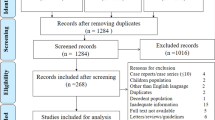
Abstract
COVID-19 has globally impacted millions. This study investigates DHEAS (dehydroepiandrosterone sulfate) as a factor for COVID-19 progression, analyzing its relationship with disease status, temporal patterns, age, gender, and comorbidities to improve outcomes. DHEAS was quantified with a competitive chemiluminescent immunoassay. We conducted DHEAS analysis across different days. COVID-19 patients, particularly inpatients, have lower DHEAS levels compared to controls. DHEAS levels in COVID-19 patients showed a dynamic pattern, with an initial decline followed by recovery. The scatter plot analysis suggested COVID-19 could increase the age-related decline in DHEAS among males. Comorbidities, including hypertension, heart disease, and diabetes mellitus, were prevalent among COVID-19 patients and correlated with disease severity. Hypertension moderated the relationship between hospitalization and DHEAS, especially in females. Our findings showed a significant association between lower DHEAS and COVID-19 severity, along with temporal dynamics. COVID-19’s potential to increase the age-related decline in DHEAS, especially in males, underscores its intricate relationship with age. Hypertension’s influence on DHEAS suggests a gender-specific effect, emphasizing tailored management approaches. These findings offer valuable insights into the interaction between COVID-19, hormonal dynamics, and demographic factors, suggesting that DHEAS levels may play a role in the pathophysiology of the disease and could be considered alongside other markers.
Similar content being viewed by others
Introduction
The emergence of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) at the end of 2019 led to the global COVID-19 pandemic, characterized by high infectivity and rapid transmission, even in asymptomatic individuals1. As of October 2023, COVID-19 has infected over 696 million people worldwide2.
Notably, a significant proportion of COVID-19 patients already have one of the underlying diseases, such as cardiovascular diseases (CVDs), hypertension (HTN), diabetes mellitus (DM), and others3,4. The pathophysiology of COVID-19 involves complex immune system dysregulation, characterized by inflammation, oxidative stress, and endothelial dysfunction. Evidence has shown that COVID-19 is associated with CVD and hypertension, although their specific interaction has not been established5,6,7. However, the question is whether the underlying CVD and hypertension are independent risks or whether other factors, such as genetics, age, gender, etc., affect their roles. Intriguingly, evidence indicates that low levels of dehydroepiandrosterone (DHEA) and its sulfate ester (DHEAS) are significant risk factors for CVD8,9. DHEA and DHEAS are the most abundant circulating steroids present in mammals, derived from cholesterol synthesized by the adrenal glands. They are precursors of the sex hormones, androgens in men and estrogens in women10. Epidemiological studies have found that DHEA(S) is associated with reduced mortality in women and men, while low serum levels are associated with a higher risk of diabetes, coronary heart disease, and lower physical vitality and function. Furthermore, it shows a significant decrease with age in both sexes11.
Reports suggest that DHEAS plays a protective role in various physiological and pathophysiological conditions by mitigating aging, oxidative stress, inflammation, diabetes, and chronic heart failure. It has also been shown to reverse vascular remodeling in pulmonary arterial hypertension and enhance vascular endothelial cell function12,13,14. In the context of viral infections, DHEAS is crucial in immune modulation. Reduced levels of DHEAS are associated with heightened inflammation and a dysregulated immune response, which can exacerbate the severity of viral infections15. Specifically, lower DHEAS levels have been shown to impair the body’s ability to control inflammatory cytokine storms, a key feature of severe COVID-19 cases16,17.
This study investigates serum DHEAS levels in Iranian COVID-19 patients with underlying diseases to determine its correlation with disease severity and explore differences by sex, age, and comorbidities. By examining these relationships, we aim to better understand DHEAS’s role in COVID-19 outcomes and assess its potential as a biomarker for disease progression.
Results
The comparison of COVID-19 patients and the control group
The results (Table 1) indicated a significantly lower DHEAS in COVID-19 patients vs. control group (p < 0.001, Cohen’s d = 0.297). This reduction was most pronounced in inpatients, whose DHEAS levels were significantly lower than both outpatients (p < 0.001, Cohen’s d = 0.438) and controls (p < 0.001, Cohen’s d = 0.484). Notably, no significant difference in DHEAS levels was observed between outpatients and controls.
Receiver operating characteristic (ROC) analysis (Fig. 1) indicated DHEAS could differentiate COVID-19 patients from controls (77% sensitivity, 36% specificity using a cutpoint of 51.35 µg/dL), inpatients from controls (77% sensitivity, 46% specificity at the same cutpoint (51.35 µg/dL)), outpatients from controls (12% sensitivity, 94% specificity with a cutpoint of 265 µg/dL), and inpatients from outpatients (65% sensitivity, 60% specificity at a cutpoint of 75.45 µg/dL).
Our results showed no significant difference in DHEAS levels between inpatient survivors and non-survivors, nor based on ICU need. Similarly, DHEAS did not significantly differ between intubated ICU survivors and non-survivors (Table 1).
The serum level of DHEAS in different groups of COVID-19 patients based on gender: Analysis of serum DHEAS levels on the first day of sampling (Table S1) revealed that females had significantly lower DHEAS levels compared to males in both the patient and control groups (both p < 0.001). This significant difference in DHEAS levels between male and female inpatients was also observed (p < 0.001). However, no significant gender-based difference in DHEAS levels was found among inpatients admitted to ICU care. Interestingly, while there was no significant difference in DHEAS levels between female patients and controls, male patients had significantly lower DHEAS levels than male controls (p < 0.001). Moreover, both male and female inpatients had significantly lower DHEAS than outpatients (both p < 0.001).
DHEAS trends in COVID-19 patients: Fig. 2 illustrates the changes in DHEAS levels in all COVID-19 patients throughout the study, relative to the first day of sampling. We also examined these trends separately for inpatients and outpatients. Overall, DHEAS levels showed a significant decreasing trend until the third day of illness. Subsequently, from the seventh day, DHEAS levels began to increase, with a significant rise observed after one month. This increasing trend continued into the second month, although this later increase was not statistically significant (not shown in Fig. 2). As depicted in Fig. 2, inpatients exhibited a decrease in DHEAS levels up to the 14th day of illness, with a slow recovery thereafter. In contrast, DHEAS levels in outpatients continued to increase.
Consideration of the age of COVID-19 patients based on disease status: Table 1 showed COVID-19 patients were significantly older than controls (p = 0.001). Inpatients were significantly older than outpatients and controls (both p < 0.001). While ICU need didn’t correlate with age in inpatients, deceased inpatients were significantly older than survivors (p < 0.001).
Consideration of the age of COVID-19 patients based on gender: Age did not significantly differ between males and females within patient and control groups, or across inpatient, outpatient, and ICU subgroups. However, both male and female patients were significantly older than controls (males: p = 0.021; females: p = 0.020). This was consistent when comparing hospitalized vs. non-hospitalized males and females separately (both p < 0.001) (Table S1).
The correlation between DHEAS and age: The scatter plot in Fig. 3A illustrates the correlation between DHEAS and age in both the control and COVID-19 patient groups. This plot reveals a clear inverse relationship between DHEAS and age in patients, mirroring that observed in the control group, but with a less steep slope. A comparison of the graph in females of both groups (Fig. 3B) showed the relationship between DHEAS with age with the same slope, however, the plot of the male subgroups (Fig. 3C) revealed a distinct difference in the relationship between DHEAS and age, with the male patient group exhibiting a less intense slope compared to the control group. Notably, the inverse correlation observed in males was stronger and more significant than in females for both patient and control groups.
Evaluating the hospitalization rate of COVID-19 patients with underlying disease
As seen in Fig. 4, hospitalization rates for HTN, DM, and HD were significantly higher among COVID-19 inpatients than outpatients.
Evaluating the impact of hypertension (HTN)
The serum level of DHEAS based on disease status: Table 2 showed significantly lower DHEAS on day 1 in COVID-19 patients with HTN vs. those without (p < 0.001, Cohen’s d = 0.401). HTN-positive outpatients also had lower DHEAS than HTN-negative outpatients (p = 0.001, Cohen’s d = 0.696). Additionally, HTN-negative inpatients had significantly different DHEAS levels compared to HTN-negative outpatients (p = 0.001, Cohen’s d = 0.536) and controls (p = 0.011, Cohen’s d = 0.339).
The serum level of DHEAS based on gender: According to Table 2, serum DHEAS levels were significantly higher in males than in females among HTN-positive patients (p = 0.001, Cohen’s d = 0.554), HTN-negative patients (p < 0.001, Cohen’s d = 0.456), and HTN-negative controls (p = 0.002, Cohen’s d = 0.881). Furthermore, among both males and females without HTN (Table S2, the mean DHEAS concentrations were significantly higher in outpatients compared to inpatients (p < 0.001). In female patients with HTN, the mean DHEAS concentration was lower for outpatients compared to inpatients, although this difference was not statistically significant. Conversely, among male patients with HTN, the mean DHEAS concentration was higher for outpatients compared to inpatients. Notably, a significantly higher DHEAS level in patients without HTN compared to those with HTN was observed only in female outpatients.
About the effect of age: Table 3 showed HTN patients were significantly older than non-HTN patients overall and within inpatient/outpatient subgroups (all p < 0.001). Furthermore, a significant age difference was observed between HTN-positive inpatients and outpatients (p = 0.048), as well as between HTN-negative inpatients and outpatients (p < 0.001).
The results also revealed a significant difference in mean age between males and females only in patients without HTN (p = 0.012), but not in patients with HTN or the control group (Table 3).
Evaluating the impact of heart disease (HD)
The serum level of DHEAS based on disease status: Table 4 showed significantly lower DHEAS in patients with HD vs. without (p = 0.001, Cohen’s d = 0.397). This was also true for outpatients (p = 0.020, Cohen’s d = 0.599). HD-negative inpatients had lower DHEAS than HD-negative outpatients (p < 0.001, Cohen’s d = 0.510) and controls (p = 0.005, Cohen’s d = 0.379).
Among the HD-positive COVID-19 patients, no significant difference was observed in DHEAS serum levels between male and female patients. However, in HD-negative patients and controls, females had significantly lower DHEAS than males (Table 4).
About the effect of age: Table 5 showed HD patients were significantly older than non-HD patients (p < 0.001). Significant differences were confirmed when comparing HD-negative inpatients with HD-positive inpatients (p < 0.001) and with the HD-negative control group (p = 0.003). Similar significant differences were also observed in the comparison of different subgroups of outpatients (p < 0.001).
There was no significant difference in age between males and females in both COVID-19 subgroups with or without HD (Table 5).
Evaluating the impact of diabetes mellitus (DM)
The serum level of DHEAS based on disease status: As shown in Table 6, the serum DHEAS level in patients with underlying DM was significantly lower compared to both DM-negative patients (p = 0.001, Cohen’s d = 0.397) and the control group (p = 0.001, Cohen’s d = 0.525). Within both outpatient and inpatient groups, DM patients experienced a greater deficiency of DHEAS than DM-negative patients (outpatients: p = 0.039, Cohen’s d = 0.189; inpatients: p = 0.010, Cohen’s d = 0.510). Furthermore, DM-negative inpatients had significantly lower DHEAS levels compared to DM-negative outpatients (p < 0.001, Cohen’s d = 0.510).
In DM-positive COVID-19 patients, DHEAS levels didn’t significantly differ between sexes. However, in non-DM patients and controls, females had significantly lower DHEAS than males (both p = 0.001, Cohen’s d = 0.535 and 0.832 respectively) (Table 6).
About the effect of age: Table 7 showed DM patients were significantly older than non-DM patients and controls (both p < 0.001). Noteworthy age differences were observed when comparing DM-negative inpatients with DM-positive inpatients and DM-negative controls (both p < 0.001). Similar age differences existed within outpatient subgroups (p < 0.001). No significant age difference was found between DM-positive inpatients and outpatients.
There was no significant age difference between males and females in both subgroups of COVID-19, regardless of the presence of DM (Table 7).
A multivariate regression analysis was conducted using DHEAs as the dependent variable and baseline variables, comorbidities, and COVID-19 severity as predictors. The analysis confirmed that age (P < 0.001), gender (P < 0.001), hypertension (P = 0.035), and COVID severity (P = 0.004) had a significant impact on DHEAS levels. In contrast, diabetes and heart disease did not (Table S3).
Interaction between severity and comorbidities
In Table 8, the simultaneous relationship between DHEAS and COVID-19 severity has been investigated in patients with and without HTN. The analysis revealed a significant interaction between comorbidities and severity (p = 0.035). Specifically, the correlation between DHEAS and severity was stronger in HTN-negative patients compared to HTN-positive patients. In contrast, no significant interaction was observed between severity and either HD or DM comorbidities (p = 0.255 and p = 0.106, respectively) (Tables S4, S5).
Discussion
Our study discovered a significant difference in DHEAS levels between COVID-19 patients and controls, supporting our previous findings linking low DHEAS with severe viral infections, including COVID-19 17. To our knowledge, this is the first study to examine DHEAS levels in COVID patients from multiple perspectives and across various regions in Iran, beyond Tehran in our previous study. This broader sampling enhances the generalizability of our findings and aligns with studies emphasizing demographic variability in DHEAS levels. Furthermore, we examined the role of gender and comorbidities in impacting DHEAS levels for a more comprehensive understanding of the COVID-19 complexities.
Our data suggest that DHEAS levels may decrease under the influence of COVID-19 infection, especially in hospitalized subjects. The ROC analysis demonstrated that DHEAS has limited diagnostic value due to its low AUC and specificity; indicating its possible role in identifying disease severity, particularly in distinguishing inpatients from controls or inpatients from outpatients alongside other marker. However, DHEAS levels did not effectively differentiate outpatients from controls, suggesting a stronger link between low DHEAS levels and more severe disease. DHEAS was also not associated with mortality or the need for intensive care, suggesting that the association between DHEAS levels and disease severity may be attenuated or modified in the ICU setting, possibly due to factors specific to the intensive care environment and interventions. This contrasts with findings from Das et al., who reported an increased hypocortisolism and lower DHEAS levels in moderate to severe COVID-19 cases compared to mild ones18. However, a report found no direct COVID-19-related effects in ACTH, aldosterone, and DHEA levels, suggesting no direct impact of the virus on the hypothalamic-pituitary-adrenal (HPA) axis or the adrenal cortex19.
Women generally exhibit lower DHEAS levels than men, consistent with the known mechanisms where estrogen and inherent lower adrenal function in females contribute to lower DHEAS levels, while testosterone stimulates DHEAS synthesis20. Beyond these biological factors, social factors such as lifestyle, stress, health behaviors, and socioeconomic conditions can further contribute to these gender inequalities21.
Our gender-based analysis confirmed markedly lower DHEAS levels in females especially between patients, control groups, and between male and female inpatients. However, this significant gender-based difference was not observed among ICU patients, suggesting that ICU care might impact DHEAS levels in both sexes. A gender-specific comparison by matching females with females and males with males showed a significant decrease in DHEAS levels in male patients compared to male controls, but not in females. This indicates that DHEAS levels may be used as a factor in differentiating between male patients and control males, although further research is warranted.
Age-related hormonal deficiencies, particularly in DHEA, could significantly influence morbidity and mortality, especially among older individuals22. Increased secretion of DHEA/DHEAS has been associated with better health outcomes in individuals and greater longevity in males23.
Typically, DHEAS levels decline with age. Our findings show a potential deviation from this expected pattern, representing a slower rate of decline in male COVID-19 patients, which may point to a SARS-CoV-2-related alteration in age-related decline in DHEAS levels. The implication of these gender and age effects for disease management requires further research.
Our findings showed a dynamic pattern in DHEAS levels throughout COVID-19 as follows: an initial significant decline followed by a subsequent recovery towards baseline levels. This decline could be attributed to acute stress response triggered by viral infections, including COVID-19 24,25, which can influence hormonal pathways, including testosterone, prolactin, and cortisol, thereby regulating the immune response and inflammatory processes. For instance, decreased testosterone levels during COVID-19 infection may contribute to the immune dysregulation26and elevated prolactin may exacerbate inflammation and negatively impact COVID-19 disease outcomes27,28. Supporting this, Vaez Mahdavi et al. showed that while cortisol levels did not significantly increase in COVID-19 patients, DHEAS levels and the DHEAS/cortisol ratio decreased with increasing disease severity17.
The subsequent recovery in DHEAS levels observed with the disease progression may signify the restoration of hormonal balance as the immune system regains control. Supporting this idea, Tommo et al. (2022) suggested that DHEAS may play a crucial role in immune defense against COVID-19 by inhibiting IL-6 and activating regulatory T cells (Tregs), which are essential for modulating the immune response24. The recovery of DHEAS levels towards the normal range may signal a favorable reaction to the infection. The recovery of DHEAS levels was different between inpatients and outpatients. A prolonged decrease in DHEAS levels lasting up to 14 days was seen in inpatients, while a decline in DHEAS levels up to three days, followed by a faster recovery to control levels, was seen in outpatients. This contrast may indicate differences in disease severity, physiological responses, and hospitalization-related factors.
We observed that COVID-19 patients were significantly older than the controls, and inpatients were older than both outpatients and controls, indicating that age plays a role in the susceptibility to the disease, its severity, and worse outcomes, consistent with other research29. However, no significant age difference was observed in inpatients who required ICU and who did not, suggesting that factors other than age, such as comorbidities, may influence ICU admission. However, inpatients who died were significantly older than survivors, emphasizing the role of age in mortality risk among COVID-19 inpatients.
Gender-constructed behaviors in both men and women, along with sociocultural factors and gender disparities, can impact the likelihood of exposure to infections, disease incidence, and their subsequent outcomes30,31. Based on our results, no significant age difference was between males and females in either patients and their subgroups or controls, suggesting that other factors, such as comorbidities, genetic predisposition, environmental exposure, or lifestyle choices, may have more prominent roles than age in the disease’s onset and severity. Furthermore, significant age differences were only observed between males and not females in the control and patient groups. This age disparity underscores the need for gender- and age-specific comparisons to properly interpret clinical and hormonal data.
Hypertension, HD, and DM stand out as prevalent conditions affecting a substantial number of individuals worldwide32,33,34,35,36,37. However, the specific association between these diseases and the increased risk of SARS-CoV-2 infection and COVID-19 severity remains unclear. In our study, approximately 24.7% of patients had HTN, 20.7% had DM, and 12.9% had pre-existing HD. Furthermore, we observed a higher prevalence of HTN and DM among hospitalized patients compared to outpatients (2.27 and 2.33 times higher, respectively), while pre-existing HD was significantly more common in inpatients compared to outpatients (3.90 times higher). This suggests that these conditions increase susceptibility to experiencing severe COVID-19.
A positive correlation was reported between endogenous DHEAS levels and blood pressure and hypertension status38. DHEAS may directly affect blood vessels by modulating intracellular calcium metabolism39. DHEAS may also regulate vascular responsiveness to various hormonal stimuli, inducing depolarization and constriction. While the precise mechanism by which DHEAS exerts its effects on vascular function remains unclear, it has been proposed that it triggers the production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS) and stimulates the release of NO from vascular endothelial cells40. DHEA may act as a cardioprotective agent41 and an anti-inflammatory agent42. However, studies linking DHEA and cardiovascular disease (CVD) have yielded inconsistent results. While some meta-analyses have reported significantly lower average DHEAS levels in coronary heart disease (CHD) patients, a significant and consistent association between DHEA levels and CHD or CVD risk has not been established43,44.
Other studies have indicated that low serum DHEA levels in men may increase the risk of CHD, although the relationship between DHEAS and CHD remains conflicting45,46,47. Similarly, findings regarding the association of both DHEA and DHEAS with CVD in women have also been inconsistent48,49.
The relationship between endogenous DHEA and DM is also complex. Decreased DHEA levels have been linked to diabetes, impaired glucose tolerance, hyperglycemia, and insulin resistance50,51. Conversely, in a study, higher serum DHEA levels were associated with a lower risk of type 2 diabetes52. However, the protective effects of DHEAS in women compared to men remain inconclusive53. Another study reported low or undetectable levels of urine DHEA in obese patients with diabetes54. While DHEA has been cross-sectionally associated with impaired fasting glucose in postmenopausal women, no significant association with type 2 diabetes was observed55.
COVID-19 inpatients often experience significant stress, inflammation, and receive various medications, which can impact hormone levels56. We found significantly decreased levels of DHEAS in COVID-19 patients with HTN, HD, and DM. These comorbidities appeared to disrupt hormonal balance and decrease DHEAS concentration, particularly among outpatients. The interaction of HTN, but not HD or DM, and changes in DHEAS levels had a notable influence on disease severity, highlighting the unique role of HTN as a moderator in the correlation of DHEAS and severity in the context of COVID-19 and suggest that the influence of this comorbidity on hormonal profiles may vary across different underlying conditions. The immune system’s response to COVID-19, stress, and underlying diseases might collectively contribute to this decrease in DHEAS, which requires further investigation. The severity of COVID-19 and/or hospitalization could also override the influence of these comorbidities on DHEAS levels.
Our result indicated a gender-specific difference in the hormonal response to COVID-19 among patients with hypertension. In both males and females with HTN, the mean DHEAS concentrations were similar between inpatients and outpatients, especially in females. This suggests that hospitalization status did not influence DHEAS levels in these hypertensive groups. However, among female patients without HTN, a notable difference was observed, with mean DHEAS concentration being lower for inpatients compared to outpatients. A similar trend, although not statistically significant, emerged in male patients without HTN.
Furthermore, female outpatients with HTN had a significantly lower DHEAS compared to those without HTN. This suggests that HTN notably impacts DHEAS levels, specifically in this subgroup. Maybe hypertension-induced physiological changes affect hormone production and regulation57,58. Sex hormones, particularly estrogens, influencing the renin-angiotensin-aldosterone system, may affect DHEAS metabolism differently in men and women59. Furthermore, sex differences exist in the pathophysiology of hypertension, including variations in inflammatory responses and endothelial function, which could influence DHEAS levels60,61alongside genetic and epigenetic factors62,63. These results suggest that HTN may moderate the relationship between hospitalization status and DHEAS levels, but this effect is more pronounced among female patients.
Whether low DHEAS causes or results from disease severity remains unclear. However, this reduction likely stems from several interconnected biological mechanisms. During critical illness, adrenal function often shifts steroid production towards cortisol to support anti-inflammatory and metabolic demands, thus reducing DHEAS synthesis64. Severe inflammation and cytokine storm can inhibit adrenal enzymes, disrupt the HPA axis, and further favor cortisol production. Oxidative stress in severe cases can also damage adrenal cells and key enzymes in DHEAS synthesis64. Additionally, SARS-CoV-2 may directly or indirectly affect adrenal function through ACE2-mediated entry or endothelial damage65. Conversely, lower DHEAS levels may exacerbate disease severity by impairing the immune response and inflammation control, potentially exacerbating the cytokine storm through reduced IL-6 inhibition64. These lower DHEAS levels could also worsen metabolic disturbances and diminish endothelial protection, contributing to a dysregulated stress response66. More research is needed to clarify the cause-and-effect relationship between DHEAS decline and COVID-19 severity.
This study did not measure other anti-inflammatory neurosteroids, such as allopregnanolone, pregnenolone, and progesterone, which could provide a more comprehensive understanding of the hormonal response in COVID-19. The study’s geographic and demographic focus may limit the generalizability of the findings. Lack of follow-up beyond 30 days prevents the assessment of long-term neurosteroid changes and their impact on recovery. Finally, potential confounders, such as medication use, nutritional status, and pre-existing metabolic or endocrine disorders, were not controlled, which may affect the results.
Future studies are required to examine the roles of these neurosteroids in COVID-19 outcomes, particularly in different geographic and demographic groups, extend follow-up periods, and control for key confounders to better understand the neuroendocrine response in COVID-19.
Conclusion
This study highlights the significant role of the dynamic relationship between DHEAS and COVID-19 progression, shaped by age, gender, comorbidities, and hospitalization status. We suggest that monitoring DHEAS may help categorize patients and facilitate early, personalized interventions. Gender-specific differences highlight the influence of hormonal factors on immune responses, suggesting the importance of considering gender in clinical data interpretation. The lower DHEAS in hospitalized patients suggests an association with severity. However, given the ROC analysis’s limited diagnostic value (low specificity), DHEAS is better viewed as a potential biomarker reflecting disease severity, warranting further study with other indicators.
The prolonged DHEAS reduction in hospitalized patients raises the hypothesis of potential benefits from therapies aimed at restoring hormonal balance, such as DHEAS supplementation, particularly in the context of comorbid conditions like hypertension. Gender-specific reduced DHEAS, particularly in hypertensive females, and the age-related decrease in DHEAS in males emphasize the need for hormonal assessments and tailored clinical approaches.
Therefore, further larger, longitudinal studies are required to validate the clinical utility of DHEAS monitoring and its links with comorbidities for patient stratification and tailored interventions. Future research should also elucidate the underlying pathways and assess the potential clinical benefits of DHEA supplementation or other hormonal interventions in specific patient populations.
Materials and methods
Study design and participants
This is an observational cohort study of 686 COVID-19 patients and 209 controls with no evidence of SARS-CoV-2 infection in Iran17. This study cooperated with Imam Khomeini Hospital Complex and the Immunoregulation Research Center of Shahed University from February 12, 2020, to April 4, 2020. Patients were included based on pulmonary infiltration detected on chest X-ray, a positive COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) test, and clinical evaluation by an infectious disease specialist. Control participants were identified as PCR-negative individuals monitored for one week to confirm their status as non-infected. Samples lost during the experiment were the only exclusion criterion. COVID-19 patients were identified based on gender and the status of all COVID-19 patients, including disease severity (non-hospitalized (outpatient), and hospitalized (inpatient)), based on WHO guidelines, https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1, which emphasizes the importance of clinical assessment and symptom severity in determining care settings. We further categorized inpatients into ICU care and non-ICU care to reflect the level of medical intervention required.
In this cohort study, the participants were followed for 6 months. Various factors were investigated, but our focus in this article is on investigating serum levels of DHEAS. For the DHEAS assay, we presented results only for up to 30 days. During this period, serum samples from the same patients were collected each day in the early morning, and DHEAS levels were measured on the same day (three to four hours after sampling) to monitor changes over time. Here, ‘day 1’ or first day signifies a day after symptom onset.
We compared COVID-19 patients with a history of HTN, HD, or DM to COVID-19 patients and controls without these conditions, irrespective of other underlying diseases, considering all COVID-19 severity levels.
The study was approved by the National Ethics Committee on Research in Medical Sciences of the Iranian Ministry of Health (Ethical Code: IR.NIMAD.REC. 1399.041). All methods were carried out by relevant guidelines and regulations. Patients filled out a written form of consent and also electronic standardized questionnaires about comorbidities associated with COVID-19, including DM, HTN, HD, and other diseases.
DHEAS analysis
Serum DHEAS levels were measured with the Siemens Kit by IMMULITE 2000, German automated system. This system uses a competitive chemiluminescent immunoassay to measure DHEAS levels in serum. Patient samples are incubated with a chemiluminescent-labeled DHEAS analog and an antibody-coated solid phase, where both the patient’s DHEAS and the labeled analog compete for antibody binding. After incubation, unbound substances are washed away, and the bound chemiluminescent signal is measured. The emitted light is inversely proportional to the DHEAS concentration, which is calculated using a calibration curve. The fully automated IMMULITE 2000 system was used for precise and efficient DHEAS measurement, with quality controls. Accuracy depends on proper sample handling (preparation, storage, avoiding freeze-thaw).
Statistical analysis
SPSS (version 26.0, IBM SPSS Co., Armonk, NY) was used for statistical analysis. Data are presented as mean ± SD (with median, Q1, Q3). Group differences were assessed with Welch’s t-test and Tukey post hoc (after ANOVA), validated by non-parametric tests and False Discovery Rate (FDR) p-value adjustment for multiple comparisons, detailed in the supplementary and tables S6-S12. Cohen’s d and ROC analysis were performed. Multivariate regression explored factors impacting DHEAS. Considering the interaction between severity and HTN, HD, or DM history, we generated an interaction variable product severity (inpatients/outpatients) by comorbidities history (with/without). In the final regression analysis, a significant interaction between severity and comorbidities would be indicated by a significant interaction variable, as detailed in the supplementary material. A P-value of less than 0.05 was considered statistically significant.
P-value1 compares Covid-19 patients with control group, inpatients with outpatients and control, No ICU Care and ICU Care, Expire and Alive. P-value2 compares inpatients and out patients. P-value of ≤ 0.05 is considered significant.
Data availability
The datasets analyzed during the current study are not publicly available due our institutes’s policy but are available from the corresponding author on reasonable request.
References
Lai, C. C., Shih, T. P., Ko, W. C., Tang, H. J. & Hsueh, P. R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 55, 105924 (2020).
https://covid19.who.int/.
Gerc, V., Masic, I., Salihefendic, N. & Zildzic, M. Cardiovascular diseases (CVDs) in COVID-19 pandemic era. Materia socio-medica 32, 158 (2020).
Pepera, G., Tribali, M. S., Batalik, L., Petrov, I. & Papathanasiou, J. Epidemiology, risk factors and prognosis of cardiovascular disease in the coronavirus disease 2019 (COVID-19) pandemic era: A systematic review. Rev. Cardiovasc. Med. 23, 28 (2022).
Radke, R. M., Frenzel, T., Baumgartner, H. & Diller G.-P. Adult congenital heart disease and the COVID-19 pandemic. Heart 106, 1302–1309 (2020).
Vidal-Perez, R. et al. Cardiovascular disease and COVID-19, a deadly combination: A review about direct and indirect impact of a pandemic. World J. Clin. Cases. 10, 9556 (2022).
Cannata, A. et al. Impact of the COVID-19 pandemic on in-hospital mortality in cardiovascular disease: a meta-analysis. Eur. J. Prev. Cardiol. 29, 1266–1274 (2022).
Feldman, H. A. et al. Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: prospective results from the Massachusetts male aging study. Am. J. Epidemiol. 153, 79–89 (2001).
Jiménez, M. C. et al. Cardiovascular risk factors and dehydroepiandrosterone sulfate among Latinos in the Boston Puerto Rican health study. J. Endocr. Soc. 3, 291–303 (2019).
Simpson, E. R. Sources of Estrogen and their importance. J. Steroid Biochem. Mol. Biol. 86, 225–230 (2003).
Yavropoulou, M. P. & Sfikakis, P. P. Cortisol and DHEAS in COVID-19. Hormones 22, 13–14 (2023).
Mannella, P., Simoncini, T., Caretto, M. & Genazzani, A. in Vitamins and hormones Vol. 108 333–353Elsevier, (2018).
Yamaguchi, Y. et al. Reduced serum dehydroepiandrosterone levels in diabetic patients with hyperinsulinaemia. Clin. Endocrinol. 49, 377–383 (1998).
Ardestani, S. K. et al. Changes in hormones, leukocyte telomere length (LTL), and p16INK4a expression in SM-exposed individuals in favor of the cellular senescence. Drug Chem. Toxicology, 1–7 (2022).
Gola, G., Bruttomesso, A., Barquero, A. & Ramirez, J. The new role of steroids in viral infections. Front. Clin. Drug Res. 4, 93–141 (2017).
Tomo, S., Banerjee, M., Sharma, P. & Garg, M. Does dehydroepiandrosterone sulfate have a role in COVID-19 prognosis and treatment? Endocr. Regul. 55, 174–181 (2021).
Vaez Mahdavi, M. R. et al. COVID-19 patients suffer from DHEA-S deficiency. Immunoregulation 3, 135–144 (2020).
Das, L. et al. Spectrum of endocrine dysfunction and association with disease severity in patients with COVID-19: insights from a cross-sectional, observational study. Front. Endocrinol. 12, 645787 (2021).
Yavropoulou, M. P. et al. Alterations in cortisol and interleukin-6 secretion in patients with COVID-19 suggestive of neuroendocrine-immune adaptations. Endocrine 75, 317–327. https://doi.org/10.1007/s12020-021-02968-8 (2022).
Traish, A. M., Kang, H. P., Saad, F. & Guay, A. T. Dehydroepiandrosterone (DHEA)—a precursor steroid or an active hormone in human physiology (CME). J. Sex. Med. 8, 2960–2982 (2011).
Chen, C. Y., Wu, C. C., Huang, Y. C., Hung, C. F. & Wang, L. J. Gender differences in the relationships among neurosteroid serum levels, cognitive function, and quality of life. Neuropsychiatric Disease Treatment, 2389–2399 (2018).
Samaras, N., Samaras, D., Frangos, E., Forster, A. & Philippe, J. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuven. Res. 16, 285–294 (2013).
Yen, S. S. Dehydroepiandrosterone sulfate and longevity: new clues for an old friend. Proceedings of the National Academy of Sciences 98, 8167–8169 (2001).
Tomo, S. et al. Assessment of DHEAS, cortisol, and dheas/cortisol ratio in patients with COVID-19: a pilot study. Hormones 21, 515–518 (2022).
Pal, R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 68, 251–252 (2020).
Al-Kuraishy, H. M., Al-Gareeb, A. I., Faidah, H., Alexiou, A. & Batiha, G. E.-S. Testosterone in COVID-19: an adversary Bane or comrade Boon. Front. Cell. Infect. Microbiol. 11, 666987 (2021).
Al-Kuraishy, H. M., Al-Gareeb, A. I., Butnariu, M. & Batiha, G. E.-S. The crucial role of prolactin-lactogenic hormone in Covid-19. Mol. Cell. Biochem. 477, 1381–1392 (2022).
Al-Kuraishy, H. M. et al. Long COVID and risk of erectile dysfunction in recovered patients from mild to moderate COVID-19. Sci. Rep. 13, 5977 (2023).
Pawlikowski, M. & Winczyk, K. Endocrine and metabolic aspects of COVID-19. Endokrynologia Polska. 72, 256–260 (2021).
Gebhard, C., Regitz-Zagrosek, V., Neuhauser, H. K., Morgan, R. & Klein, S. L. Impact of sex and gender on COVID-19 outcomes in Europe. Biology Sex. Differences. 11, 1–13 (2020).
Mourosi, J. T., Anwar, S. & Hosen, M. J. The sex and gender dimensions of COVID-19: A narrative review of the potential underlying factors. Infection Genet. Evolution, 105338 (2022).
Bansal, M., Cardiovascular disease & COVID-19. and Diabetes Metabolic Syndrome: Clin. Res. Reviews 14, 247–250 (2020).
Zheng, Y. Y., Ma, Y. T., Zhang, J. Y. & Xie, X. COVID-19 and the cardiovascular system. Nat. Reviews Cardiol. 17, 259–260 (2020).
Ruan, Q., Yang, K., Wang, W., Jiang, L. & Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from wuhan, China. Intensive Care Med. 46, 846–848 (2020).
Yang, C. & Jin, Z. An acute respiratory infection runs into the most common noncommunicable epidemic—COVID-19 and cardiovascular diseases. JAMA Cardiol. 5, 743–744 (2020).
Gupta, R., Ghosh, A., Singh, A. K. & Misra, A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metabolic Syndrome. 14, 211 (2020).
Organization, W. H. Hypertension and COVID-19: Scientific Brief, 17 June 2021 (World Health Organization, 2021).
Schunkert, H., Hense, H. W., Andus, T., Riegger, G. A. & Straub, R. H. Relation between dehydroepiandrosterone sulfate and blood pressure levels in a population-based sample. Am. J. Hypertens. 12, 1140–1143 (1999).
Barbagallo, M., Shan, J., Pang, P. K. & Resnick, L. M. Effects of dehydroepiandrosterone sulfate on cellular calcium responsiveness and vascular contractility. Hypertension 26, 1065–1069 (1995).
Tousoulis, D., Kampoli, A. M., Nikolaos Papageorgiou, T., Stefanadis, C. & C. & The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 10, 4–18 (2012).
Nakamura, S. et al. Possible association of heart failure status with synthetic balance between aldosterone and dehydroepiandrosterone in human heart. Circulation 110, 1787–1793 (2004).
Rutkowski, K., Sowa, P., Rutkowska-Talipska, J., Kuryliszyn-Moskal, A. & Rutkowski, R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs 74, 1195–1207. https://doi.org/10.1007/s40265-014-0259-8 (2014).
Wu, T. T., Gao, Y., Zheng, Y. Y., Ma, Y. T. & Xie, X. Association of endogenous DHEA/DHEAS with coronary heart disease: A systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 46, 984–994. https://doi.org/10.1111/1440-1681.13146 (2019).
Wu, T., Zheng, Y. & Xie, X. GW29-e1427 the correlations between endogenous DHEA/DHEAS and coronary artery diseases: A systematic review and Meta-Analysis. J. Am. Coll. Cardiol. 72, C246–C246 (2018).
Tivesten, Å. et al. Dehydroepiandrosterone and its sulfate predict the 5-year risk of coronary heart disease events in elderly men. J. Am. Coll. Cardiol. 64, 1801–1810 (2014).
Jia, X. et al. Plasma dehydroepiandrosterone sulfate and cardiovascular disease risk in older men and women. J. Clin. Endocrinol. Metabolism. 105, e4304–e4327 (2020).
Zhang, X. et al. Low serum dehydroepiandrosterone and dehydroepiandrosterone sulfate are associated with coronary heart disease in men with type 2 diabetes mellitus. Front. Endocrinol. (Lausanne). 13, 890029. https://doi.org/10.3389/fendo.2022.890029 (2022).
Meun, C. et al. High androgens in postmenopausal women and the risk for atherosclerosis and cardiovascular disease: the Rotterdam study. J. Clin. Endocrinol. Metabolism. 103, 1622–1630 (2018).
Page, J. H. et al. Plasma dehydroepiandrosterone and risk of myocardial infarction in women. Clin. Chem. 54, 1190–1196. https://doi.org/10.1373/clinchem.2007.099291 (2008).
Wellman, M., Shane-McWhorter, L., Orlando, P. L. & Jennings, J. P. The role of dehydroepiandrosterone in diabetes mellitus. Pharmacotherapy: J. Hum. Pharmacol. Drug Therapy. 19, 582–591 (1999).
Kim, C. & Halter, J. B. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr. Cardiol. Rep. 16, 467. https://doi.org/10.1007/s11886-014-0467-6 (2014).
Brahimaj, A. et al. Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: the Rotterdam study. Diabetologia 60, 98–106. https://doi.org/10.1007/s00125-016-4136-8 (2017).
Rasmussen, J. J. et al. Endogenous testosterone levels are associated with risk of type 2 diabetes in women without established comorbidity. J. Endocr. Soc. 4, bvaa050 (2020).
Aoki, K. & Terauchi, Y. Effect of dehydroepiandrosterone (DHEA) on diabetes mellitus and obesity. Vitam. Horm. 108, 355–365 (2018).
Kalyani, R. R. et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J. Clin. Endocrinol. Metab. 94, 4127–4135. https://doi.org/10.1210/jc.2009-0910 (2009).
Bajwa, S. J. S., Sharma, R. & Kurdi, M. S. The stress of COVID-19: playing havoc with the hormones-A review. J. Endocrinol. Res. 2, 1–8 (2020).
Levanovich, P. E., Diaczok, A. & Rossi, N. F. Clinical and molecular perspectives of Monogenic hypertension. Curr. Hypertens. Reviews. 16, 91–107 (2020).
Hester, J., Ventetuolo, C. & Lahm, T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr. Physiol. 10, 125 (2019).
O’Donnell, E., Floras, J. S. & Harvey, P. J. Estrogen status and the Renin angiotensin aldosterone system. Am. J. Physiology-Regulatory Integr. Comp. Physiol. 307, R498–R500 (2014).
Gillis, E. E. & Sullivan, J. C. Sex differences in hypertension: recent advances. Hypertension 68, 1322–1327 (2016).
Virdis, A. et al. Endothelial function in hypertension: role of gender. J. Hypertens. 20, S11–S16 (2002).
O’Donnell, C. J. et al. Evidence for association and genetic linkage of the angiotensin-converting enzyme locus with hypertension and blood pressure in men but not women in the Framingham heart study. Circulation 97, 1766–1772 (1998).
Bourgeois, C. T., Satou, R. & Prieto, M. C. HDAC9 is an epigenetic repressor of kidney angiotensinogen Establishing a sex difference. Biology Sex. Differences. 8, 1–10 (2017).
Prall, S. P. & Muehlenbein, M. P. DHEA modulates immune function: a review of evidence. Vitam. Horm. 108, 125–144 (2018).
Luo, D., Bai, M., Zhang, W. & Wang, J. The possible mechanism and research progress of ACE2 involved in cardiovascular injury caused by COVID-19: a review. Front. Cardiovasc. Med. 11, 1409723 (2024).
Oberbeck, R. & Kobbe, P. Dehydroepiandrosterone (DHEA): a steroid with multiple effects. Is there any possible option in the treatment of critical illness? Curr. Med. Chem. 17, 1039–1047 (2010).
Author information
Authors and Affiliations
Contributions
T.J. wrote the main manuscript text, prepared figures, and considered the data scientifically.S.K.A. and T.Gh. designed the research, supervised the study, and considered the data.M.V.M. designed the researchA.R. considered the manuscript scientifically and edited it.M.M.N. performed the statistical analysis.F.T. performed the statistical analysis.H.Kh. and A.Kh. and B.Kh. and K.L. performed the experiments.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jamali, T., Ardestani, S.K., Vaez-Mahdavi, MR. et al. Association of DHEAS levels with COVID19 severity, gender, age, comorbidities, and management strategies. Sci Rep 15, 21348 (2025). https://doi.org/10.1038/s41598-025-05919-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05919-9