Abstract
Given the proven efficacy of hormonal treatment, more women with endometrial hyperplasia (EH) are choosing to preserve their fertility. Previous studies have focused primarily on the progression of EH to endometrial cancer or pregnancy success rates in these women post-treatment. However, limited research has examined pregnancy outcomes in women with EH. Therefore, we analyzed the association between EH and adverse pregnancy outcomes using Korean National Health Insurance claims data from 2006 to 2023, focusing on women with obstetric outcomes classified using the International Classification of Disease, Tenth Edition. Adverse obstetric outcomes included pregnancy-associated hypertension, gestational diabetes mellitus, placenta previa, placenta accreta, placental abruption, preterm labor, intrauterine growth restriction, uterine rupture, threatened abortion, preterm premature rupture of membranes, polyhydramnios, oligohydramnios, postpartum hemorrhage, and fetal death in utero. Multivariate logistic regression was used to evaluate the association between EH and adverse obstetric outcomes. Among the 199 143 women included in the study, 1655 were diagnosed with EH before pregnancy. After adjusting for age, body mass index, and preexisting conditions, women with EH showed a significantly higher risk of adverse obstetric outcomes. Our findings indicate that EH is a significant risk factor for adverse obstetric outcomes, necessitating obstetric surveillance.
Similar content being viewed by others
Introduction
Endometrial cancer (EC) is the most common gynecological malignancy in industrialized countries1. Notably, its incidence has risen in recent years among women in their 30s2. The precursor lesion for the predominant endometrioid adenocarcinoma subtype is endometrial hyperplasia (EH)1.
Excessive estrogen exposure without the protective counteracting effect of progestin is a significant risk factor for EH and EC3. Factors contributing to excessive estrogen exposure include obesity, chronic anovulation, early menarche, late menopause, and estrogen-secreting tumors1.
In 2014, the World Health Organization classified EH based on cellular atypia into two types: Endometrial hyperplasia without atypia and endometrial atypical hyperplasia4. The cancer risk of EH without atypia is 1–2% but can reach up to 60% for atypical cases of endometrial intraepithelial neoplasia (EIN)5. The definitive treatment for EIN is total hysterectomy, which causes loss of fertility. However, hormonal therapy has emerged as an alternative for young women who have not yet completed their fertility plans6. With the increasing diagnosis of EH in younger women and the proven efficacy of hormonal treatments, more women are opting to preserve their fertility and to attempt pregnancy later in life7.
Even after EH shows a complete response to hormonal therapy, underlying factors causing hormonal imbalances, such as obesity, may persist in women diagnosed with EH. Previous studies have predominantly focused on the risk of progression to EC or the pregnancy success rate in women diagnosed with EH8,9.
Therefore, in this study, we aimed to analyze pregnancy outcomes in women diagnosed with EH who are likely to become pregnant in the future.
Methods
Data characteristics
The Korean National Health Insurance (KNHI) claims database encompasses health information for the majority of Koreans, except for 3% of the population covered by the Medical Aid Program. This comprehensive database includes data on women’s health conditions, particularly obstetric and gynecological diagnoses, identified using the International Classification of Diseases, Tenth Edition (ICD-10) codes, except for uninsured procedures, such as plastic surgeries.
Dataset
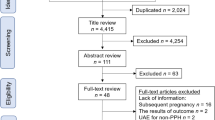
The study analyzed the KNHI claims data from 2006 to 2023. This study was approved by the Institutional Review Boards of the Catholic Medical Centre (OC23ZISI0058). Because this was a retrospective cohort study and because all data were anonymized, the need for informed consent was waived by the Institutional Review Board of The Catholic University of Korea. This study was conducted in accordance with the guidelines of the Declaration of Helsinki, and the rights of all patients were protected. We included women diagnosed with EH before pregnancy and excluded those with missing data. The study population consisted of 1 778 529 women who gave birth from 2006 to 2023. After applying the exclusion criteria, 199 143 women were included in the study (Fig. 1). Of these, 1655 (0.83%) had EH with or without atypia and 197 488 (99.17%) women were in the non-EH group.
The baseline characteristics are shown in Table 1.. Obstetric diagnoses were identified using specific ICD-10 codes: Pregnancy associated hypertension (PAH; O13–O16), gestational diabetes mellitus (GDM; O24.4, O24.9), placenta previa (O44), placenta abruption (O45), placenta accreta spectrum (O432, O720, O73), oligohydramnios (O41), polyhydramnios (O40), postpartum hemorrhage (O72), preterm premature rupture of membranes (PPROM; O42), intrauterine growth restriction (IUGR; O365) and fetal death in uterus (FDIU) (O364). Neonatal outcomes, including preterm birth and birth weight, were extracted from the National Health Screening Program for Infants and Children database. PAH included gestational hypertension, pre-eclampsia, eclampsia, and superimposed pre-eclampsia. Preterm birth was defined as delivery before 37 weeks of gestation.
The diagnostic criteria for obstetrics and gynecological conditions in Korea are based on the standards outlined in Williams Obstetrics.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables as percentages. Clinical characteristics were compared using analysis of variance for continuous variables and the chi-squared test for categorical variables. A multivariate logistic regression analysis model was used to estimate the adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for adverse pregnancy outcomes. Maternal age (≥ 35years), BMI (≥25kg/m2), maternal disease (high blood pressure, diabetes mellitus, thyroid disease), previous uterine surgery (myomectomy, hysteroscopy, dilatation and curettage) were adjusted as potential confounders.
Statistical analyses were performed using statistical analysis system software for Windows (version 9.4, SAS Inc., Cary, NC, USA). The study protocol was approved by the Institutional Review Board of the Catholic University of Korea at Incheon St. Mary’s Hospital (approval no. OC23ZISI0058).
Results
Baseline characteristics of the study population
The EH group exhibited significantly higher maternal age and body mass index (BMI; P < 0.05) compared with the non-EH group. Additionally, the proportion of women with maternal age ≥ 35 years and BMI ≥ 25 kg/m2 was significantly greater in the EH group versus the non-EH group (P <0.001). The prevalence of pre-existing maternal diseases, including polycystic ovary syndrome (PCOS), hypertension, diabetes, thyroid disease, and autoimmune disease was significantly higher in the EH group than in the non-EH group (P < 0.001). The EH group showed a significantly higher rate of previous uterine surgery compared to the non-EH group.
Obstetric outcomes according to maternal EH
Data on obstetric outcomes are presented in Table 2.. The EH group had significantly higher rates of pregnancy-associated hypertension and GDM, compared with the non-EH group (P < 0.001). Additionally, the EH group showed significantly increased incidences of threatened abortion, preterm labor, PPROM, polyhydramnios, oligohydramnios, placenta previa, and placenta accreta, compared with the non-EH group (P < 0.001). Rates of IUGR and FDIU were also significantly elevated in the EH group than in the non-EH group (P < 0.001). Furthermore, the EH group also experienced higher rates of postpartum hemorrhage than the non-EH group (P < 0.001), whereas no significant intergroup differences were observed in the frequencies of placental abruption and uterine rupture.
Multivariate analysis of obstetric outcomes according to EH
Table 3. shows the risk of adverse obstetric outcomes associated with EH. In the unadjusted analysis, compared with the non-EH group, the EH group showed significantly higher risks for PAH (OR 2.182, 95% CI 1.792–2.656), GDM (OR 2.102, 95% CI 1.876–2.34), placenta previa (OR 2.650, 95% CI 1.951–3.599), placenta accreta (OR 4.795, 95% CI 2.855–8.052), preterm labor (OR 2.416, 95% CI 2.154–2.711), IUGR (OR 2.184, 95% CI 1.698–2.810), threatened abortion (OR 2.244, 95% CI 1.988–2.534), PPROM (OR 3.389, 95% CI 2.745–4.185), polyhydramnios (OR 2.541, 95% CI 1.49–4.323), oligohydramnios (OR 1.898, 95% CI 1.473–2.446), postpartum hemorrhage (OR 1.463, 95% CI 1.209–1.770) and FDIU (OR 3.345, 95% CI 2.334–4.796). In the adjusted analysis that controlled for maternal age, compared with the non-EH group, the EH group continued to demonstrate significantly higher risks for PAH (OR 1.675, 95% CI 1.374–1.2.042), GDM (OR 1.508, 95% CI 1.342–1.93), placenta previa (OR 1.707, 95% CI 1.253–2.325), placenta accreta (OR 3.020, 95% CI 1.790–5.095), preterm labor (OR 1.805, 95% CI 1.605–2.030), IUGR (OR 1.619, 95% CI 1.256–2.087), threatened abortion (OR 1.769, 95% CI 1.604–1.952), PPROM (OR 2.542, 95% CI 2.055–3.145), polyhydramnios (OR 1.721, 95% CI 1.009–2.938), oligohydramnios (OR 1.674, 95% CI 1.298–2.160), postpartum hemorrhage (OR 1.405, 95% CI 1.161–1.701) and FDIU (OR 2.383, 95% CI 1.657–3.425). In an adjusted analysis that controlled for maternal age and BMI, the EH group, compared with the non-EH group, showed a significantly higher risks for PAH (OR 1.459, 95% CI 1.192–1.786), GDM (OR 1.470, 95% CI 1.308–1.652), placenta previa (OR 1.730, 95% CI 1.270–2.357), placenta accreta (OR 3.014, 95% CI 1.785–5.088), preterm labor (OR 1.828, 95% CI 1.626–2.056), IUGR (OR 1.652, 95% CI 1.282–2.130), threatened abortion (OR 1.766, 95% CI 1.600–1.948), PPROM (OR 2.542, 95% CI 2.054–3.146), oligohydramnios (OR 1.660, 95% CI 1.286–2.141), postpartum hemorrhage (OR 1.404, 95% CI 1.160–1.700) and FDIU (OR 2.335, 95% CI 1.623–3.358). In the adjusted analysis that controlled for maternal age, BMI, hypertension and diabetes, the EH group, compared with the non-EH group, showed significantly higher risks for GDM (OR 1.249, 95% CI 1.107–1.409), placenta previa (OR 1.665, 95% CI 1.221–2.271), placenta accreta (OR 2.610, 95% CI 1.539–4.426), preterm labor (OR 1.664, 95% CI 1.478–1.874), IUGR (OR 1.473, 95% CI 1.140–1.902), threatened abortion (OR 1.711, 95% CI 1.550–1.888), PPROM (OR 2.325, 95% CI 1.876–2.881), oligohydramnios (OR 1.573, 95% CI 1.218–2.031), postpartum hemorrhage (OR 1.358, 95% CI 1.121–1.645) and FDIU (OR 2.112, 95% CI 1.465–3.046). In the adjusted analysis that controlled for maternal age, BMI, hypertension, diabetes, hyperthyroidism and hypothyroidism, the EH group, compared with the non-EH group, demonstrated significantly higher risks for GDM (OR 1.196, 95% CI 1.060–1.350), placenta previa (OR 1.610, 95% CI 1.180–2.197), placenta accreta (OR 2.361, 95% CI 1.390–4.009), preterm labor (OR 1.542, 95% CI 1.368–1.738), IUGR (OR 1.404, 95% CI 1.086–1.814), threatened abortion (OR 1.669, 95% CI 1.512–1.842), PPROM (OR 2.178, 95% CI 1.756–2.701), oligohydramnios (OR 1.543, 95% CI 1.195–1.994), postpartum hemorrhage (OR 1.343, 95% CI 1.109–1.627) and FDIU (OR 2.032, 95% CI 1.408–2.933). In the adjusted analysis that controlled for maternal age, BMI, hypertension, diabetes, hyperthyroidism and hypothyroidism and previous uterine surgery, the EH group, compared with the non-EH group, demonstrated significantly higher risks for GDM (OR 1.182, 95% CI 1.047–1.334), placenta previa (OR 1.555, 95% CI 1.139–2.122), placenta accreta (OR 2.277, 95% CI 1.341–3.865), preterm labor (OR 1.504, 95% CI 1.334–1.696), IUGR (OR 1.92, 95% CI 1.33–2.771), threatened abortion (OR 1.646, 95% CI 1.479–1.833), PPROM (OR 2.099, 95% CI 1.693–2.604), oligohydramnios (OR 1.535, 95% CI 1.181–1.984), postpartum hemorrhage (OR 1.275, 95% CI 1.052–1.545) and FDIU (OR 1.92, 95% CI 1.33–2.771).
Discussion
This study revealed that EH is a significant risk factor for adverse obstetric outcomes. Women with EH exhibited clinical characteristics such as advanced maternal age, higher BMI, and higher incidence of PCOS, hypertension, diabetes, thyroid disorders, and autoimmune diseases. After adjusting for confounding factors, the EH group showed significantly higher rates of adverse obstetric outcomes, including GDM, placenta previa, placenta accreta, preterm labor, IUGR, threatened abortion, PPROM, oligohydramnios, postpartum hemorrhage, and FDIU, compared with the non-EH group.
Excessive estrogen stimulation of the endometrium, without the protective effects of progestins, induces proliferative glandular epithelial changes leading to hyperplasia. Moreover, many women receiving gynecological treatment for EH also have other underlying conditions, such as obesity, PCOS, hypertension, and type 2 diabetes10,11,12,13,14.
A systematic review and meta-analysis evaluating fertility in individuals with EH following progesterone therapy reported pregnancy rates ranging from 26.3 to 41.0%15,16. As many patients with EH also experience conditions that may result in chronic anovulation (e.g., polycystic ovary syndrome, obesity, and diabetes), the live birth rate achieved through assisted reproductive technology is higher than that achieved through spontaneous pregnancy (39.4% vs. 14.9%, P<0.001)17. Another study showed that the cumulative risk of progression to EC steadily increased over time in women with EH after completion of progesterone therapy (8.2% for 4 years versus 27.5% for 19 years)18. However, guidelines regarding the optimal duration for using levonorgestrel-releasing intrauterine devices or systemic progestogens to prevent long-term recurrence have not yet been established19. The American College of Obstetricians and Gynecologists recommends that medical management with progestogens be continued in patients at risk of EC after initial treatment with these drugs for the following reasons: Advanced age; late menopause; anovulation or chronic anovulation; high-risk conditions (Lynch syndrome, Cowden syndrome); modifiable risk factors, such as the use of unopposed estrogen therapy; and potentially modifiable risk factors, such as obesity and type 2 diabetes19. If these patient-specific risk factors remain unchanged, EH is likely to negatively affect obstetric outcomes, regardless of whether it regresses or not.
Maternal obesity increases the risk of complications such as miscarriage, gestational diabetes, preterm delivery, venous thromboembolism, induced labor, and caesarean section20. The effects of high-dose progestins on subsequent pregnancies remain unclear. High-dose progestins may downregulate the expression of progesterone receptors in EH, and reduced endometrial thickness (< 6 mm) is associated with decreased live birth rates21.
Recent research has determined that patients with EC or EIN experience significantly higher rates of GDM and postpartum hemorrhage. In addition, a previous study of Korean patients with EC or EIN reported significantly higher rates of twin pregnancy, preterm delivery, and caesarean section22.
Although EH with or without atypia shares risk factors with EC, previous studies have primarily focused on EC, leaving a gap in our understanding of the obstetric implications of EH.
Only a few studies have been conducted on the pregnancy outcome of endometrial hyperplasia. One propensity score matched study found that EH group has higher rate of abortion and preterm delivery23. Other studies have shown that the EH group had higher rates of induction of labor, cesarean section, and postpartum hemorrhage compared to the non-EH group24. However, research on endometrial hyperplasia and adverse obstetric outcomes is still lacking.
Our study demonstrated that not only EC but also EH with or without atypia are significant risk factors for adverse obstetric outcomes. Women with EH exhibited characteristics such as advanced maternal age, higher BMI, a greater prevalence of pre-existing disease and adverse obstetric outcomes. Notably, even after adjusting for confounding factors, differences in pregnancy outcomes persisted, except for preeclampsia and polyhydramnios. This study showed significantly higher rate of FDIU and placenta accreta in EH group than non-EH group. Previous studies have not investigated the association between EH and FDIU. However, several studies targeting the EH group showed that the rate of late pregnancy loss (13–28 weeks of pregnancy) was higher than that in the general population. Late pregnancy loss included FDIU that occurred after 20 weeks of pregnancy23,24. Another study found that EH group has a higher rate of placenta accreta than control group. They described that the EH group had a high frequency of dilatation and curettage (D&C), which may contribute to placenta accreta25. In our study, we analyzed the results by adding previous uterine surgery that may cause placental accreta (Model 5), and even after adjusting for previous uterine surgery, the EH group showed a higher risk of placental accreta than the control group. As the incidence of placenta accreta increases, the risk of postpartum hemorrhage also increases. High-dose progestin administration during EH treatment may result in thinning of endometrium, which may interfere with proper implantation and lead to placental dysfunction26,27. Thin endometrial thickness is related to adverse obstetric outcomes including threatened abortion, preterm birth, IUGR and placenta accreta25,28. These findings are consistent with those of the present study, suggesting the need for the close clinical monitoring of women with a history of EH. Nevertheless, further research is warranted to clarify these associations.
This study has some limitations. Control group was defined as individuals without any diagnostic codes for endometrial hyperplasia. However, we cannot completely exclude the possibility that some individuals in the control group may have had undiagnosed disease, which may introduce misclassification bias. Its retrospective design and the inclusion of multicenter hospitals from various regions made it challenging to standardize diagnostic tools and treatment protocols, potentially introducing detection bias. Moreover, the use of national claims data limited our access to detailed individual information regarding physical, underlying medical conditions and endometrial thickness through ultrasonography.
However, this study also has notable strengths. The large sample size, encompassing over 199 000 deliveries, and the utilization of data from the KNHI Program, which covers 97% of the Korean population, enhances the generalizability of our findings. Importantly, this study represents a large analysis of EH, addressing an area whose clinical importance has been relatively underexplored.
Our study indicates that EH is a significant risk factor for adverse obstetric outcomes. The results of this study suggest that women with EH require close obstetric surveillance, regardless of EH regression. Prospective, large-scale studies are required to further validate these hypotheses.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available because of the sensitivity of the data, but are available from the corresponding author upon reasonable request.
References
Nees, L. K. et al. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch Gynecol. Obstet 306, 407–421 (2022).
Matsuo, K. et al. Ovarian conservation for young women with early-stage, low-grade endometrial cancer: A 2-step schema. Am. J. Obstet. Gynecol. 224, 574–584 (2021).
Brinton, L. A. et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: Results from a case-control study. Am. J. Obstet. Gynecol. 167, 1317–1325 (1992).
Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. WHO classification of tumours, fourth edition, 6, 2014.
Auclair MH, Yong PJ, Salvador S, Thurston J, Colgan TTJ, Sebastianelli A. Guideline No. 390-Classification and management of endometrial hyperplasia. J. Obstet. Gynaecol. Can. 2019;41:1789–1800. Erratum in: J. Obstet. Gynaecol. Can. 42,1287 (2020).
An, H. et al. Pregnancy outcomes in infertile patients with endometrial hyperplasia with or without atypia undergoing in vitro fertilization: The early-follicular long protocol is superior to midluteal long protocol. Front. Endocrinol. (Lausanne) 15, 1314432 (2024).
Suzuki, Y. et al. Fertility-preserving treatment for stage IA endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 231, 599-610.e17 (2024).
Prip, C. M., Stentebjerg, M., Bennetsen, M. H., Petersen, L. K. & Bor, P. Risk of atypical hyperplasia and endometrial carcinoma after initial diagnosis of non-atypical endometrial hyperplasia: A long-term follow-up study. PLoS ONE 17, e0266339 (2022).
De Rocco, S. et al. Reproductive and pregnancy outcomes of fertility-sparing treatments for early-stage endometrial cancer or atypical hyperplasia: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 273, 90–97 (2022).
Jernigan, A. M. et al. Referring survivors of endometrial cancer and complex atypical hyperplasia to bariatric specialists: A prospective cohort study. Am. J. Obstet Gynecol. 213(350), e1-10 (2015).
Linkov, F. et al. Endometrial hyperplasia, endometrial cancer and prevention: Gaps in existing research of modifiable risk factors. Eur. J. Cancer 44, 1632–1644 (2008).
Haggerty, A. F. et al. The use of novel technology-based weight loss interventions for obese women with endometrial hyperplasia and cancer. Gynecol. Oncol. 140, 239–244 (2016).
Haggerty, A. F. et al. Obesity and endometrial cancer: A lack of knowledge but opportunity for intervention. Nutr. Cancer 69, 990–995 (2017).
Raffone, A. et al. Diabetes mellitus is associated with occult cancer in endometrial hyperplasia. Pathol. Oncol. Res. 26, 1377–1384 (2020).
Gunderson, C. C., Fader, A. N., Carson, K. A. & Bristow, R. E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol. Oncol. 125, 477–482 (2012).
Gallos, I. D. et al. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 207, 266.e1-266.e12 (2012).
Koskas, M., Uzan, J., Luton, D., Rouzier, R. & Daraï, E. Prognostic factors of oncologic and reproductive outcomes in fertility-sparing management of endometrial atypical hyperplasia and adenocarcinoma: Systematic review and meta-analysis. Fertil. Steril. 101, 785–794 (2014).
Lacey, J. V. Jr. et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J. Clin. Oncol. 28, 788–792 (2010).
Management of endometrial intraepithelial neoplasia or atypical endometrial hyperplasia: ACOG Clinical Consensus No. 5. Obstet Gynecol 142, 735–44 (2023).
Catalano, P. M. & Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 356, j1 (2017).
Mahutte, N. et al. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: An analysis of live birth rates from 96,000 autologous embryo transfers. Fertil. Steril. 117, 792–800 (2022).
Shim, S.-H. et al. Risk of adverse obstetric outcomes in patients with a history of endometrial cancer: A nationwide population-based cohort study. BJOG 130, 1662–1668 (2023).
Song, W. et al. The impact of previous conservative treatment of atypical hyperplasia on pregnancy outcomes after IVF/ICSI-embryo transfer: A propensity score-matched retrospective cohort study. Hum. Reprod. 38(12), 2447–2455 (2023).
Vasileva, R. et al. Pregnancy and obstetric outcomes after fertility-sparing management of endometrial cancer and atypical hyperplasia: A multicentre cohort study. Hum. Reprod. 39(6), 1231–1238 (2024).
Lai, S. et al. A sonographic endometrial thickness <7 mm in women undergoing in vitro fertilization increases the risk of placenta accreta spectrum. Am. J. Obstet. Gynecol. 231(5), 557.e1-557.e18 (2024).
Casper, R. F. It’s time to pay attention to the endometrium. Fertil. Steril. 96, 519–521 (2011).
Zhang, J. et al. Effect of endometrial thickness on birthweight in frozen embryo transfer cycles: An analysis including 6181 singleton newborns. Hum. Reprod. 34, 1707–1715 (2019).
Fang, Z., Huang, J., Mao, J., Yu, L. & Wang, X. Effect of endometrial thickness on obstetric and neonatal outcomes in assisted reproduction: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 21(1), 55 (2023).
Acknowledgments
We would like to thank Byung-Ryong Ahn for his help with data analysis.
Author information
Authors and Affiliations
Contributions
SUL and YWK conceived and designed the study. SUL, SKC, HS, and YWK collected and verified the data. SUL wrote the initial draft of the manuscript. SUL and YWK revised the manuscript for intellectual content. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Review Boards of the Catholic Medical Centre (OC23ZISI0058). The requirement for informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, S.U., Choi, S.K., Song, H. et al. Pregnancy outcomes of endometrial hyperplasia with or without atypia. Sci Rep 15, 23231 (2025). https://doi.org/10.1038/s41598-025-05906-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05906-0