activation of azvudine in the thymus tissue with immune protection effect. Acta Pharm. Sin B. 14, 3140–3154. https://doi.org/10.1016/j.apsb.2024.03.032 (2024).
Li, G., Hilgenfeld, R., Whitley, R. & De Clercq, E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat. Rev. Drug Discov. 22, 449–475. https://doi.org/10.1038/s41573-023-00672-y (2023).
Kabinger, F. et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 28, 740–746. https://doi.org/10.1038/s41594-021-00651-0 (2021).
Xie, H. et al. Effectiveness and safety of azvudine versus nirmatrelvir-ritonavir in adult patients infected with COVID-19 Omicron strains: a retrospective study in Beijing. Sci. Rep. 14, 23974. https://doi.org/10.1038/s41598-024-74502-5 (2024).
Chandrasekar, V., Mohammad, S., Aboumarzouk, O., Singh, A. V. & Dakua, S. P. Quantitative prediction of toxicological points of departure using two-stage machine learning models: A new approach methodology (NAM) for chemical risk assessment. J. Hazard. Mater. 487, 137071. https://doi.org/10.1016/j.jhazmat.2024.137071 (2025).
Singh, A. et al. Investigating tattoo pigments composition with UV-Vis and FT-IR spectroscopy supported by chemometric modelling. Curr. Anal. Chem. 20 https://doi.org/10.2174/0115734110316443240725051037 (2024).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl. J. Med. 382, 1708–1720. https://doi.org/10.1056/NEJMoa2002032 (2020).
Docherty, A. B. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 369, m1985. https://doi.org/10.1136/bmj.m1985 (2020).
Meslé, M. M. I. et al. Estimated number of lives directly saved by COVID-19 vaccination programmes in the WHO European region from december, 2020, to march, 2023: a retrospective surveillance study. Lancet Respir Med. 12, 714–727. https://doi.org/10.1016/s2213-2600(24)00179-6 (2024).
Markovskaya, Y., Gavioli, E. M., Cusumano, J. A. & Glatt, A. E. Coronavirus disease 2019 (COVID-19): secondary bacterial infections and the impact on antimicrobial resistance during the COVID-19 pandemic. Antimicrob. Steward Healthc. Epidemiol. 2, e114. https://doi.org/10.1017/ash.2022.253 (2022).
Tian, X. et al. Efficacy and safety of azvudine in symptomatic adult COVID-19 participants who are at increased risk of progressing to critical illness: a study protocol for a multicentre randomized double-blind placebo-controlled phase III trial. Trials 25, 77. https://doi.org/10.1186/s13063-024-07914-3 (2024).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457. https://doi.org/10.1016/s0140-6736(07)61602-x (2007).
Zhang, Z., Kim, H. J., Lonjon, G. & Zhu, Y. Balance diagnostics after propensity score matching. Ann. Transl Med. 7, 16. https://doi.org/10.21037/atm.2018.12.10 (2019).
Austin, P. C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 38, 1228–1234. https://doi.org/10.1080/03610910902859574 (2009).
Acknowledgements
We thank Dingding Zhang for guidance on constructing the methodology for this retrospective study. We thank all the hospital staff members for their efforts in COVID-19 treatment and data collecting that used in this study; thank the patients who participated in this study.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2023YFC3041900), National High Level Hospital Clinical Research Funding (grant number 2023-PUMCH-G-001), and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number 2021-I2M-1-048).
Author information
Authors and Affiliations
Contributions
L.W., J.F., Y.X., X.T., M.W. and S.Z. designed the study. Y.W., C.C. and H.X. did the analyses. L.W. and Y.W. drafted the manuscript. The other authors revised the initial draft. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (I-23PJ946). As this was a retrospective study, the requirement for informed consent was waived by Institutional Review Board of Peking Union Medical College Hospital.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, L., Wang, Y., Xu, Y. et al. Impact of early and delayed azvudine administration on COVID-19 mortality: a retrospective study. Sci Rep 15, 21729 (2025). https://doi.org/10.1038/s41598-025-05381-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05381-7