Abstract
Therapeutic strategies for advanced renal cell carcinoma have advanced dramatically since the approval of immune checkpoint inhibitors. To date, four PD-L1 assays have been established as companion diagnostic tools for checkpoint inhibitors. However, all PD-L1 assays present several limitations in terms of concordance and compatibility. In this study, we constructed tissue microarrays from 286 clear cell renal cell carcinoma (ccRCC) tissue samples and evaluated PD-L1 expression using the 22C3, 28 − 8, SP142, and SP263 PD-L1 assays. Detection of PD-L1 expression in tumor cells was very low for all PD-L1 assays tested, although expression of PD-L1 in immune cells in the tumor area was 14.7% for 22C3, 16.1% for 28 − 8, 2.1% for SP142, and 15.0% for SP263. PD-L1 expression on immune cells assessed by 28 − 8 showed a moderate pairwise concordance with other assays (κ statistics: 0.52 with 22C3, 0.16 with SP142, and 0.46 with SP263). Patients with PD-L1 expression in immune cells evaluated using 22C3, 28 − 8, and SP263 showed significantly worse cancer-specific survival. PD-L1 expression on renal cell carcinoma tissues assessed using 22C3, 28 − 8, SP142, and SP263 did not show good concordance. In conclusion, differences between these assays should be considered when evaluating the results of immunohistochemical findings.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) is the fourteenth most frequently diagnosed cancer worldwide1. In 2020, an estimated 431,288 new RCC and associated 179,368 deaths were reported. In its early stages, RCC can be cured by surgical resection. However, patients with advanced RCC have a poor prognosis because of tumor resistance to chemotherapy and radiotherapy2. The 5-year survival rate has been reported to be over 90% for patients with localized RCC and approximately 10% for those with advanced disease3. Patients with advanced RCC have benefited greatly from recent advancements in molecular-targeted drugs4. Over the past decade, the introduction of immune checkpoint inhibitors (ICIs) has changed the management of patients with advanced RCC5. Although clinical trials of ICIs have demonstrated significant improvements in survival and response, their efficacy remains limited6,7,8,9,10,11.
Recently, the development of companion diagnostics methods has been exploited concurrently with that of molecular-targeted drugs. Several biomarkers, such as tumor mutation burden (TMB), microsatellite instability, expression of programmed death-ligand 1 (PD-L1), and lymphocyte infiltration in cancer tissues, have been reported to be significantly correlated with the efficacy of ICIs. In general, tumor types with a higher TMB achieve a better response to ICI therapy12,13. Despite its relatively low TMB, RCC can benefit from ICI therapy14,15. PD-L1 expression, assessed using immunohistochemical (IHC) staining, was developed as a companion diagnostic tool for ICIs targeting programmed cell death 1 (PD-1) or PD-L1. ICI therapy improved the clinical outcome in RCC patients in the clinical trials and PD-L1 expression was assessed in these trials6,7,8,9,11. The Food and Drug Administration (FDA) has currently approved four PD-L1 IHC assays (PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Inc., Santa Clara, CA), PD-L1 IHC 28 − 8 pharmDx (Agilent Technologies, Inc.), VENTANA PD-L1 (SP142) assay (Roche Diagnostics K.K., Tokyo, Japan), and VENTANA PD-L1 (SP263) assay (Roche Diagnostics K.K.)) for different drugs in treating different cancer types16. However, suitable PD-L1 IHC assays have not been approved to aid the selection of therapeutic candidates for ICI therapy in patients with advanced RCC. Table 1 summarizes the details and outcomes of the four currently available PD-L1 IHC assays in clinical trials evaluating ICIs for advanced RCC. The rate of PD-L1 expression detected differed according to the assay used. These four assays were developed independently and present several limitations in their application. For instance, each assay is based on different antibody clones, target evaluation, and method of evaluation, which impose a notable burden on pathology laboratories. Therefore, a trend towards harmonizing the results of different PD-L1 IHC assays has emerged, particularly for the diagnosis of lung cancer. Nonetheless, the concordance in the evaluation of PD-L1 expression using the different assays has not been well examined. The four FDA-approved assays have been automated and require specialized equipment. Furthermore, the reagents are expensive, which makes assess multiple samples very costly.
In the present study, we used tissue specimens from patients with clear cell renal cell carcinoma (ccRCC) and constructed a tissue microarray (TMA) that allowed evaluation of multiple samples under the standardized conditions. Next, we examined the concordance of PD-L1 expression in ccRCC tissues across these four PD-L1 IHC assays. Finally, we performed next-generation sequencing (NGS) using tumor tissues and assessed genomic characteristics for a subset of patients to identify structural variant of PD-L1.
Results
Patient characteristics
The clinicopathological characteristics of 286 patients enrolled in the study are summarized in Table 2. The entire cohort consisted of 203 male and 83 female patients with ccRCC, and the median age at surgery was 65 years (range 24–89 years). Nephrectomies and partial nephrectomies were performed in 190 and 96 patients, respectively. All patients were pathologically diagnosed with ccRCC, and 7 cases were accompanied by sarcomatoid changes. Overall, 181 patients presented with pathological stage 1, 16 with stage 2, 86 with stage 3, and 3 with stage 4. According to the WHO/ISUP grading system, 34 cases were grade 1, 170 were grade 2, 54 were grade 3, and 28 were grade 4. Necrosis was observed in 69 tumors. The median follow-up duration was 1572 days (range 13–5323 days). Among the 91 patients with metastases or RCC recurrence, 49 died of disease progression.
PD-L1 expression using tissue microarray
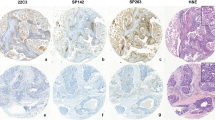
The representative images of the positive and negative cases are shown in Fig. 1 and Figure S1. The evaluation criteria and PD-L1 positivity for each PD-L1 IHC assay are summarized in Table 3. Under the evaluation criteria specific to the clinical trials each IHC assay was used, antibodies 28 − 8 and SP142 showed low positivity (2.1% each), whereas antibodies 22C3 and SP263 showed higher positivity (18.9% and 15.0%, respectively). Using uniform assessment criteria, PD-L1 positivity assessed in the tumor cells (TCs) was extremely low across all four assays. In contrast, PD-L1 positivity assessed in the tumor-infiltrating immune cells (ICs) was approximately 15.0%, except for SP142. We also observed the similar positivity of PD-L1 expression when combined TCs and ICs using 22C3, 28 − 8, and SP263 antibodies. The expression of PD-L1 in TCs was low for all PD-L1 IHC assays tested. Furthermore, PD-L1 expression assessed using SP142, was remarkably low for both TCs and ICs.
Representative images of PD-L1 IHC assays in TMA. Four PD-L1 IHC assays (28 − 8, 22C3, SP142, and SP263) were performed on TMAs consisting of 3 cores from each case. The images of the entire cores of TMA were generated by compositing sectioned images using All-in-one fluorescence microscope (BZ-X710, KEYENCE, Osaka, Japan). One representative example of a PD-L1-positive and PD-L1-negative case is shown. This positive case showed PD-L1 expression in both TCs and ICs. The bottom images are the high-powered field of PD-L1 staining in TCs and ICs, respectively. TC, tumor cells; IC, immune cell.
Pairwise concordance of PD-L1 expression among the four FDA-approved IHC assays
Next, we performed pairwise comparisons between the assays using PD-L1 expression in TCs and ICs (Table 4 and Figure S2). Overall, the kappa coefficient was higher for PD-L1 expression in TCs than in ICs. The 28 − 8 assay demonstrated the highest agreement with the other assays. The SP142 assay was considered unsuitable for evaluating concordance owing to the very low expression of PD-L1 (Figure S3).
Association between PD-L1 IHC expression and prognosis
We next examined the prognostic impact of PD-L1 expression associated with different clinical outcome stratifying the patients based on positive and negative PD-L1 expression. When assessing PD-L1 expression in ICs, the overall survival (OS) was significantly shorter in patients with PD-L1-positivity based on the 22C3 and 28 − 8 assays (Fig. 2). When the TCs were included in the assessment, the SP263 assay showed a significant difference. The cancer-specific survival (CSS) was significantly shorter in patients with PD-L1 positivity for all evaluation criteria in the 22C3, 28 − 8 and SP263 assays (Fig. 3).
Identification of tumor mutation profile and tumor mutation burden in CcRCC tissue specimens
Among 286 patients with available specimens for TMA, we performed NGS on 113 patients with appropriate tissue samples. The mutation profiles detected in ccRCC tissues are summarized in Fig. 4. As previously reported in patients with ccRCC, the most frequently mutated gene was VHL (70.8%), followed by PBRM1 (34.5%), BAP1 (17.7%), and SETD2 (13.3%). The distribution of the tumor mutation burden (TMB) is shown in Fig. 5. The median TMB levels were 1.64 mut/mb (0.06–18.22). Only one patient (K15) had a high TMB. This patient was PD-L1 negative for all four assays. When we evaluated the correlation between TMB and PD-L1 positivity, we were not able to confirm any correlation between these two parameters which are already known to be the predictor of ICIs (Figure S4). The total number of frameshift deletions and insertions is shown in Figure S5. The median number of frameshift deletions and insertions was 0.18 mut/mb (0.00–0.94).
Identification of structural variant of PD-L1
Next, we evaluated structural variants of 3’-UTR of PD-L1 using RNA-seq data in 79 samples, which cause cleavage of cytoplasmic domain of PD-L1 protein. To explore the difference of PD-L1 positivity using four antibodies determined by the RNA-seq data in 79 samples. The ratio of exon 4 over 3’-UTR expression was assessed as described previously17. However, there were no cases with a high exon 4 to 3’-UTR ratio indicating 3’-UTR disruption (Data not shown), suggesting there was another mechanism that cause the difference of PD-L1 positivity using four antibodies.
Discussion
ICIs have been established as treatments for several cancer types and have shown consistent therapeutic efficacy18. However, improvement in prognosis is still limited; furthermore, ICI therapy can lead to immune-related adverse events, sometimes resulting in serious outcomes19. Therefore, predictive biomarkers have been developed to identify patients that would most benefit from the treatment. PD-L1 expression in tumor tissues is an established biomarker for predicting the therapeutic efficacy of ICIs and has been approved by the FDA for several cancer types20,21. In the JAVELIN Renal 101 trial, PD-L1 positivity was associated with an improved PFS10. However, other clinical trials have also demonstrated that PD-L1 status was not associated with oncological outcomes8,11. This difference in results may be caused by a lack of standardization in the assessment of PD-L1 expression. Nonetheless, the role of determining PD-L1 expression in patients with RCC remains unclear. For patients with ccRCC, only one report to date has focused on advanced-stage samples, albeit with a small sample size22. In the present study, we clarified the TMB profile of ccRCC in a real-world setting, analyzed PD-L1 expression in RCC tissues using four FDA-approved PD-L1 IHC assays, and performed pairwise comparisons between these assays. This was the first study to evaluate and compare PD-L1 expression across all ccRCC stages using a large sample size.
In accordance with previous reports, the TMB of ccRCC was low in our cohort, except for one patient. The mutation profiles showed similar trends. Furthermore, our results revealed that PD-L1 expression in TCs was remarkably low based on the results of all IHC assays tested. When specimens were evaluated using the SP142 antibody, PD-L1 staining was not observed in either TCs or ICs. In the Blueprint Project, which attempted to harmonize PD-L1 IHC assays in lung cancer samples, the 22C3, 28 − 8 and SP263 assays demonstrated highly comparable staining, whereas the SP142 assay demonstrated less sensitivity23. A similar trend was observed for urothelial carcinoma, breast cancer, and other types of cancers24,25. Our results are consistent with those of previous studies of other cancer types. In previous studies, higher PD-L1 expression levels have been associated with poor prognosis in ccRCC26. The current study showed that higher PD-L1 expression, as assessed using the 22C3, 28 − 8, and SP263 assays, was significantly correlated with worse CSS. However, it was difficult to use the SP142 assay because of the extremely low positivity rate of PD-L1 in this study. Antibodies 22C3, 28 − 8 and SP263 were used to assess PD-L1 expression in RCC samples.
The reason for these differences in detection sensitivity is unclear. PD-L1 is a transmembrane protein which has two extracellular domains and one cytoplasmic domain. In the IHC assay, each antibody binds to a specific site on the PD-L1 protein. According to the previous study to clarify the binding site of each clone using PD-L1 IHC assays, 22C3 and 28 − 8 bind to epitopes on distinct surfaces of the extracellular domain of PD-L1, whereas SP142 and SP263 recognize identical epitopes in the cytoplasmic domain of PD-L127. Recently, several groups have reported changes in PD-L1 protein that may alter the structure of the binding regions of the anti-PD-L1 antibody. Kataoka, et al. showed structural variations within the 3′ region of the PD-L1 gene resulted in cleavage of the cytoplasmic domain of PD-L1 protein17. In samples with this aberration, PD-L1 expression was detected only with an antibody binding to the extracellular domain and not to the cytoplasmic domain. None of the patients in our cohort presented structural variations within the 3′ region of PD-L1. Another group demonstrated that N-linked glycosylation prevented PD-L1 antibody binding to the protein, while deglycosylation enhanced PD-L1 detection in the IHC assay28. Considering that these findings may explain the difference of mechanism of PD-L1 detection, further studies are warranted.
The present study had some limitations. In addition to its retrospective nature, we utilized TMA, which enabled the evaluation of PD-L1 expression in multiple samples. However, the TMA may not reflect the entire specimen block, especially in samples with low PD-L1 expression, owing to the low limit of detection of PD-L1 for each antibody, and the heterogeneity of RCC tissues. Future studies should validate our findings using whole formalin-fixed paraffin-embedded (FFPE) blocks. Additionally, it is necessary to perform analyses investigating the therapeutic efficacy of ICI treatment to examine the utility of the PD-L1 IHC assay as a diagnostic tool.
In summary, our findings demonstrate that PD-L1 expression in TCs in ccRCC tissues was equally low in all four FDA-approved IHC assays. The 22C3, 28 − 8, and SP263 assays detected PD-L1 expression in ICs and showed a significant association with CSS. Attention is warranted when interpreting the results assessed by SP142 because of the low positive rates of PD-L1 in ccRCC specimens.
Conclusions
In this study, we assessed the known biomarkers of ICIs in patients with ccRCC. The TMB in ccRCC tissues was low. IHC for detecting PD-L1 expression in FFPE tissues was performed using the 22C3, 28 − 8, SP142, and SP263 assays. Almost no PD-L1 expression was observed when assessed using the SP142 assay. The PD-L1 expression in TCs was also low, as assessed using the other assays. Although 28 − 8 showed a moderate concordance of PD-L1 expression in ccRCC tissue with the 22C3 and SP263 assays, PD-L1 expression evaluated by all four assays did not show consistent results.
Materials and methods
Patient cohort and tissue microarray construction
The cohort included 286 patients who underwent nephrectomy or partial nephrectomy for unilateral sporadic ccRCC between 2005 and 2018 at the Osaka University Medical Hospital. Histopathological diagnosis was performed based on standard hematoxylin and eosin (H&E)-stained sections, as assessed by two or more experienced senior pathologists according to the 8th Union for International Cancer Control TNM staging system29 and the WHO/ISUP grading system30. The pathologists selected FFPE blocks for the TMA construction. Briefly, TMAs were constructed using three cores of 1 mm diameter from the corresponding part of the FFPE blocks using a tissue arrayer (Pathology Institute Corp, Toyama, Japan). These cores were taken from the regions with the highest WHO/ISUP grades in the sample.
PD-L1 immunohistochemical staining
PD-L1 IHC staining was performed in a CLIA-certified laboratory (SRL Inc., Tokyo, Japan). A series of 4-µm serial sections sliced from TMAs were stained using four FDA-approved PD-L1 IHC assays; PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Inc.), PD-L1 IHC 28 − 8 pharmDx (Agilent Technologies, Inc.), VENTANA PD-L1 (SP142) Assay (Roche Diagnostics K.K.), and VENTANA PD-L1 (SP263) Assay (Roche Diagnostics K.K.) according to the manufacturers’ protocols.
Evaluation of PD-L1 expression in tissue microarray
To evaluate PD-L1 expression, we performed membrane staining of TCs or ICs in TMAs. First, PD-L1 expression was assessed in tumor tissues using evaluation methods specific to each IHC assay by the expert urogenital pathologist. The region including the morphologically viable cells was assessed. The stroma and the necrotic area were excluded from the assessment. PD-L1 expression was evaluated based on the 3 unified criteria: PD-L1 positive ICs in the tumor region, PD-L1 positive TCs in the tumor region, and PD-L1 positive ICs and TCs in the tumor region. PD-L1 expression was determined by calculating the average PD-L1 expression rate of the three cores corrected for area. Each case was considered PD-L1 positive if the expression rate showed 1% or more, in accordance with data from clinical trials6,7,8,9,11,31.
Whole exome sequencing for germline and tumor DNA
Tumor DNA was isolated from frozen ccRCC tissues using the Allprep DNA/RNA Mini Kit (QIAGEN, Hilden, Germany), whereas germline DNA was isolated from whole blood samples using the Qiagen DNA Blood Mini Kit (QIAGEN). Whole exome sequencing (WES) of germline and tumor DNA was performed using target capture with Agilent SureSelect XT Human All Exon V6 (Agilent Technologies, Inc.). Raw data were generated using an Illumina NovaSeq6000 platform (Illumina, San Diego, CA) with a standard 150-bp paired-end read protocol at Macrogen Japan (Tokyo, Japan). FASTQ files were generated using bcl2fastq2 (v.2.20.0) for the sequence data.
Analysis of somatic mutations and Estimation of tumor mutation burden
Somatic and germline mutations in tumor DNA were identified using the Genomon pipeline (https://genomon-project.github.io/GenomonPagesR/). GRCh37 was used as a reference genome. Of the mutations detected in the tumor DNA, mutations with overlapping germline mutations were excluded as SNPs or errors. We used the following criteria: (1) Fisher’s exact P ≥0.1, (2) ≥4 variant reads in the tumor sample, (3) MAF in the tumor sample ≥0.02, and (4) MAF of the matched normal sample < 0.1, with the exclusion of synonymous single nucleotide variants (SNVs) and known variants listed in NCBI dbSNP build 131.
The TMB was defined as the total number of non-synonymous SNVs, frameshift deletions, and frameshift insertions with a depth above 20x per megabase of the examined genome (muts/Mb). The total number of frameshift deletions and insertions was also assessed.
RNA sequencing for tumor RNA
Total RNA was isolated from ccRCC tissues preserved in RNAlater (Thermo Fisher Scientific) using an RNeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol. Total RNA was subjected to polyA + selection and chemical fragmentation, and the 100-bp RNA fraction was used to construct cDNA libraries using the TruSeq Stranded mRNA Prep Kit (Illumina). These paired-end libraries were sequenced using the Illumina NovaSeq6000 platform with a standard 100-bp paired-end protocol (Macrogen, Japan).
Analysis of gene expression
RNA-seq data were processed using the Hisat2/StringTie pipeline32. Mapping was performed with hisat2 2.2.1 against the GRCh37 human genome, and gene expression values were estimated using StringTie v.2.1.7. Sequencing reads in the PD-L1 gene region were visually reviewed using Integrative Genomics Viewer33 .
Statistical analysis
All statistical analyses were performed using R v.4.2.1. A waterfall plot was generated using GenVisR v.1.28.034. All graphs were drawn using ggplot2 v.3.3.635. We assessed pairwise concordance between assays using κ values. The OS and CSS of patients positive and negative for PD-L1 expression were estimated using the Kaplan–Meier method, and statistical differences were compared using the log-rank test. p < 0.05 were considered statistically significant.
Ethics approval
This study was approved by the Institutional Review Board of Osaka University Hospital (#12187-5 and #13397-19). Written informed consent was obtained from all patients. All experiments were conducted in compliance with the guidelines and regulations of the Research Ethics Committee of Osaka University.
Data availability
The datasets generated and/or analyzed during the current study were registered in the Gene Expression Omnibus repository under accession number CSE294575 and are available at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE294575.
Abbreviations
- ccRCC:
-
Clear cell renal cell carcinoma
- CSS:
-
Cancer specific survival
- FDA:
-
Food and Drug Administration
- FFPE:
-
Formalin-fixed paraffin-embedded
- IC:
-
Immune cell
- ICI:
-
Immune checkpoint inhibitor
- NGS:
-
Next-generation sequencing
- OS:
-
Overall survival
- PD-L1:
-
Programmed death-ligand 1
- RCC:
-
Renal cell carcinoma
- TC:
-
Tumor cell
- TMA:
-
Tissue microarray
- TMB:
-
Tumor mutation burden
References
Bukavina, L. et al. Epidemiology of renal cell carcinoma: 2022 update. Eur. Urol. 82, 529–542 (2022).
Choueiri, T. K. & Motzer, R. J. Systemic therapy for metastatic renal-cell carcinoma. New. Engl. J. Med. 376, 354–366 (2017).
Padala, S. A. et al. Epidemiology of renal cell carcinoma. World J. Oncol. 11, 79–87 (2020).
Lalani, A. K. A. et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: current paradigms, use of immunotherapy, and future directions. Eur. Urol. 75, 100–110 (2019).
Singla, N. A. New therapeutic era for metastatic renal cell carcinoma. JAMA Oncol. 6, 633–634 (2020).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl. J. Med. 373, 1803–1813 (2015).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl. J. Med. 378, 1277–1290 (2018).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl. J. Med. 380, 1116–1127 (2019).
Rini, B. I. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393, 2404–2415 (2019).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl. J. Med. 380, 1103–1115 (2019).
Choueiri, T. K. et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl. J. Med. 384, 829–841 (2021).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. New. Engl. J. Med. 377, 2500–2501 (2017).
Shiravand, Y. et al. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 29, 3044–3060 (2022).
Miao, D. et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359, 801–806 (2018).
Braun, D. A. et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat. Med. 26, 909–918 (2020).
Jørgensen, J. T. An update on companion and complementary diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert Rev. Mol. Diagn. 21, 445–454 (2021).
Kataoka, K. et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 534, 402–406 (2016).
Dine, J., Gordon, R., Shames, Y., Kasler, M. K. & Barton-Burke, M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia-Pac J. Oncol. Nurs. 4, 127–135 (2017).
Johnson, D. B., Nebhan, C. A., Moslehi, J. J. & Balko, J. M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022).
Yamaguchi, H., Hsu, J. M., Sun, L., Wang, S. C. & Hung, M. C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell. Rep. Med. 5, 101621 (2024).
Gjoerup, O. et al. Identification and utilization of biomarkers to predict response to immune checkpoint inhibitors. AAPS J. 22, 132 (2020).
Brown, L. C. et al. PD-L1 assay concordance in metastatic renal cell carcinoma and metastatic urothelial carcinoma. Clin. Genitourin. Cancer. 18, 509–513 (2020).
Tsao, M. S. et al. PD-L1 immunohistochemistry comparability study in real-Life clinical samples: results of blueprint phase 2 project. J. Thorac. Oncol. 13, 1302–1311 (2018).
Huang, T. H., Cheng, W. & Wang, Y. H. Interpretation according to Clone-Specific PD-L1 cutoffs reveals better concordance in muscle-invasive urothelial carcinoma. Diagnostics 11, 448 (2021).
Huang, X. et al. Comparison of three FDA-approved diagnostic immunohistochemistry assays of PD-L1 in triple-negative breast carcinoma. Hum. Pathol. 108, 42–50 (2021).
Lu, Y. et al. The prevalence and prognostic and clinicopathological value of PD-L1 and PD-L2 in renal cell carcinoma patients: a systematic review and meta-analysis involving 3,389 patients. Transl Androl. Urol. 9, 367–381 (2020).
Lawson, N. L. et al. Mapping the binding sites of antibodies utilized in programmed cell death ligand-1 predictive immunohistochemical assays for use with immuno-oncology therapies. Mod. Pathol. 33, 518–530 (2020).
Lee, H. H. et al. Removal of N-Linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 36, 168–178e4 (2019).
Brierley, J. D., Gospodarowicz, M. K. & Wittekind, C. TNM Classification of Malignant Tumours, 8th Edition. (Wiley-Blackwell, (2017).
Delahunt, B. et al. The international society of urological pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 37, 1490–1504 (2013).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN renal 101 trial. Nat. Med. 26, 1733–1741 (2020).
Pertea, M., Kim, D., Pertea, G. M., Leek, J. T. & Salzberg, S. L. Transcript-level expression analysis of RNA-seq experiments with HISAT, stringtie and ballgown. Nat. Protoc. 11, 1650–1667 (2016).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Skidmore, Z. L. et al. GenVisR: Genomic Visualizations in R. Bioinformatics 32, 3012–3014 (2016).
Wickham, H. ggplot2, Elegant Graphics for Data Analysis. R. https://doi.org/10.1007/978-3-319-24277-4 (2016).
Funding
This work was supported by JSPS KAKENHI Grant Number JP 20K18113.
Author information
Authors and Affiliations
Contributions
Y.K., T.K., and M.U. contributed to the study design and conceptualization. Y.K., G.Y., E.T., and M.M. performed specimen collection. Y.K. and M.M. constructed the TMA. Y.K. prepared the samples for NGS, performed the IHC assays, analyzed the data, and drafted the manuscript. T.K., K.H., A.K., M.U., and N.N. supervised the study. All authors have read and agreed to the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Osaka University Hospital (Approval No. #12187-5, #13397-19, and #668-4).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Koh, Y., Kato, T., Yamamichi, G. et al. Evaluation of the diagnostic concordance of FDA-approved PD-L1 assays in clear cell renal cell carcinoma. Sci Rep 15, 21253 (2025). https://doi.org/10.1038/s41598-025-05697-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05697-4