Abstract
Cross-sectional data from a sample of older adults with obesity was used to determine how peripheral insulin resistance (PIR) and neuronal insulin signaling abnormalities (NISAs) relate to executive function and functional brain network topology. Older adults (n = 71) with obesity but without type 2 diabetes were included. PIR was quantified by HOMA2-IR. NISAs were quantified according to an established neuron-derived small-extracellular-vesicle-based metric, R. An executive function composite score, summed scores to the Auditory Verbal Learning Test (AVLT) trials 1–5, and functional brain networks generated from resting-state functional magnetic resonance imaging data were outcomes in analyses. We used general linear models and a novel regression framework for brain network analysis to identify relationships between insulin-related biomarkers and brain-related outcomes. HOMA2-IR, but not R, was negatively associated with executive function. Neither measure was associated with AVLT score. HOMA2-IR was also related to hippocampal network topology in participants who had undergone functional neuroimaging. Neither HOMA2-IR nor R were significantly related to network topology of the central executive network. This study provides further evidence that PIR is associated with aging brain function. NISAs were not found to be related to PIR, cognition, or functional brain network topology.
Similar content being viewed by others
Introduction
Peripheral insulin resistance (PIR) has long been considered a risk factor for cognitive decline in aging adults. PIR was first described in type 2 diabetes, where peripheral tissues (such as muscle and fat) show reduced sensitivity to insulin, resulting in hyperglycemia. Decades of human research have shown that PIR is related to unfavorable aging outcomes1,2,3,4 including lower cognitive performance5,6,7, reduced brain glucose uptake8,9, and altered functional connectivity in several brain regions, including the hippocampus10,11. These findings suggest that PIR may be involved in brain changes that contribute to cognitive decline. The search for mechanisms explaining the relationship between PIR and brain function has contributed to interest in “brain insulin resistance”, a broad term that refers to the failure of cells located in the brain (including neurons, glia, and vascular cells) to respond to insulin12,13. However, it remains unclear how PIR may contribute to brain insulin resistance and cognitive decline.
Talbot et al. demonstrated histological evidence that the brains of Alzheimer’s disease (AD) patients demonstrate “insulin resistance” (defined by dysfunctional phosphorylation of type 1 Insulin Receptor Substrate [IRS-1]), particularly in the hippocampus13. However, this finding came from the brains of deceased AD patients—therefore, the methodology from this study cannot be used in living humans. To address this limitation, a method for measuring in vivo “neuronal insulin resistance” using neuronal-derived extracellular vesicles (NDEs) was proposed14. We note that while this method claims to measure “neuronal insulin resistance”, this approach does not measure insulin’s effects on neuronal glucose uptake and therefore does not meet classical criteria for quantifying insulin resistance. Therefore, a more specific and appropriate term for the physiological characteristic that this method quantifies is “neuronal insulin signaling abnormalities” (NISAs), which we will use hereafter. Data collected using this technique suggest that NISAs are elevated in both type 2 diabetes and AD14. While these findings suggest that NDEs may be a useful tool for studying NISAs in living humans, outcomes depend upon the surface markers used to isolate NDEs. Currently, there is not a universally accepted surface marker to isolate neuron-specific or central-nervous-system-derived extracellular vesicles15. Further, only a few published studies have assessed how insulin signaling biomarkers in NDEs relate to PIR, cognition, or functional brain connectivity16,17.
In the present work, we investigate how PIR and NISAs relate to each other and to brain-related outcomes in a sample of older adults with obesity. First, we assessed whether PIR and NISAs were statistically related. Second, we determined whether PIR and/or NISAs were associated with cognitive ability. Finally, we determined whether PIR and/or NISAs were associated with functional brain network metrics in regions typically associated with the cognitive outcomes measured in our second aim. Those regions are the central executive network (CEN), which is typically engaged during working memory tasks18, and the hippocampus, which is well-known for its role in learning and memory formation19. While it is true that insulin receptors are found in widespread regions of the brain, the hippocampus was of particular interest as it expresses the insulin-dependent glucose transport protein GLUT4 at a high density20,21, is one of the first brain regions to atrophy in AD22, and is thought to be particularly vulnerable to insulin resistance13.
Methods
Participants
Participants were enrollees of INFINITE, a study of weight loss and exercise in older adults (aged 65–80 years) with obesity23. Specifically, participants were drawn from an ancillary study, INFINITE MIND, that collected structural and functional brain imaging and cognitive data in a subset of the INFINITE participants24. All data included in analyses were from baseline visits of the study, prior to any interventions. Exclusion criteria for the parent study included Mini-Mental State Exam (MMSE) scores < 24, osteoporosis, smoking within the past year, insulin-dependent diabetes, hip fracture, hip or knee replacement, or spinal surgery in the past 6 months, or clinical evidence of depression, heart disease, cancer, liver disease, renal disease, chronic pulmonary disease, uncontrolled hypertension, major physical impairment, or contraindication for exercise or weight loss upon exam. Figure 1 demonstrates how participants from the parent study were included in cognitive and functional neuroimaging analyses for this work. Of the 180 participants enrolled in the parent study, NISAs were quantified in 85 participants, 14 of which were excluded due to prior diagnosis of type 2 diabetes to avoid confounding effects of diabetes medication. Of the 71 participants without type 2 diabetes, 69 completed cognitive testing and 51 completed structural and functional MRI scans.
Sample selection for cognitive and imaging analyses. Of the 180 participants enrolled in the parent study, 69 had sufficient data to be included in cognitive analyses and 51 had sufficient data to be included in functional neuroimaging analyses. 49 of the participants between these two analysis groups were the same.
All human subjects research in this study was approved by the Wake Forest University Institutional Review Board. Informed consent was obtained from all participants. All aspects of data collection and analyses in this work adhered to the standards put forth in the Declaration of Helsinki and Belmont reports. All data used for analyses in this work will be made available in de-identified form upon reasonable request from the corresponding author.
Peripheral insulin resistance (PIR)
Participants completed a blood draw following a 12-h fast at their baseline visit for the parent study. From these blood draws, fasting plasma glucose and fasting plasma insulin were used to calculate index values for the computer-based homeostatic model assessment for insulin resistance (HOMA2-IR)25. HOMA2-IR is a widely accepted index of insulin resistance in peripheral tissue.
Neuronal insulin signaling abnormalities (NISAs)
NDEs were isolated from plasma following earlier established protocols14,26,27. Approximately 500ul of plasma was incubated with 150ul of thromboplastin‐D (Fisher Scientific, Inc., Hanover Park, IL) at room temperature for 60 min, followed by the addition of 350ul of calcium‐ and magnesium‐free Dulbecco’s balanced salt solution (DPBS) with protease inhibitor cocktail (Roche Applied Sciences, Inc., Indianapolis, IN) and phosphatase inhibitor cocktail (Pierce Halt, Thermo Scientific, Inc., Rockford, IL). After centrifugation at 1500 × g for 20 min, supernatant were mixed with 252 μl of ExoQuick precipitation solution (EXOQ; System Biosciences, Inc., Mountain View, CA), and incubated for 1 h at 4 °C. Resultant total extracellular vesicle (TE) suspensions were centrifuged at 1500 × g for 30 min at 4 °C and each pellet was resuspended in 200 μl of DPBS with inhibitor cocktails.
TE suspensions were incubated with 2 μg of mouse anti-human CD171 (L1CAM, transmembrane L1 cell adhesion molecule) biotinylated antibody (clone 5G3, eBioscience, San Diego, CA) for 1 h at 4 °C, then 25 μl of streptavidin‐agarose resin (Thermo Scientific, Inc.) plus 50 μl of 3% bovine serum albumin (BSA) incubation for 2 h at 4 °C. After centrifugation at 200 × g for 10 min at 4 °C and removal of the supernatant, each pellet was suspended in 50 µl of 0.05 M glycine–HCl (pH 3.0) by vortexing for 10 s, incubated at 4 °C for 30 min, and re-centrifuged at 200 × g for 15 min at 4 °C. Each suspension then received 450ul M-PER mammalian protein extraction reagent (Thermo Fisher Scientific, Incorporated) that had been adjusted to pH 8 with 1 M Tris HCL (pH 8.6) and contained the cocktails of protease and phosphatase inhibitors. These suspensions were incubated at 37 °C for 10 min with vortex-mixing before storage at − 80 °C until use in ELISAs.
Protein concentration was determined using the BCA method as per manufacturer’s protocol (Pierce, Rockford IL). The expression of specific proteins in NDEs were quantified by ELISA kits for human P-serine 312-IRS-1 (Life Technologies Corporation, Carlsbad, CA, USA), human P-pan-tyrosine-IRS-1 (Cell Signaling Technology, Danvers, MA, USA), human total IRS-1 (AMSBIO, Incorporated, Cambridge, MA, USA), according to suppliers’ directions. Extensive characterization of the purity of NDEs isolated with this approach has been completed in previous work from our group28,29,30.
An insulin resistance index was calculated based on the methods in previous work14. Total IRS-1, pan tyrosine phosphorylated IRS-1, and serine 312 phosphorylated IRS-1 were quantified with ELISA. Values were adjusted by the total amount of protein. The ratio of phosphorylated serine 312 to pan-tyrosine was calculated and used as an NDE-based insulin function metric. While this method is designed to quantify NISAs (as explained earlier), the original work describing the approach referred to the final measure as the “neuronal insulin resistance factor (R)”. To remain consistent with the work that established this measure14, we will refer to this measure as “R” throughout the remainder of this work.
Neuropsychological testing
Prior to completing the cognitive assessment battery, participants were asked whether they had followed their normal daily eating and medication regimens and completed a finger stick glucose test to confirm blood glucose levels were > 60 mg/dl. All participants had glucose levels higher than this cutoff.
An executive function composite score was calculated based on performance in a cognitive battery including the Digit Symbol Coding task (DSC), the Trail Making Test (TMT) parts A and B, the Stroop task, phonemic fluency, and semantic fluency. The 90-s version of the DSC was administered; the outcome was the number of correct responses. The TMT outcome was difference in time in parts A and B in seconds. The interference score (interference score = [(time(s) needed for subtask 3) − (time(s) needed for subtasks 1 + 2)]/2) from the 40-item version of the Stroop task was used. For phonemic fluency, participants verbally generated as many words beginning with the letter F as they could in one minute, and then did likewise for the letters A and S. The sum of all 3 trials was used for analysis. For semantic fluency, participants verbally listed as many animals as possible within one minute and then did likewise for kitchen items. The sum of both trials was used for analysis. The composite score was calculated by summing the z-scores of DSC score, TMT B-A time, Stroop interference score, phonemic fluency, and semantic fluency. The sign of the z-score was reversed prior to summing when necessary, so that positive z-scores indicate better performance. The executive function composite was the pre-specified outcome of the INFINITE MIND ancillary.
In addition to the executive function composite score, participants completed the Auditory Verbal Learning Task (AVLT) trials 1–5 to quantify learning ability. The summed score of the first five trials was used for analyses31.
MRI acquisition
Fifty-one participants completed 60-min MRI scans at baseline. MRI scans were acquired on a 3.0T Siemens Skyra scanner with a 32-channel head coil (Siemens, Germany). High resolution T1-weighted images were acquired using an MPRAGE-GRAPPA sequence (TR = 1900 ms, TE = 2.93 ms, TI = 900 ms, flip angle = 9 degrees, 176 slices). Resting state blood-oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) images were acquired using a whole-brain gradient-echo echo planar imaging sequence (35 contiguous slices; slice thickness = 3.75 mm; in-plane resolution = 3.75 × 3.75 mm, TR = 2.0).
fMRI preprocessing
T1-weighted images were warped to the Montreal Neurological Institute (MNI) template using the longitudinal image processing pipeline in Advanced Normalization Tools (ANTs) software (ANTs, https://www.nitrc.org/projects/ants). Because this was an interventional study with two timepoints, for each participant, a single-subject (halfway-space) image was created by warping T1 images from timepoint 1 and timepoint 2 together. Single-subject templates were then combined to create a study-specific template using the antsMultivariateTemplateConstruction command. Brain extraction, N4 bias field correction, and tissue segmentation were performed using ANTs Cortical Thickness. The warp between the study-specific template and the MNI template was calculated, and individual T1-weighted images in native space were transformed into MNI space by simultaneously applying transforms from native space to the study-specific template and the study-specific template to the MNI template.
Preprocessing of BOLD fMRI images was performed using FEAT in FMRIB’s Software Library (FSL, www.fmrib.ox.ac.uk/fsl). N4 bias field correction in ANTS was applied to BOLD images prior to preprocessing and the first 10 volumes of the BOLD image were removed to eliminate irregularities in signal due to the stabilization of the magnetic field. The function epireg was used to apply fieldmaps for distortion correction and perform realignment and slice timing correction. Data were band-pass filtered (0.009–0.08 Hz) to further remove physiological noise and low frequency drift from the functional signal. Images were smoothed with a 5mm Gaussian kernel. Potential confounding for non-neural signal due to motion and other physiological signals was addressed by removing global, white matter, and cerebrospinal fluid signal using the compcorr method32 implemented through the Conn Toolbox33. Fluctuations in signal remaining after controlling for head motion were identified using ART software33 and time points with meaningful deviations in signal were regressed out. BOLD images were coregistered to their native space structural image and then warped to the MNI template using the same parameters used to warp the T1-weighted structural image to MNI space.
Network generation and efficiency inference
Functional brain networks were generated such that each network node represented a voxel. The choice to use voxel-based nodes was based on previous work demonstrating substantial inhomogeneity in the BOLD signal of atlas-based nodes across a variety of structural and functional brain atlases34,35. Functional connections are based on the extent of synchrony between voxel time series. This is quantified by calculating the Pearson correlation coefficient of each pairing of two nodes’ time series36,37. Pearson correlation was used because it performs well in identifying functional connections from simulated data35 and is less computationally expensive than alternatives (i.e., partial correlation). Computational efficiency is crucial when working with high-dimensional networks of many nodes (i.e., voxel-based networks).
Edge weights were thresholded and binarized to create an adjacency matrix, Aij. For each participant, edges were first thresholded to retain only the strongest positive edges while satisfying S = 2.5 for each participant, where S = log(N)/log(k) with N being the number of nodes in the network and k being average degree (i.e., the average number of nodes a given node shares a connection with). A threshold of S = 2.5 was used because this has previously been shown to produce networks with minimal fragmentation which are comparable to other naturally generated networks38. Using a proportional threshold ensures that each network has approximately the same density. The alternative—having a specific threshold Pearson’s r value that applies across the sample—would result in participants having brain networks with very different densities. Networks with different densities cannot be meaningfully compared using existing methods39. Sensitivity analyses were performed with networks with thresholds of S = 3.0 and S = 3.5 to show robustness of findings to different edge densities. Only positive edges were maintained because negative edge weights are incompatible with the graph metrics we investigated (described below), and the physiological interpretation of a negative edge is unclear40. Each participant’s binarized adjacency matrix was used for functional brain network analyses.
Global and local efficiency were computed for each network. Global efficiency can be measured for any node i by finding the average inverse of the path length between i and every other node in the network. Network-level global efficiency can be calculated by averaging all nodal global efficiency values. The local efficiency of node j is the network-level global efficiency of the subgraph of nodes which are directly connected to j. Similarly to path length, global efficiency quantifies global information flow but only allows values to range from 0 to 1 whereas path lengths can be infinite. Local efficiency is closely related to clustering coefficient41, but also rescales values from 0 to 1. For a full review of network efficiency measures, see42. While many other graph metrics could be computed at the nodal level, global and local efficiency were the focus of this work because they are very simple measures that capture network characteristics at both the global and local level. Additionally, the distribution of node-level global and local efficiency values are approximately normally distributed while many other common metrics, such as node degree, are not (Supplementary Fig. 1). Having an approximately normal distribution bounded from 0 to 1 helps to avoid spurious results in the brain network regression approach described below.
Non-imaging statistical analyses
To determine whether HOMA2-IR and R have a linear relationship, we calculated the Pearson correlation between values. Because the absence of a relationship between R and HOMA2-IR was considered to be as important as the presence of a relationship for this study, we additionally computed the Bayes factor (using the BayesFactor R package) for the correlation between these two variables to gather supporting evidence in favor of or against the null hypothesis that R and HOMA2-IR are not linearly related.
To determine whether PIR and/or NISAs are associated with executive function, HOMA2-IR and R were evaluated for associations with the executive function composite score using a linear regression model. This model adjusted for age, sex, years of education, and BMI.
Imaging statistical analyses
We used BANTOR43, a novel regression framework for brain network distance metrics to measure the associations of HOMA2-IR and R with global and local efficiency. First, to examine the associations between insulin function measures and global efficiency for the central executive network (CEN) and hippocampus, two separate distance regression models (i.e., one model for the CEN and one for the hippocampus) were constructed with a 3-dimensional global efficiency map serving as the dependent variable. The independent variables for each participant were HOMA2-IR, R, age, sex, years of education, and BMI. The distances between the global efficiency maps were calculated with Jaccard distance. The Jaccard distance comes from the Jaccardized Czekanowski similarity index44(also known as the Ružička index45). To convert these similarity indices into distance measures, we calculated the Jaccard distance as 1-Jaccardized Czekanowski index. For each independent variable, the distance was an absolute value difference between participants. These distances were computed between every subject pair to generate a distance matrix for each variable in the model. Then, the independent variable distances were regressed against the global efficiency distance using a linear statistical model with individual-level fixed effects43. Because every possible pairing of participants is used to fit this regression model, the goal is not to predict a brain related outcome, but to determine whether there is a significant linear relationship between any included independent variables and brain network measures such as global efficiency. The interpretation of a significant positive β value from this framework is that a larger distance between an independent variable (e.g., HOMA2-IR) in two participants would tend to be accompanied by more distant brain network metrics between the two participants.
This process was repeated with local efficiency maps as the dependent variable. Statistical significance was set at α = 0.05 for all analyses. As neuroimaging analyses were exploratory for future hypothesis generation, we considered type-II error to be at least as impactful as type-I error46 and report p-values which are unadjusted for multiple comparisons.
Results
Participant characteristics
Participant characteristics for the cognition and functional neuroimaging samples are shown in Table 1. Of the 51 participants included in imaging analyses, 49 were also represented in the cognitive analysis sample.
Relationship of peripheral and neuronal insulin resistance
HOMA2-IR and R were not correlated (r = − 0.036, p = 0.768, Fig. 2). The Bayes factor of the correlation between HOMA2-IR and R was 0.285 ± 0%, suggesting stronger evidence in favor of the null model (i.e., that R and HOMA2-IR are not linearly related) than against the null model.
Cognitive outcomes
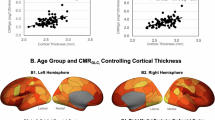
Associations between measures of insulin function and cognitive scores are shown in Table 2. A linear regression model controlling for age, sex, years of education, and BMI revealed that HOMA2-IR was negatively associated with executive function (β = − 0.798, SE = 0.319, p = 0.015, Fig. 3a). R, which was also a covariate in the same model, was not associated with executive function (β = 0.005, SE = 0.083, p = 0.954, Fig. 3b). Neither HOMA2-IR (β = − 1.201, SE = 0.946, p = 0.209) nor R (β = 0.109, SE = 0.247, p = 0.660) was significantly associated with AVLT scores in a second regression model with AVLT score as the outcome.
Associations between insulin function markers and executive function. Plots show participants’ insulin function levels (x-axes) versus their predicted executive function composite score (y-axis) from linear regression models adjusting for age, years of education, sex, and BMI. Panel (a) shows the significant negative association between HOMA2-IR and executive function. Panel (b) shows the statistically insignificant relationship between R and executive function.
Global and local efficiency of functional brain networks
Associations between measures of insulin function and network efficiency are shown in Table 3. HOMA2-IR was not significantly related to global efficiency in the CEN (p = 0.097) or hippocampus (p = 0.724). R also was not related to global efficiency in the CEN (p = 0.380) or hippocampus (p = 0.752).
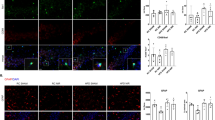
HOMA2-IR exhibited a relationship with local efficiency in the hippocampus (β = 0.0035, SE = 0.0014, p = 0.011). To visualize this result, the average local efficiency maps of participants in the highest and lowest HOMA2-IR tertile are shown in Fig. 4. Sensitivity analyses on networks with different densities showed very similar results (Supplementary Tables 1 and 2). Because HOMA2-IR had been found to be associated with executive function composite scores, a post-hoc BANTOR model with executive function as the outcome and the interaction between HOMA2-IR and hippocampal local efficiency as the independent variable of interest was fit. This model also included age, sex, education, HOMA2-IR, and local efficiency as independent variables. In this model, the interaction between HOMA2-IR and hippocampal local efficiency was significantly related to executive function score (β = 1.943, SE = 0.760, p = 0.011).
HOMA2-IR and hippocampal local efficiency. Color scale refers to the average local efficiency value of voxels. Warmer colors indicate higher local efficiency. The right hippocampus of participants with low HOMA2-IR has higher local efficiency than in participants with high HOMA2-IR. (MNI Y coordinates: -26 [left], 26 [right]).
HOMA2-IR was not significantly related to local efficiency in the CEN (p = 0.104), and R was not associated with local efficiency in the CEN (p = 0.897) or hippocampus (p = 0.782).
Discussion
In this study, we found that PIR (quantified by HOMA2-IR) was not linearly related to NISAs (quantified by R14) in our sample of older adults with obesity. While we replicated existing findings that PIR is negatively associated with executive function7,47 in a unique population, we did not find that R was associated with any cognitive outcomes. Finally, functional brain network analyses showed that HOMA2-IR, but not R, was related to hippocampal functional network topology, and the interaction between HOMA2-IR and hippocampal local efficiency was related to executive function ability. We expand on the implications of our findings below.
Previous work14 demonstrated that R is elevated in individuals with type 2 diabetes relative to non-diabetic controls. Data collection for this study occurred soon after publication of the proposed method for measuring R. In the time between data collection and analyses for the present work, the use of L1CAM as a marker of NDEs has received criticism from some15 while the group that introduced this measure has published work further validating its use48. In the present work, we chose to explore whether R, measured using L1CAM + NDE, was associated with brain-related outcomes of interest. In our study, we did not find any relationship between R and HOMA2-IR, cognition, or functional brain network topology. There are caveats to these results; Kapogiannis et al.14 reported higher R values (T2D: 50.4 ± 6.93, non-T2D: 16.4 ± 0.67) compared to ours (non-T2D: 6.47 ± 4.12). Our cohort was four years younger on average than the sample from14. A 4-year follow-up visit could show that R rapidly increased in the years following the baseline visit in INFINITE. Elucidating the timeframe of R escalation longitudinally would be an important next step to inform any future studies of this measure. In addition to the difference in age, our sample had a higher proportion of female participants than14. Finally, our study did not have control groups of individuals with a normal BMI or normal HOMA2-IR, nor did it have a large sample of individuals with type 2 diabetes or Alzheimer’s disease for comparison. It is possible that our cohort was too homogenous for nuanced comparisons to R values from other, potentially more heterogeneous samples.
In functional neuroimaging analyses, we observed a relationship between HOMA2-IR and local efficiency in the hippocampus. Visualizations of this result showed that participants with lower HOMA2-IR had higher local efficiency in their right hippocampus. This finding suggests that an increase in PIR is related to a less functionally-intraconnected hippocampus. In the context of an aging brain, local efficiency offers a quantification of the resiliency of the brain’s functional wiring42,49. More locally efficient networks are expected to minimize the propagation of spurious information through a network. Our finding that differences in HOMA2-IR are related to differences in hippocampal local efficiency may indicate that PIR increases risk for aberrant signaling in the hippocampus. The subsequent finding—that the interaction between HOMA2-IR and hippocampal local efficiency is related to executive function ability—underscores the relevance of PIR to brain and cognitive function. This finding suggests that intact functional connectivity of the hippocampus may be a protective factor against PIR-related executive function decline. Future work should identify mechanisms through which PIR impacts hippocampal functional connectivity. Likely mechanisms include oxidative stress and inflammation, though we do not rule out that a novel measure of NISAs (or of neuronal insulin resistance) may reveal a relationship with PIR which was not identified in the present study using an exosome-based approach.
We note that the hippocampus has historically been associated with learning and memory formation19. The finding that the interaction between hippocampal connectivity and PIR is associated with executive function (which is typically thought to be governed by the CEN in the frontal and parietal lobes18) is at first counterintuitive. Further, PIR showed no association with scores from AVLT trials 1–5, a well-established measure of learning ability. In interpreting these findings, we note that network neuroscience increasingly suggests that reductive one-to-one mappings of cognitive functions to brain regions (as with learning to the hippocampus) are oversimplifications of more complex, distributed neural processes50. For the present study, the implication is that the cognitive impact of changes to hippocampal functional connectivity has the potential to be far-reaching due to the hippocampus’ interdependence with broader subnetworks in the brain. In other words, changes to hippocampal connectivity may impact learning ability, but they may also impact other cognitive domains such as executive function.
Limitations to analyses in this work include the relatively small sample size, the limited assessment of memory ability, and the cross-sectional design of analyses, which prevent us from making conclusions about causality in any of the relationships identified. Additionally, the non-specificity of L1CAM + extracellular vesicles for CNS could hamper their utility to assess brain insulin function. Notably, we found no evidence that R was associated with HOMA2-IR, cognition, or functional brain connectivity. This does not necessarily mean that NISAs are not related to cognitive decline. If more reliable measures of NISAs are developed, recreating analyses in the present work may provide a more accurate view of the relationship between NISAs and cognitive decline. Additionally, studies could explore the effects of NISAs in different populations and assess their impact on a wider variety of cognitive outcomes. In addition to investigation of alternative measures of NISAs and neuronal insulin resistance, future work could take advantage of alternative imaging modalities, such as fluorodeoxyglucose positron emission tomography (FDG-PET) imaging or SV2A imaging, which assess glucose uptake and synaptic density, respectively.
We conclude by summarizing that PIR was negatively associated with executive function and different functional connectivity in the hippocampus. Further, the statistical interaction between PIR and hippocampal local efficiency was associated with executive function. Ultimately, the findings of this study support the existence of a relationship between PIR and brain function, but limitations to the method for measuring NISAs prevent us from making conclusive statements about the role of NISAs or neuronal insulin resistance in cognitive decline.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arnold, S. E. et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 14(3), 168–181 (2018).
Kodl, C. T. & Seaquist, E. R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 29(4), 494–511 (2008).
Perlmuter, L. C. et al. Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am. J. Med. 77(6), 1043–1048 (1984).
Salameh, T. S. et al. Insulin resistance, dyslipidemia, and apolipoprotein E interactions as mechanisms in cognitive impairment and Alzheimer’s disease. Exp. Biol. Med. (Maywood) 241(15), 1676–1683 (2016).
Kim, T. E. et al. The relationship between cognitive performance and insulin resistance in non-diabetic patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry 30(6), 551–557 (2015).
Willmann, C. et al. Insulin sensitivity predicts cognitive decline in individuals with prediabetes. BMJ Open Diabetes Res. Care 8(2), e001741 (2020).
McIntyre, C. C. et al. Insulin homeostasis mediates the relationship between cardiorespiratory fitness and cognitive speed in aging adults. J. Alzheimers Dis. 93(2), 577–584 (2023).
Baker, L. D. et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68(1), 51–57 (2011).
Willette, A. A. et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol. 72(9), 1013–1020 (2015).
Chen, Y. C. et al. Aberrant brain functional connectivity related to insulin resistance in type 2 diabetes: A resting-state fMRI study. Diabetes Care 37(6), 1689–1696 (2014).
Liu, T. et al. Altered effective connectivity of bilateral hippocampus in type 2 diabetes mellitus. Front. Neurosci. 14, 657 (2020).
Rhea, E. M., et al., State of the science on brain insulin resistance and cognitive decline due to Alzheimer’s Disease. Aging Dis., (2023).
Talbot, K. et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 122(4), 1316–1338 (2012).
Kapogiannis, D. et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 29(2), 589–596 (2015).
Norman, M. et al. L1CAM is not associated with extracellular vesicles in human cerebrospinal fluid or plasma. Nat. Methods 18(6), 631 (2021).
Mullins, R. J. et al. Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer’s disease. Hum. Brain Mapp. 38(4), 1933–1940 (2017).
Mustapic, M. et al. Extracellular vesicle biomarkers track cognitive changes following intranasal insulin in Alzheimer’s disease. J. Alzheimers Dis. 69(2), 489–498 (2019).
Marek, S. & Dosenbach, N. U. F. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. 20(2), 133–140 (2018).
Bird, C. M. & Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 9(3), 182–194 (2008).
McNay, E. C. et al. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol. Learn. Mem. 93(4), 546–553 (2010).
McNay, E. C. & Pearson-Leary, J. GluT4: A central player in hippocampal memory and brain insulin resistance. Exp. Neurol. 323, 113076 (2020).
Planche, V. et al. Structural progression of Alzheimer’s disease over decades: The MRI staging scheme. Brain Commun. 4(3), fcac109 (2022).
Nicklas, B. J. et al. Effects of caloric restriction on cardiorespiratory fitness, fatigue, and disability responses to aerobic exercise in older adults with obesity: A randomized controlled trial. J. Gerontol. Ser. a-Biol. Sci. Med. Sci. 74(7), 1084–1090 (2019).
Hugenschmidt, C. E. et al. Cognitive effects of adding caloric restriction to aerobic exercise training in older adults with obesity. Obesity (Silver Spring) 27(8), 1266–1274 (2019).
Levy, J. C., Matthews, D. R. & Hermans, M. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21(12), 2191–2192 (1998).
Fiandaca, M. S. et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement 11(6), 600–607 (2015).
Patterson, S. A., Deep, G. & Brinkley, T. E. Detection of the receptor for advanced glycation endproducts in neuronally-derived exosomes in plasma. Biochem. Biophys. Res. Commun. 500(4), 892–896 (2018).
Kumar, A. et al. Brain cell-derived exosomes in plasma serve as neurodegeneration biomarkers in male cynomolgus monkeys self-administrating oxycodone. EBioMedicine 63, 103192 (2021).
Kumar, A. et al. Small extracellular vesicles in plasma reveal molecular effects of modified Mediterranean-ketogenic diet in participants with mild cognitive impairment. Brain Commun 4(6), fcac262 (2022).
Kumar, A. et al. MicroRNA expression in extracellular vesicles as a novel blood-based biomarker for Alzheimer’s disease. Alzheimers Dement 19(11), 4952–4966 (2023).
Williamson, J. D. et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: A randomized clinical trial. JAMA Intern. Med. 174(3), 324–333 (2014).
Behzadi, Y. et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37(1), 90–101 (2007).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2(3), 125–141 (2012).
Stanley, M. L. et al. Defining nodes in complex brain networks. Front. Comput. Neurosci. 7, 169 (2013).
Smith, S. M. et al. Network modelling methods for FMRI. Neuroimage 54(2), 875–891 (2011).
Telesford, Q. K. et al. The Brain as a complex system: Using network science as a tool for understanding the brain. Brain Connect. 1(4), 295–308 (2011).
Friston, K. J. Functional and effective connectivity: A review. Brain Connect. 1(1), 13–36 (2011).
Hayasaka, S. & Laurienti, J. Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data. Neuroimage 50(2), 499–508 (2010).
van Wijk, B. C. M., Stam, C. J. & Daffertshofer, A. Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE 5(10), e13701 (2010).
Schwarz, A. J. & McGonigle, J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage 55(3), 1132–1146 (2011).
Watts, D. J. & Strogatz, S. H. Collective dynamics of “small-world” networks. Nature 393(6684), 440–442 (1998).
Latora, V. & Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 87(19), 198701 (2001).
Tomlinson, C. E. et al. A regression framework for brain network distance metrics. Netw. Neurosci. 6(1), 49–68 (2022).
Schubert, A. Measuring the similarity between the reference and citation distributions of journals. Scientometrics 96(1), 305–313 (2013).
Schubert, A. & Telcs, A. A note on the Jaccardized Czekanowski similarity index. Scientometrics 98(2), 1397–1399 (2014).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1(1), 43–46 (1990).
Kim, A. B. & Arvanitakis, Z. Insulin resistance, cognition, and Alzheimer disease. Obesity 31(6), 1486–1498 (2023).
Nogueras-Ortiz, C. J. et al. Single-extracellular vesicle (EV) analyses validate the use of L1 Cell Adhesion Molecule (L1CAM) as a reliable biomarker of neuron-derived EVs. J. Extracell Vesicles 13(6), e12459 (2024).
Bullmore, E. & Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10(3), 186–198 (2009).
Pessoa, L. Understanding brain networks and brain organization. Phys. Life Rev. 11(3), 400–435 (2014).
Acknowledgements
The authors would like to thank all study participants and their families, as well as study staff, for their essential contributions to this work. This work was funded by NIH grants R01HL093713 (BJN), K01AG030506 (CEH), UL1TR001420 (Wake Forest Clinical and Translational Science Award), and P30AG21332 (Wake Forest Claude D. Pepper Older Americans Independence Center), American Federation for Aging Research (AFAR) grant RAG12516 (CEH), the Translational Science Center at Wake Forest University, and National Center for Advancing Translational Sciences (NCATS).
Author information
Authors and Affiliations
Contributions
CEH and BJN provided funding and designed the study. YS and GD carried out lab work to quantify exosome-based neuronal insulin resistance from blood samples. RGL and CEH oversaw fMRI data preprocessing. CCM and CEH curated data for analyses. CCM, RGL, SLS, and CEH completed all quantitative analyses. SLM assisted with interpretation of results. CCM wrote the manuscript with input from all authors. All authors contributed to manuscript revision.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
McIntyre, C.C., Lyday, R.G., Su, Y. et al. Insulin resistance, cognition, and functional brain network topology in older adults with obesity. Sci Rep 15, 22612 (2025). https://doi.org/10.1038/s41598-025-06038-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06038-1