Abstract
We aimed to investigate the association between respiratory function and intraocular pressure (IOP). We included the Jikei and Japan Ningen Dock Study (JNDS) datasets that included data from 10,361 (50.3 ± 11.0 years) and 283,199 (51.7 ± 10.3 years) participants, respectively. IOP was measured using non-contact tonometry, and respiratory function was assessed using spirometry, focusing on the forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio, percent predicted values of FEV1 (ppFEV1), and percent predicted values of FVC (ppFVC). The relationship between respiratory function indices and IOP was assessed using multiple linear regression. The mean IOP was 12.7 ± 2.8 and 13.3 ± 2.9 mmHg in the Jikei and JNDS datasets, respectively, with a significant positive linear correlation between FEV1/FVC and IOP (β = 0.020, 95% confidence interval [CI]: 0.011 − 0.029, P < 0.001 in the Jikei dataset; β = 0.015, 95% CI: 0.013 − 0.016, P < 0.001 in the JNDS dataset). Compared with healthy individuals, 505 and 2,228 participants with FEV1/FVC < 70% (Jikei dataset) and FEV1/FVC < 60% (JNDS dataset) had lower IOPs of 0.641 (95% CI: 0.286 − 0.996, P < 0.001) mmHg and 0.888 (95% CI: 0.729 − 1.047, P < 0.001) mmHg, respectively. Both ppFEV1 and ppFVC showed no association with IOP. This possible association between obstructive ventilatory disorders and IOP suggests the importance of considering respiratory function in IOP management.
Similar content being viewed by others
Introduction
Glaucoma is the second most common cause of blindness worldwide1, and the global incidence of glaucoma is estimated to increase to 111.8 million by 20402. Intraocular pressure (IOP) plays a pivotal role in glaucoma development. Elevated IOP is considered the most important and clinically modifiable risk factor for glaucoma development and progression3. At present, IOP reduction is the only proven treatment for glaucoma. Therefore, it is important to fully understand the characteristics of IOP, including its influencing factors. IOP is affected by physical variables. Several epidemiologic studies have demonstrated the association of factors, such as blood pressure4,5, fasting plasma glucose (FPG)6, body mass index (BMI)7, and age4,5,8,9 with IOP. However, there are only a few reports on the relationship between respiratory function and IOP. Lee et al. suggested that women with chronic obstructive pulmonary disease (COPD) may have lower IOP than healthy individuals do10. However, aside from that report, there is a lack of studies examining the relationship between respiratory dysfunction and IOP, and the underlying mechanism of any such association remains unclear. If more severe respiratory impairment is indeed associated with lower IOP, it raises the question of whether this could be beneficial for patients with glaucoma. To address these uncertainties, we sought to replicate and expand upon the findings of Lee et al. using a larger sample to further clarify the relationship between respiratory dysfunction and IOP.
Japan has a unique health checkup system known as Ningen Dock, which is conducted on a large scale nationwide9,11,12,13. Approximately 3 million Japanese individuals are included in this system annually. Ningen Dock emphasizes lifestyle education for the primary prevention of life-threatening cerebrovascular and cardiovascular diseases, which might play a role in the high level of healthy life expectancy in Japan13. Ophthalmic examinations, including visual acuity tests, IOP measurements, fundus imaging, and respiratory function tests, are also performed within Ningen Dock.
This study aimed to investigate the association between pulmonary function and IOP.
Methods
Study design and population
This cross-sectional study used the Jikei University and Japan Ningen Dock Study (JNDS) datasets. Data were retrospectively collected and anonymized by decoding identifying information from each institute. We obtained demographic, systemic, and anthropometric data, including IOP measurements and spirometry results, from the Jikei University and Ningen Dock database. The study protocol complied with the Declaration of Helsinki and was approved by the Ethics Committee of Jikei University School of Medicine (33–144(10759) and the Ethical Committee of the Japan Society of Ningen Dock (JSND-EC: 2013-0001). Prior written informed consent was obtained from all participants in the Jikei University Hospital, and informed consent was obtained using the opt-out method in JNDS.
The Jikei dataset included examinees who underwent a series of medical examinations at the Center for Preventive Medicine of Jikei University Hospital between January 1, 2015 and December 31, 2018. The comprehensive health checkup included physical measurements; blood pressure check; electrocardiogram; heart rate measurement; audiometry; spirometry; blood, urine, and fecal tests; chest radiography and upper gastrointestinal series; abdominal ultrasound; physical examination by physicians (chest auscultation and neck and abdominal palpation); and interviews for collecting medical history and data on lifestyle habits13. The ophthalmic examinations included visual acuity test, tonometry, and fundus photography. The Jikei dataset included data from 37,064 individuals. Duplicate individuals and those with ocular diseases, including glaucoma, were excluded. Finally, we included participants who had complete data for IOP in both eyes: percent predicted values of forced expiratory volume in 1 s (ppFEV1) and percent predicted values of forced vital capacity (ppFVC); forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio; BMI; waist circumference (WC); systolic blood pressure (SBP); diastolic blood pressure (DBP); heart rate; FPG; glycated hemoglobin (HbA1c); hematocrit; and a self-administered questionnaire that included details on smoking, drinking, and exercise habits. This resulted in the inclusion of 10,361 participants.
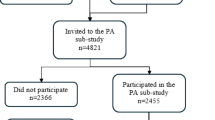
Details of the JNDS dataset have been described in a previous study9. A total of 129 institutes participated in the 2014 JNDS, which was conducted from April 1, 2014 to March 31, 2015. The medical examinations conducted were identical to those conducted for collecting data for the Jikei dataset. In the present study, we included participants with complete data for IOP in both eyes: ppFEV1; ppFVC; FEV1/FVC; BMI; SBP; DBP; heart rate; FPG; HbA1c; hematocrit; and a self-administered questionnaire that included details on smoking, drinking, and exercise habits. Among the 1,073,922 participants in the JNDS dataset who were initially evaluated, 283,199 eligible participants from 58 institutions were included in the final analysis. The participant selection flowchart for both datasets is shown in Fig. 1.
Flowchart of the study participant selection. This study used two datasets: the Jikei and JNDS datasets. The number of participants in each dataset is shown. The Jikei dataset spans multiple years, with some individuals undergoing multiple health checks; therefore, only initial data were used. The number of examinees with all clinical information for analysis was 10,341 and 283,199 in the Jikei and JNDS datasets, respectively.
JNDS, Japan Ningen Dock Study.
Measurements
IOP was measured using a non-contact tonometer. The TX-10 tonometer (Canon Inc., Tokyo, Japan) was used at Jikei University Hospital; however, the devices varied among facilities in the JNDS, as mentioned in the previous study9. Respiratory function was assessed using spirometry, with three key indices measured: ppFEV1, ppFVC, and FEV1/FVC. Jikei University Hospital is accredited under ISO 15,189, and spirometry is performed by nationally licensed medical technologists under physician supervision. All facilities participating in the JNDS are accredited by the Japan Society of Ningen Dock and Preventive Medical Care and have obtained ISO 15,189 certification. No bronchodilators were administered. FVC was defined as the amount of air expelled in a single breath at maximum speed after inhalation to full capacity. ppFVC was calculated by dividing the FVC by the predicted FVC. ppFVC was used to evaluate restrictive ventilation disorders. FEV1 was defined as the amount of air expelled in the first second after exhaling as fast as possible from maximum inhalation. FEV1/FVC was used as an index to evaluate obstructive ventilation disorders. ppFEV1 was calculated by dividing FEV1 by the predicted FEV1. This metric was used to determine the severity of COPD. The predicted values for the spirometry parameters were calculated using reference equations developed by Kubota et al., which were adjusted for age, sex, and height14. Participants were categorized into two groups based on the presence or absence of obstructive ventilatory disorder. An FEV1/FVC ratio below the lower limit of normal (LLN) was considered indicative of obstructive ventilatory impairment. The LLN can be calculated for each individual using the prediction Eq.14.
Data included SBP, DBP, and anthropometric parameters, such as weight, height, and WC. BMI was calculated as weight (kg) divided by height squared (m2). FPG was measured using auto-analyzers, and HbA1c was measured using a latex immunoturbidimetric method or high-performance liquid chromatography. The four seasons were classified as spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). Smoking and drinking status and exercise habits were determined based on the patients’ answers to the questionnaire, which was in accordance with the “specific medical checkup” prescribed by the Ministry of Health, Labor and Welfare in Japan.
Statistical analysis
Continuous variables were presented as mean ± standard deviation. The 95% confidence intervals (CIs) were included as appropriate. An independent Student’s t-test or a Mann–Whitney U test was used to compare continuous variables between the two groups after assessing data normality. Meanwhile, categorical variables were compared using the chi-squared test. Multiple linear regression analysis was performed with the IOP value for the right eye as the dependent variable. The independent variables are listed in Table 1 and Supplementary Table (1) We selected factors associated with IOP from previously published data4,5,15,16,17,18. Given the strong correlation between the data for the right and left eyes (r = 0.847, P < 0.001 in the Jikei dataset; r = 0.829, P < 0.001 in the JNDS dataset), the data for the right eye were used for the analysis. Age, ppFEV1, ppFVC, FEV1/FVC, BMI, WC, SBP, DBP, heart rate, FPG, and HbA1c were treated as continuous variables, whereas sex, smoking, drinking status, exercise, and season were treated as categorical variables and converted to dummy variables. Subsequently, univariate linear regression analysis was performed to identify potential individual factors. Finally, significant variables in the univariate analysis (i.e., those with P < 0.05) were included in the multivariable analysis to identify independent factors. Considering multicollinearity, variables that were significantly correlated were not included simultaneously, and two models were constructed. Model 1 excluded WC, DBP, FPG, and ppFEV1 due to multicollinearity; these variables were included in Model (2) As FEV1/FVC is a raw ratio not adjusted for sex, age, and height, the z-scores for FEV1, FVC, and FEV1/FVC were calculated to address this limitation. Using these z-scores, an additional multivariate analysis was performed following the same approach as previously described, including the development of Models 1 and 2 while accounting for multicollinearity. All statistical analyses were performed using R software (version 4.2.1; http://r-project.org).
Results
The characteristics of the 10,361 study participants in the Jikei dataset are summarized in Table 1. The participants’ mean age was 50.3 ± 11.0 (range, 20–94) years, and 3,511 (33.9%) of them were women. The mean IOP in the right eye was 12.7 ± 2.8 mmHg. Compared with the no-obstructive-ventilatory-disorder group, the obstructive-ventilatory-disorder group (characterized by an FEV1/FVC ratio below the LLN) included a significantly higher proportion of current and former smokers. Moreover, the obstructive-ventilatory-disorder group exhibited lower IOP, compared with the no-obstructive-ventilatory-disorder group. The IOP distributions for the right and left eyes are shown in Fig. 2. A total of 4,437 participants were excluded from the analysis due to missing data (Fig. 1). A comparison between participants with incomplete and complete data suggested that the exclusion of the participants with missing data did not introduce significant selection bias (Supplementary Table 2).
After adjusting for covariates, Model 1 of the Jikei dataset demonstrated that IOP linearly decreased as the FEV1/FVC decreased (β = 0.020, 95% CI: 0.011–0.029, P < 0.001) (Fig. 3A). The IOP was lower by 0.35 mmHg and 0.51 mmHg in 1,188 and 505 participants with an FEV1/FVC ratio of 70–75% and < 70%, respectively, than in 668 participants with an FEV1/FVC ratio of 90–100% (Supplementary Table 3). Model 2 also showed significant positive associations between IOP and FEV1/FVC. The IOP was lower by 0.64 mmHg in participants with an FEV1/FVC ratio of < 70% than in participants with an FEV1/FVC ratio of 90–100%. The other two metrics, ppFEV1 and ppFVC, did not show a clear correlation with IOP (Supplementary Fig. 1). The multivariate analysis using the z-scores revealed a significant positive correlation between IOP and the z-score of FEV1/FVC in Model 1 (β = 0.091, 95% CI: 0.041–0.142, P < 0.001). A similar association was observed in Model 2 (β = 0.122, 95% CI: 0.068–0.177, P < 0.001). These findings were consistent with the analysis based on ppFEV1, ppFVC, and FEV1/FVC.
Relationship between IOP and FEV1/FVC. Study participants are stratified to show how IOP changes with decreasing FEV1/FVC in the Jikei dataset (A), using the group with FEV1/FVC above 90% as a reference, and in the JNDS dataset (B), using the group with FEV1/FVC above 95% as reference. A positive linear correlation is observed between FEV1/FVC and IOP. IOP, intraocular pressure; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; JNDS, Japan Ningen Dock Study.
The characteristics of the study participants in the JNDS dataset are presented in Supplementary Table 1. The participants’ mean age was 51.7 ± 10.3 (range, 20–93) years, and 106,780 (37.7%) of them were women. The mean IOP in the right eye was 13.3 ± 2.9 mmHg. The obstructive-ventilatory-disorder group had higher BMI and SBP levels, a greater proportion of current smokers, and lower IOP, compared with the no-obstructive-ventilatory-disorder group. The results of the multivariate analysis are presented in Supplementary Tables 4 and Fig. 3B. IOP was positively correlated with FEV1/FVC (β = 0.015, 95% CI: 0.013–0.016, P < 0.001), consistent with the results obtained from the Jikei dataset. Due to the larger sample size of the JNDS dataset, stratification based on FEV1/FVC could be performed with greater granularity than that in the Jikei dataset. In Model 1, the IOP was 0.65 mmHg lower in the 2,288 participants with an FEV1/FVC ratio < 60% compared with the 4,940 participants with an FEV1/FVC ratio of 95–100%. In Model 2, the IOP was lower by 0.89 mmHg. There was a consistent linear correlation between IOP and FEV1/FVC, even in patients with very low FEV1/FVC values.
Discussion
The effect of respiratory dysfunction on the eye remains unclear to date. After adjusting for known IOP-related factors, our results suggested a positive correlation between FEV1/FVC and IOP. This relationship was consistently observed in the two independent datasets. However, neither ppFEV1 nor ppFVC showed a clear association with the IOP. Even after adjusting for the spirometric parameters for sex, age, and height using z-scores, similar results were obtained. The findings might help enhance the understanding of the characteristics of IOP and ultimately contribute to a more precise management of IOP in ocular diseases.
In the Jikei dataset, 505 participants had obstructive ventilatory impairments. Although several diseases may account for this impairment, a precise breakdown could not be performed in the present study. COPD and asthma are among the most common diseases causing such impairments. In this dataset, the rate of current smokers was higher in the obstructive-ventilatory-disorder group than in the no-obstructive-ventilatory-disorder group (66.0% vs. 45.2%). This suggests that many patients with obstructive ventilatory impairment are likely to develop COPD. Therefore, it is crucial to consider the relationship between COPD and IOP to fully understand the results of the present study.
Respiratory dysfunction may affect the eye. Choroidal and retinal thinning and decreased vascular density have been reported in patients with COPD19,20,21,22,23. Decreased respiratory function, particularly decreased FVC, has also been recently suggested to be associated with glaucoma24. However, the effect of respiratory dysfunction on IOP has not been thoroughly examined. Lee et al. suggested that women with COPD may have lower IOP than healthy individuals do10. Their report supports our finding that IOP decreases as obstructive respiratory impairment becomes more severe. In the current study, participants with an FEV1/FVC ratio < 60% had an IOP that was 0.89 mmHg lower than that in healthy participants. This difference is clinically significant, particularly in patients with ocular diseases requiring IOP management.
The decreased IOP in patients with COPD could be attributed to impaired ocular blood circulation and consequent choroidal dysfunction. The balance between endothelin-1 (ET1), which promotes vasoconstriction, and nitric oxide, which facilitates vasodilation, is crucial for maintaining healthy ocular blood flow25,26,27,28. Compared with healthy individuals, patients with COPD often have higher serum and urinary ET1 levels, potentially leading to reduced ocular blood flow and chronic hypoxia29,30. Studies using optical coherence tomography angiography revealed reduced vascular density and increased foveal avascular zone size in patients with COPD22. Furthermore, color Doppler ultrasonography studies have demonstrated disrupted retrobulbar hemodynamics and reduced blood flow in patients with COPD, along with increased resistance in the retinal and choroidal circulation31,32,33. Collectively, these findings suggest that COPD may adversely affect retinal and choroidal function, possibly affecting aqueous humor dynamics, thereby reducing IOP.
The ppFEV1 was used as an indicator to assess the severity of COPD; however, no association with IOP was found. However, ppFEV1 is not a direct measure of obstructive impairment but rather FEV1/FVC. This distinction between the two indices may have contributed to the differences in the results. In addition, no correlation was found between ppFVC and IOP, indicating that there was no clear association between restrictive ventilatory disorders and IOP. We analyzed two large, independent datasets, each with distinct strengths and limitations. The Jikei dataset, though smaller, excluded patients with glaucoma and thus avoided the influence of IOP-lowering medications while utilizing a uniform IOP-measurement device. Contrastingly, the JNDS dataset employed multiple devices across participating facilities and included individuals taking IOP-lowering agents but offered a much larger population for more detailed stratification analyses. By examining both datasets with these complementary characteristics, we strengthened the robustness of our findings. The impact of FEV1/FVC, which is weaker than that of known IOP-related factors, such as blood pressure and BMI, may not have been discernible without validation in a large sample size.
Despite our promising results, the present study has some limitations. First, the IOP was measured using non-contact tonometry, which is generally considered less accurate than applanation tonometry. Second, this was a cross-sectional study. Investigating how IOP changes in the same patient when obstructive ventilatory disorders worsen, in a longitudinal manner, may further clarify the relationship between respiratory function and IOP. In the future, the JNDS plans to accumulate annual health check data over multiple years, which may provide a more comprehensive understanding of the relationship between respiratory dysfunction and IOP. Additionally, incorporating optical coherence tomography imaging data, including retinal and choroidal thickness and vascular structures, could offer novel insights beyond IOP alone.
In conclusion, the present study established a correlation between obstructive ventilatory disorders and reduced IOP. These findings were consistent across two independent datasets. Importantly, they suggest the importance of considering respiratory function for accurate IOP management. Although the decrease in ocular blood flow due to COPD and the consequent reduction in aqueous humor production may explain the results of the present study, the specific mechanisms are not yet fully understood, indicating the need for further research.
Independent Student-t test or chi-square test was used to compare characteristics between normal and obstructive ventilatory disorder.
Data availability
The data supporting the findings of this study are available from The Jikei University School of Medicine and the Japan Society of Ningen Dock. However, restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data can be made available upon reasonable request with permission from these institutions by contacting the corresponding author.
References
Resnikoff, S. et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 82, 844–851 (2004).
Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090 (2014).
Leidl, M. C., Choi, C. J., Syed, Z. A. & Melki, S. A. Intraocular pressure fluctuation and glaucoma progression: what do we know? Br. J. Ophthalmol. 98, 1315–1319 (2014).
Klein, B. E., Klein, R. & Linton, K. L. Intraocular pressure in an American community. The beaver dam eye study. Investig. Ophthalmol. Vis. Sci. 33, 2224–2228 (1992).
Chan, M. P. et al. Associations with intraocular pressure in a large cohort: results from the UK biobank. Ophthalmology 123, 771–782 (2016).
Zhao, D., Cho, J., Kim, M. H., Friedman, D. S. & Guallar, E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology 122, 72–78 (2015).
Khawaja, A. P. et al. Associations with intraocular pressure across europe: the European eye epidemiology (E(3)) consortium. Eur. J. Epidemiol. 31, 1101–1111 (2016).
Nakano, T., Tatemichi, M., Miura, Y., Sugita, M. & Kitahara, K. Long-Term physiologic changes of intraocular pressure: A 10-year longitudinal analysis in young and middle-aged Japanese men. Ophthalmology 112, 609–616 (2005).
Terauchi, R. et al. Age-Related changes in intraocular pressure: Japan Ningen dock study. J. Glaucoma. 31, 927–934 (2022).
Lee, J. S. et al. Increased risk of open-angle glaucoma in non-smoking women with obstructive pattern of spirometric tests. Sci. Rep. 12, 16915 (2022).
Terauchi, R. et al. Association between days of the week and intraocular pressure: Japan Ningen dock study. J Glaucoma (2023).
Terauchi, R. et al. FDT Perimetry for Glaucoma Detection in Comprehensive Health Checkup Service. J Ophthalmol. 4687398 (2020). (2020).
Ikeda, N. et al. What Has Made Popul. Japan Healthy?? Lancet 378, 1094–1105 (2011).
Kubota, M. et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig. 52, 242–250 (2014).
Memarzadeh, F., Ying-Lai, M., Azen, S. P. & Varma, R. Associations with intraocular pressure in latinos: the Los Angeles Latino eye study. Am. J. Ophthalmol. 146, 69–76 (2008).
Wu, S. Y. & Leske, M. C. Associations with intraocular pressure in the Barbados eye study. Arch. Ophthalmol. 115, 1572–1576 (1997).
Kawase, K., Tomidokoro, A., Araie, M., Iwase, A. & Yamamoto, T. Ocular and systemic factors related to intraocular pressure in Japanese adults: the Tajimi study. Br. J. Ophthalmol. 92, 1175–1179 (2008).
Wong, T. T. et al. The relationship of intraocular pressure with age, systolic blood pressure, and central corneal thickness in an Asian population. Invest. Ophthalmol. Vis. Sci. 50, 4097–4102 (2009).
Alim, S., Demir, H. D., Yilmaz, A., Demir, S. & Güneş, A. To Evaluate the Effect of Chronic Obstructive Pulmonary Disease on Retinal and Choroidal Thicknesses Measured by Optical Coherence Tomography. J Ophthalmol. 7463815 (2019). (2019).
Ozcimen, M. et al. Peripapillary choroidal thickness in patients with chronic obstructive pulmonary disease. Cutan. Ocul Toxicol. 35, 26–30 (2016).
Gok, M., Ozer, M. A., Ozen, S. & Botan Yildirim, B. The evaluation of retinal and choroidal structural changes by optical coherence tomography in patients with chronic obstructive pulmonary disease. Curr. Eye Res. 43, 116–121 (2018).
Songur, M. S. et al. The alterations of retinal vasculature detected on optical coherence tomography angiography associated with chronic obstructive pulmonary disease. Clin. Respir J. 16, 284–292 (2022).
Ahmed, N. O., Shaaban, Y. M. & Ezzelregal, H. G. Evaluation of the impact of COPD severity grading and oxygen saturation on the retinal nerve fiber layer thickness and subfoveal choroidal thickness in COPD patients. Egypt. J. Bronchol. 15, 45 (2021).
Mehta, P. et al. Automated detection of Glaucoma with interpretable machine learning using clinical data and multimodal retinal images. Am. J. Ophthalmol. 231, 154–169 (2021).
Schmetterer, L. et al. Effects of endothelin-1 (ET-1) on ocular hemodynamics. Curr. Eye Res. 16, 687–692 (1997).
Polak, K. et al. Regulation of human retinal blood flow by endothelin-1. Exp. Eye Res. 76, 633–640 (2003).
Donati, G. et al. Nitric oxide controls arteriolar tone in the retina of the miniature pig. Invest. Ophthalmol. Vis. Sci. 36, 2228–2237 (1995).
Pournaras, C. J., Rungger-Brändle, E., Riva, C. E., Hardarson, S. H. & Stefansson, E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 27, 284–330 (2008).
Sofia, M. et al. Increased 24-hour endothelin-1 urinary excretion in patients with chronic obstructive pulmonary disease. Respiration 61, 263–268 (1994).
Roland, M. et al. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax 56, 30–35 (2001).
Ozer, T. et al. Color doppler evaluation of the ocular arterial flow changes in chronic obstructive pulmonary disease. Eur. J. Radiol. 57, 63–68 (2006).
Yakut, Z. I., Karadağ, R., Ozol, D. & Senturk, A. Evaluation of arterial blood flow changes by orbital doppler in chronic obstructive pulmonary disease and asthma. J. Investig Med. 63, 12–16 (2015).
Celik, C. et al. Color doppler evaluation of the retrobulbar hemodynamic changes in chronic obstructive pulmonary disease: COPD and retrobulbar hemodynamic changes. Ultrason. Imaging. 36, 177–186 (2014).
Acknowledgements
The authors thank the colleagues at the participating institutes for providing the data for individuals receiving annual health examinations.
Author information
Authors and Affiliations
Contributions
R.T.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, and Roles/Writing - original draft. K.F.: Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, and Writing - review & editing. S.F.: Investigation, Methodology, Validation, and Writing - review & editing. K.I.: Data curation, Resources, and Writing - review & editing. T.K.: Data curation, Resources, and Writing - review & editing. K.K.: Data curation, Resources, and Writing - review & editing. M.T.: Methodology, Software, Supervision, Validation, and Writing - review & editing. J.A.: Methodology, Supervision, Validation, and Writing - review & editing. Y.K.: Resources, Validation, and Writing - review & editing. T.Na.: Resources, Software, Supervision, Validation, and Writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Terauchi, R., Fukai, K., Fujimoto, S. et al. Relationship between intraocular pressure and pulmonary function. Sci Rep 15, 21187 (2025). https://doi.org/10.1038/s41598-025-05731-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-05731-5