Abstract
Killer whales (Orcinus orca) in the North Pacific have diverged into subspecies with distinct diets, social structures, and vocal behaviors. Understanding the rate at which killer whales of different subspecies vocalize provides insight into their communication with conspecifics as well as the environmental factors shaping vocalization patterns. Estimating a population’s calling rate can also enable the number of animals present to be estimated acoustically. We used killer whale dialects, long-term photo-identification records, and year-round autonomous acoustic data across multiple locations to estimate call detection rates during vocal bouts for three killer whale populations, one resident (fish-eating) and two transient (mammal-eating). We focused on call detection rates, or the number of calls detected rather than the true number produced, in order to capitalize on this large autonomous dataset to investigate the influence of spatiotemporal and social factors across many observations. Generalized additive mixed models showed that the call detection rate for southern Alaska resident killer whales was consistent across locations, seasons, which pod was calling, and the presence of other pods. Gulf of Alaska transient killer whale call detection rates differed across locations. We detected fewer, more tightly spaced discrete calls from AT1 transients than from Gulf of Alaska transients, and the mean AT1 call detection rate was stable across spatiotemporal factors. Although transients call less often than residents, we found that once vocalizing, they did so at a higher rate. These call detection rates provide novel insight into killer whale vocal behavior and can support passive acoustic group size estimation for these populations.
Similar content being viewed by others
Introduction
Killer whales (Orcinus orca) are top predators in the marine ecosystem. In the North Pacific, the species has divided into three genetically and ecologically distinct ecotypes that have different diets, social structures, and acoustic repertoires1,2,3,4,5,6,7. The resident ecotype eats fish, especially salmonids; the transient (also called Bigg’s) ecotype hunts marine mammals; and the offshore ecotype has been documented primarily consuming sharks1,4,6,8. Resident and transient killer whales were recently recognized as separate subspecies, O. o. ater and O. o. rectipinnus, respectively9,10. As their distinct diets suggest, each ecotype has unique functions within the marine environment.
Killer whales live in every ocean basin but are found in the highest densities in high latitude regions11. In the northern Gulf of Alaska, there are four recognized killer whale populations. The southern Alaska residents range from southeast Alaska to Kodiak Island and number about 1,000 individuals12. The Gulf of Alaska transients number a few hundred animals and similarly range from southeast Alaska to Kodiak Island13,14. The AT1 transients, an isolated subpopulation with a unique vocal dialect found in Prince William Sound and Kenai Fjords, experienced a severe decline following the Exxon Valdez oil spill15. Originally documented with 22 animals, the genetically unique AT1s now number only six individuals and all females are post-reproductive age15. The offshore population, which ranges at least from the Aleutian Islands to southern California, numbers 300 to 500 animals8.
Killer whales’ vocal behaviors are influenced by the hearing ability of their distinct prey16,17. Most fish hear poorly in the frequency range of killer whale vocalizations, so there is minimal prey avoidance cost for resident or offshore killer whales to vocalize while hunting or in the vicinity of prey16,17. Residents and offshores therefore commonly echolocate and call while foraging8,17. In contrast, most marine mammals have excellent hearing in the frequency range of killer whales, imposing a high cost for transients to vocalize16,17. Transient killer whales vocalize less often than residents, which likely helps them avoid detection by their prey16,17,18,19,20,21.
In addition, resident and transient killer whales use dialects that reflect their unique social structures (offshore ecotype dialects are less well understood)22. Both female and male resident killer whales stay with their mothers for life23,24,25,26. A female and her offspring form the highly stable, core social unit of resident social structure: the matriline. Groups of related matrilines that swim together most of the time are called pods25,26. Resident killer whale pods have specific dialects, or repertoires of discrete calls that can be reliably distinguished by the human ear5,7,27,28. Pod-specific dialects are thought to be an important mechanism for resident killer whales to recognize unrelated animals and maintain genetic diversity, as the degree of similarity among pod repertoires reflects their genetic relatedness5,7,22. Transient killer whales, in contrast, often disperse from their mothers as adults29,30,31. For transient killer whales, discrete call types can be identified to the population but not the pod, as their social structure is more fluid and genetic diversity can be maintained through dispersal16,19,22,32. Killer whales also produce whistles and variable tonal calls, which are often associated with social behavior8,17,19,20,33.
The rate at which animals vocalize can reveal patterns in their communication and social interactions. For example, in many species, call production rates depend on the number of animals present, their behavior state, social status, individual and group demographics, season, time of day, location, anthropogenic activity, and ambient noise level34,35,36,37,38,39,40,41,42,43,44. Additionally, when an acoustic cue production rate (cues/animal/unit time, \(\:\widehat{{r}_{p}}\)), has been estimated for a population, the number of animals present in a monitored area (\(\:\widehat{N}\)) over time period (\(\:T\)) can be estimated as the number of cues detected (\(\:{n}_{c}\)) divided by the cue rate and the probability of detecting a cue (\(\:\widehat{P}\)):
Equation. 1 (modified from Marques et al.45)
Various acoustic signals including whistles34,35, echolocation clicks45,46, calls16,19,47,48,49,50,51, and song notes38,43,52 have been used as cues for cetaceans. Estimating cetacean call production rates typically requires arrays of multiple hydrophones to localize vocal animals, paired acoustic and visual surveys, or acoustic tags16,19,34,35,36,37,38,49,50,51,53,54,55. These approaches have important strengths, but also often have a small sample size and limited temporal and spatial extent.
In Eq. 1, the number of cues detected (\(\:{n}_{c}\)) is divided by the probability of detection (\(\:\widehat{P}\)) to estimate the true number of cues. An alternative is to use the number of cues detected by the sensor per animal per unit time (cue detection rate, \(\:\widehat{{r}_{d}}\)). In this case, if the probability of detection is assumed to be the same for \(\:{n}_{c}\) and the cue detection rate, then number of animals present can be estimated as:
The objective of this study was to estimate southern Alaska resident, Gulf of Alaska transient, and AT1 transient killer whale cue detection rates during vocally active bouts. We do this by capitalizing on killer whales’ use of dialects to enable us to identify populations and pods in a large, multi-site, year-round autonomous acoustic dataset. We focus on discrete calls as the relevant cue and use the term call detection rate hereafter. Killer whales of the offshore ecotype were not considered because they were rarely documented in our study area in the northern Gulf of Alaska. This work generates new insight into killer whale vocal behavior and can inform estimates of the number of animals present during acoustic encounters.
Methods
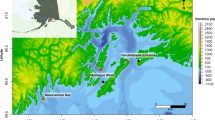
We deployed Ocean Instruments SoundTrap hydrophones (model ST300, ST500, and ST600) in four locations in the northern Gulf of Alaska (Fig. 1) between June 2019 and August 2021, though not all hydrophones recorded for the entire period (Table 1). Summer vessel-based fieldwork and telemetry data indicated high killer whale use of Hinchinbrook Entrance, Montague Strait, and Resurrection Bay, while telemetry data and anecdotal reports suggested consistent killer whale presence in Kachemak Bay56,57. Hydrophones were deployed at depths between 20 m and 40 m on primarily gravel and sand substrate. The hydrophones recorded at a 24 kHz sampling rate on a primarily five min on, 10 min off duty cycle, though during some deployments the duty cycle was shorter to conserve battery life (Table 1). Different duty cycles were not expected to bias call detection rate estimates, since rates were calculated for each 4- or 5-minute recording and were standardized by either the recording length or the calling period within the recording.
Map of hydrophone locations.Autonomous recordings were collected from June 2019 to August 2021. Inset map shows study area in the northern Gulf of Alaska. Map created using the marmap package58 in R (version 4.0.5, https://www.R-project.org)59 with bathymetric data from ETOPO 202260
We used two different applications to identify recordings (the on period within each duty cycle) with killer whale vocalizations (Table 1). Recordings from Hinchinbrook Entrance, Montague Strait, and Resurrection Bay through May 31, 2020 were previously processed using the Whistle and Moan Detector in PAMGuard version 1.15.1761 (settings information in Supplementary Methods), and killer whale presence was reported in Myers et al.62. Recordings from Kachemak Bay through July 31, 2021 were also previously processed using the PAMGuard Whistle and Moan Detector and killer whale presence was reported in National Centers for Coastal Ocean Science63. All recordings with a minimum of three PAMGuard detections were checked for killer whale presence. Remaining recordings were processed with Raven-X, a custom shallow neural network implemented using a toolbox in MATLAB and trained with southern Alaska resident recordings64. Using a sliding two-second window with 50% overlap, Raven-X assigns a probability score from 0 to 1 that each window contains killer whale calls. Windows with scores greater than 0.8 were checked. PAMGuard and Raven-X may not identify the same set of recordings with killer whale calls. However, the goal for this step of the analysis was not to identify every recording with killer whales, but instead to select a sufficient sample size of recordings across the covariates we tested. To evaluate whether the detector used to identify the recording affected (log of) call detection rates, we used a one-way ANOVA.
Each four- or five-min recording with killer whale vocalizations was annotated with the population and, for residents, pod present using published call catalogues for the southern Alaska residents5,28, Gulf of Alaska transients62, and AT1 transients19 as well as field recordings collected during vessel survey encounters when animals present were photographically identified (field methods described below). Recordings were not used if calls could not be both visually (on a spectrogram) and aurally distinguished, they did not include a minimum of three calls, or the calling period was less than 10 s. Excluding these recordings removed extreme values from the dataset. Killer whale calls were then manually annotated, as discussed below.
Statistics and reproducibility: Resident killer whale call detection rate
For resident killer whales, we used acoustic recordings of single-matriline pods to characterize call detection rate. When we recorded calls from these pods, we assumed all animals in the pod were present because these pods were made up of only one matriline each and, in the eastern North Pacific, all resident killer whales within the same matriline are observed together almost without exception24,25,26. In our study area, there are two single-matriline resident pods with clearly recognizable dialects for which we have a consistent photo-ID record: the AD8 pod and the AD16 pod. We identified the AD16 pod in autonomous acoustic recordings using their two discrete call types: AKS01 and AKS095,28. The AD16 pod was not documented producing other discrete call types. We identified the AD8 pod using call type AKS43 as well as three additional discrete call types that had not been catalogued but were consistently documented in field recordings when only the AD8 pod was present (Fig. 2, Supplementary recordings). We designated these calls AKS44, AKS45, and AKS46, following the protocol established in Yurk et al.5,28. The number of discrete calls in a pod’s repertoire varies among pods5,7,22,28 and we did not detect other discrete call types likely to be produced by these groups.
For each recording, the call detection rate (\(\:\widehat{{r}_{d}})\) was calculated as the number of calls detected (\(\:{n}_{c}\)) divided by the presumed number of animals present (\(\:N\), discussed below) and time (\(\:T\), in minutes):
All AKS01, AKS09, AKS43, AKS44, AKS45, and AKS46 calls in recordings with AD8- or AD16-pod vocalizations were manually annotated by an expert analyst (H. Myers) using Raven Pro version 1.6.465 (Fig. 3). Only discrete calls were counted because whistles, variable calls, and clicks cannot be assigned to a pod7,33. The number of whales was given by the photo-ID record of pod size for that year. The AD8 pod grew from 9 animals in 2019 to 10 animals in 202066. The AD16 pod grew from 11 to 12 animals in fall 2019 and to 14 animals in 202166. We measured time in two ways: (1) as the calling period, i.e., the end of the last call minus the beginning of the first call within a recording, and (2) as the recording length (4–5 min). Using these two distinct measures of time allowed us to compare rates with previous studies16,19, assess unique aspects of vocal behavior, and develop two approaches for applying these rates to passive acoustic group size estimation. It was also valuable to assess the robustness of relationships between call detection rate and the spatiotemporal and social factors we tested, as generalized additive mixed models (GAMMs) are sometimes prone to overfitting. Note that the rate calculated here is the call detection rate and was not corrected for probability of detection. However, we included signal-to-noise ratio (SNR) in GAMMs as a variable likely related to probability of detection.
We then used GAMMs to model the effect of six potential explanatory variables on the natural logarithm of killer whale call detection rate:
\(\:{Pod}_{i}\) was identified as either the AD8 pod or AD16 pod and \(\:{Location}_{j}\) was hydrophone site j. The two-level-factor variable \(\:{OtherPods}_{k}\) denoted the acoustic presence or absence of other pod(s). \(\:{DayOrNight}_{l}\), a two-level factor, captured whether sun angle > 0 (determined using the ‘sunAngle’ function from the oce package67 in R with the coordinates from the Montague Strait hydrophone; Table 1) at the time of the recording. We used a smooth cyclic function of day of year (\(\:{s}_{1}\left(DayOfYear\right)\)) to test for seasonal effects, which were assumed to be consistent across locations, and allowed moderate flexibility (up to four degrees of freedom) to accommodate realistic seasonal trends. We measured SNR for each discrete call using the NIST Quick method in Raven Pro68, then averaged across all calls for the recording. We estimated a smooth function of signal-to-noise ratio \(\:{s}_{2}\left(SNR\right)\) to model log(\(\:\widehat{{r}_{d}}\)). We expected the smooth function of SNR (and hence the call detection rate) to increase until reaching a horizontal asymptote at which almost all calls are likely detected. To make the effect of SNR easier to understand and apply in future analyses, when SNR was significant, we extracted the SNR value at the peak of the partial effect smooth fit for the best model (ΔAICc = 0). We then fit the log of call detection rate as a linear function of SNR using only observations with SNR values below the peak. Finally, we included a random effect of acoustic encounter, \(\:{a}_{m}\), defined as recordings from the same day and location, and, for residents, pod, to account for non-independence of observations from the same encounter. Errors (ε) were assumed to be independent and were checked for normality using diagnostic plots.
Parameters and smooth terms were estimated using a GAMM as implemented in the mgcv package69 in R version 4.0.559. We used stepwise selection to identify the best model for each population by iteratively dropping the term with the highest p-value until reaching the null model, then compared all models using Akaike’s Information Criterion controlled for small sample size (AICc). Models with ΔAICc scores less than two were considered for drawing inferences. Residuals were examined for normality, homoscedasticity, and for evidence of any remaining autocorrelation.
Finally, to test the hypothesis that call detection rates for the AD8 and AD16 pods were similar to those of other pods in the southern Alaska resident population, we compared call detection rates across field recordings collected during vessel surveys. Vessel-based fieldwork was conducted in Prince William Sound (primarily Hinchinbrook Entrance and Montague Strait) and Kenai Fjords (primarily Resurrection Bay; Fig. 1) between May and October in 2019, 2020, and 2021. When we encountered killer whales, we photographically identified as many individuals present as possible. If at least one member of a matriline was photographed, we assumed that all members of the matriline were present, as in Olsen et al.70. All whales photographed were identified to a matriline. We then moved the vessel approximately 500 m in front of the animals (or the nearest group of animals in large encounters), shut off the engine, and collected a field recording using an Ocean Instruments SoundTrap ST300 hydrophone or a High-Tech, Inc. HTI-96-Min hydrophone deployed at 8–10 m depth with a TASCAM DR100 portable digital recorder. Field recordings were collected during multi-objective fieldwork and were not optimized for estimating calling rates. This is in part because in large encounters, field recordings were often made within the vicinity of only a few of the matrilines photographically identified. However, field recordings did allow us to compare call detection rates among a greater diversity of pods.
All discrete calls in field recordings were annotated and the call detection rate calculated for each recording. We used a one-way ANOVA to test for differences in the log of mean call detection rates between field recordings from encounters that included the AD8 and/or AD16 pods and those of only other resident pods, and a two-way Kolmogorov-Smirnov test to identify whether these two sets of recordings came from significantly different distributions. Because field recordings were often longer than five minutes (the length of most autonomous recordings), if there was a break of at least five minutes between calls the field recording would have been split and two call detection rate estimates generated; however, this did not occur in the field recordings we analyzed.
Statistics and reproducibility: Transient killer whale call detection rate
We identified recordings with transient killer whales using call catalogues for the Gulf of Alaska62 and AT119 populations. We used the same methods described above to count the number of discrete calls in these recordings. However, transient killer whales do not use pod-specific dialects16,19,31,32,62, so we could not determine which pods were vocalizing—and therefore the number of animals present—by matching call types to the photo-ID record as we did with residents. Instead, to estimate the number of animals present, we used the long-term mean number of transients encountered in annual vessel surveys from 1984 to 2022. Because we encountered transient killer whales rarely in vessel surveys, we also used reports with identifiable photographs contributed by local tour boat operators, water taxi captains, and others. For the AT1 transients, we only used encounters after 2003, the year in which the population reached seven animals, its size during the study period. To ensure group sizes were representative of the study sites, we identified the nearest hydrophone to the encounter and excluded encounters for which the nearest hydrophone location was more than 50 km away. This approach requires two main assumptions: (1) transient group size is consistent across time and locations, and (2) transient call detection rates per animal are not affected by group size. We tested the first assumption by modeling group size as a function of year or nearest hydrophone location using a generalized linear model with a Poisson family and log link implemented using the lme4 package71 in R version 4.0.559. We were unable to test the second assumption; previous studies of transient calling rates address the effects of behavior states but not group size16,19.
Finally, we used a one-way ANOVA to test for differences in the log of call detection rate between the Gulf of Alaska transient and AT1 transient populations. We then used the same generalized additive mixed modeling and model selection approach as described above for residents to model the log of transient call detection rate as a function of location, day of year, time of day, and SNR. Pod and presence of other pods were not included because transients do not use pod-specific dialects.
Results
Resident killer whale call detection rates
We identified 527 autonomous recordings with calls from the AD8 pod (225 recordings) or AD16 pod (302 recordings) from 152 acoustic encounters. We annotated a total of 13,769 AD8 and AD16 discrete calls in these 527 recordings. Discrete call detection rates of southern Alaska residents were variable, but the mean rate was consistent across the explanatory variables we tested except for SNR (Table 3; Fig. 4). The overall median resident call detection rate was 0.64 calls detected/whale/min (interquartile range (IQR) = 0.41–0.94) with time defined as the calling period and 0.42 calls detected/whale/min (IQR = 0.20–0.69), by recording length (Table 2). The acoustic detector used to identify recordings with killer whale calls (PAMGuard61 or Raven-X64) had no significant effect on the log of mean call detection rate for any population (Table S1).
Southern Alaska resident killer whale call detection rates from autonomous recordings. Call detection rates are shown by each potential explanatory variable: (A) AD16 or AD8 pod, (B) hydrophone location, (C) acoustic presence of other pods in the recording, (D) day of year, (E) time of day (day or night), and (F) mean signal-to-noise ratio across all calls in a recording. In (A), (B), (C), and (E), the horizontal line represents median call detection rate and boxes show the interquartile range. Whiskers extend up to 1.5 times the interquartile range, observations beyond the whiskers are shown as individual points. In (D) and (F), each point represents a four- or five-min recording. Overlapping observations appear darker in color. Call detection rate with time as calling period is shown.
In the best GAMMs for residents (ΔAICc < 2), the explanatory variables we tested explained 4% or less of the variability in the log of call detection rate (adjusted R2 0.0184–0.0435; Table 3). Since any fixed covariate therefore explained very little variation in call detection rate, we focused on variables included in all models with ΔAICc < 2. The only explanatory variable retained in all resident models was the smooth function of SNR (p = 6.2*10− 5 – 0.0191, Table 3). Our expectation that call detection rate would increase with the mean SNR of a recording until reaching a horizontal asymptote was supported by the plot of the partial effect of SNR (Figure S1), which peaked near SNR 16. The back-transformed linear coefficient of SNR was 1.08 with time as calling period and 1.14 with time as recording length (Table S2), suggesting that call detection rate increases approximately 8 – 14% with each increase of 1 in SNR. The variability in call detection rate between acoustic encounters (i.e., the standard deviation of the random intercept) was less than one third of residual variability, or the remaining variability after accounting for fixed and random effects (residual standard deviation, Table 3). This indicates substantial variability in call detection rate within acoustic encounters. For all populations, diagnostic plots suggested that residuals from the best models were approximately normally distributed (Figure S2).
During the study period, we collected 106 field recordings of resident killer whales, 40 of which were from encounters in which the AD8 and/or AD16 matrilines were photographed. These encounters ranged from seven to 123 animals and included 27 unique matrilines (Supplementary Text). Recordings were 7.16 min long on average and ranged between 0.48 min and 22.70 min (total time 12.77 h). All catalogued resident discrete calls5,28 in these recordings were manually annotated for a total of 14,348 calls. We found no evidence that call detection rates were different for the AD8 or AD16 pods and other pods. There was no significant difference in the log of call detection rate between recordings from encounters that included the AD8 and/or AD16 pods and those that did not (one-way ANOVA, p = 0.742 for calling period or p = 0.410 for recording length; two-way Kolmogorov-Smirnov test, p = 0.467 for calling period or p = 0.095 for recording length; Fig. 5).
Transient killer whale call detection rates
In 139 photo-ID encounters with Gulf of Alaska transients from 1984 to 2022, 3.47 animals were documented per encounter on average (s = 1.88; Table 4). In 97 encounters with AT1 transients from 2003 to 2022, 2.90 AT1 transients were documented per encounter on average (s = 1.36; Table 2). For both populations, there was no significant change in group size over time (generalized linear model, Gulf of Alaska transients p = 0.388, AT1 transients p = 0.674) and group size was stable across hydrophone locations (generalized linear model, Gulf of Alaska transients p = 0.166–0.939, AT1 transients p = 0.865–0.877). We therefore applied each population’s long-term mean group size across locations to calculate call detection rate.
We identified 247 recordings from 83 acoustic encounters of the Gulf of Alaska transients and 69 recordings from 38 acoustic encounters of the AT1 transients. We annotated a total of 5,126 Gulf of Alaska transient discrete calls and 451 AT1 transient discrete calls in these recordings. We found a significant difference in the log of call detection rate between the two transient populations (one-way ANOVA, p = 4.60*10− 7 for calling period and p = 2.23*10− 14 for recording length) and therefore modeled their call detection rates separately.
Gulf of Alaska transient killer whale call detection rates during vocal bouts were higher than those of residents and were significantly different across locations (Table 3; Fig. 5). Overall, the median call detection rate for Gulf of Alaska transients was 1.54 calls detected/whale/min (IQR = 1.01–2.27) for calling period and 0.92 calls detected/whale/min (IQR = 0.52–1.50) for recording length, though the call detection rate in Montague Strait was significantly higher than in the other two locations (Table 2 and S4, Fig. 5). The higher median call detection rate in Montague Strait could not be explained by collinearity with other variables. Mean SNR was slightly lower in Montague Strait (12.48) than the other locations (12.96 in Hinchinbrook Entrance and 12.86 in Kachemak Bay) and the proportion of night-time observations in Montague Strait (0.67) was slightly lower than the overall proportion (0.70).
Gulf of Alaska and AT1 transient killer whale discrete call detection rates. Call detection rates are shown by each potential explanatory variable: (A) hydrophone location, (B) time of day (day or night), (C) mean signal-to-noise ratio, and (D) day of year. In (A) and (B), the horizontal line represents median call detection rate and boxes show the interquartile range. Whiskers extend up to 1.5 times the interquartile range, observations beyond the whiskers are shown as individual points. In (C) and (D), each point represents a recording. Gulf of Alaska transients were not acoustically detected in Resurrection Bay during the study period; AT1 transients were not detected in Kachemak Bay during the study period. Overlapping observations appear darker in color. Call detection rate with time as calling period is shown.
Fixed effects in the best Gulf of Alaska transient models explained approximately 5–10% of call detection rate variability (adjusted R2 0.0545–0.1080, Table 3). Call detection rates were significantly higher in Montague Strait in most models (p = 0.0035–0.0785, Table 3). The smooth function of mean recording SNR was also retained in all models (p = 0.0005–0.0103, Table 3). The smooth fit of SNR peaked near SNR 15 (Figure S3). For the best GAMM with time as recording length, the estimated degrees of freedom for the smooth term of SNR was 1; we therefore refit this model with a linear term for SNR. The back-transformed linear coefficient of SNR was 1.12 with time as calling period and 1.10 with time as recording length (Table S2). The estimated mean call detection rate was more stable across acoustic encounters when measured by calling period, as more of the variability was attributed to residual (within-encounter) variability (i.e., the standard deviation of the random intercept was several times lower with time as calling period than recording length, Table 3). In all models, residual variability exceeded variability between acoustic encounters (Table 3).
We detected fewer but more tightly spaced discrete calls from AT1 transient killer whales than from Gulf of Alaska transients. In other words, when time was defined as the calling period, the AT1 transient call detection rate was higher than that of the other populations, but when time was defined as the recording length, the AT1 transient call detection rate was lower than that of Gulf of Alaska transients and similar to that of residents (Table 2). There was no consistent relationship with explanatory variables (Table 3; Fig. 5), so we calculated call detection rates across all AT1 transient recordings without accounting for temporal, location, or noise effects. Median AT1 transient call detection rate was 2.33 calls detected/whale/min (IQR = 1.58–3.02) for calling period and 0.34 calls detected/whale/min (IQR = 0.21–0.48) for recording length.
The fixed effects in the best models for AT1 transients explained approximately 10–19% of variability in call detection rate (adjusted R2 0.0984–0.1850, Table 3). Day of year was included in most candidate models; however, plots of the partial effect of day of year suggested model overfitting, as the patterns for calling period and recording length were essentially opposite (Figure S4). Furthermore, the best models did not consistently outperform the null model containing only the random effect of acoustic encounter (Table 3). As with Gulf of Alaska transients, call detection rate was more consistent across acoustic encounters when measured by calling period than recording length (i.e., the standard deviation of the random intercept was lower when measured by calling period, Table 3).
Discussion
We found distinct call detection rates for resident and transient killer whale populations that were consistent over time and, for residents, across locations, social context, and vocal pod. Call detection rate variability, as measured by the quartile coefficient of dispersion (Table 2), was about half that reported for call production rates of other populations of killer whales16 as well as other species, such as bowhead whales (Balaena mysticetus)49. Although call detection rates are not directly comparable to call production rates, the lower variability we found is likely due at least in part to our focus on periods when animals were already vocalizing—i.e., vocal bouts. However, for all the killer whale populations characterized in this study, the variables we tested failed to explain almost all variation in call detection rates (approximately 80–96%). Some of the variability we observed is likely related to the true number of calls produced while some is likely related to the probability of detecting those calls. In this study, it was not possible to separate those sources of variability. For example, call production rate may vary with behavioral context or the information communicated, while probability of detection is affected by distance from and angle to the hydrophone (such as when animals are entering and leaving the detection area) and environmental conditions influencing background noise levels72,73. Changing probability of detection as animals moved through a hydrophone listening area in particular may help explain the variability in call detection rate within acoustic encounters. Summarizing over the relatively high number of observations and acoustic encounters in this study helps mitigate the influence of these factors on overall median call detection rates.
Killer whale communication
Transient killer whales probably call less often than residents to avoid detection by their marine mammal prey, which has acute hearing within the frequency range of killer whale vocalizations16,17,18,19,20,21. However, in this study we exclusively examined recordings in which killer whales were already vocalizing, and found that, when transients vocalized, they did so at a higher rate than residents. This is a different measure than the probability that the animals will call in the first place. As mentioned above, focusing on vocally active periods, instead of attempting to estimate how often animals are silent, likely contributed to lower variance in our call detection rate estimates for all populations46. Resident killer whales commonly vocalize while foraging, socializing, and traveling33whereas transient killer whales vocalize most often when socializing often after a kill16,19,20. The higher call detection rates we found for vocal transients may therefore be associated with a more excited behavior state, on average, than that of vocal residents. It is likely not explained by a higher probability of detection of transient killer whale calls than residents, as the SNR of recordings of both Gulf of Alaska transients and AT1 transients was lower than those of residents (Table S3).
This study highlights differences in call detection rates among killer whale subspecies and populations in the same areas. Different methodologies often make it challenging to compare calling rates across studies. For example, the call detection rates we estimated are, by definition, lower than call production rates, and the fact that we excluded silent periods longer than five minutes would bias our estimates higher than those with a longer cutoff. Nevertheless, in other regions, previous research on northern resident killer whales using vessel-based focal follows and including silent periods found a median call production rate of 0.34 calls produced/whale/min (n = 10 encounters, IQR = 0.09–1.23)16. This estimate is close to the call detection rate we estimated for southern Alaska residents (0.42 calls detected/whale/min, IQR = 0.20–0.69). In contrast, the call production rate reported for West Coast transients using the same vessel-based methods, 0.05 calls produced/whale/min (n = 25 encounters, IQR 0.00–0.23)16 is substantially lower than the median call detection rate we estimated for Gulf of Alaska transients (0.92, IQR = 0.52–1.50 calls detected/whale/min), though it overlaps with the IQR of AT1 transients (0.21–0.48 calls detected/whale/min). The lower median rate estimated for West Coast transients using these methods is not unexpected, since transients vocalize infrequently and we only analyzed relatively short recordings that included calls. Interestingly, using similar vessel-based methods, a mean call production rate of 0.28 calls produced/whale/min was reported for AT1 transients (n = 545 encounters, variability not reported)19, which is close to our call detection rate estimate (0.34 calls detected/whale/min, IQR = 0.21–0.48).
The fact that Gulf of Alaska transient call detection rates were significantly higher in Montague Strait may be explained in several ways. First, transient killer whale group sizes may have been larger, on average, in Montague Strait than other areas, leading to an inflated call detection rate estimate. In general, it would be reasonable to assume that some of the lower transient call detection rate observations were related to fewer animals and some of the higher observations were associated with larger groups. Summarizing over the relatively large sample size in this study helps mitigate this issue, and differences in call detection rate due to group size are captured in overall variance. However, in photo-ID encounters, Gulf of Alaska transient group size was not significantly higher in Montague Strait than in other locations (Table 4). Further validating transient group size estimates with visual observations is impractical in this area—for example, in 38 years of annual vessel fieldwork, Gulf of Alaska transients were encountered only 15 times in Montague Strait (Table 4). Second, transient vocal behavior in Montague Strait may have actually been different from other locations, which could be associated with site-specific hunting strategies or movement patterns. The Montague Strait hydrophone is located at the meeting point of two passageways (Fig. 1), so Gulf of Alaska transients in this area may produce more contact calls to regroup or communicate with others. Alternatively, it could be a more productive foraging area, as transients are known to vocalize at higher rates after a kill16,19. The higher Gulf of Alaska transient call detection rate in Montague Strait was likely not explained by a higher probability of detection in this area, as neither residents nor AT1 transients showed a similarly elevated call detection rate there.
Analyzing this large acoustic dataset also provided novel insight into other aspects of killer whale vocal communication. First, we found that resident call detection rate was stable at the individual—rather than matrilineal—level. The number of calls detected/whale/min for the AD8 and AD16 pods were not significantly different, even though these pods are made up of different numbers of animals (9–10 and 11–14 during the study period, respectively). There was also no significant difference in call detection rates between field recordings that included the AD8 and AD16 matrilines and those that did not, nor between autonomous recordings of just the AD8 or AD16 pods and those that included other vocal pods. This suggests that the information conveyed in discrete calls is related to the individual. For example, if a notable function of discrete calls is as contact calls5,7, then conveying the presence of each individual in the matriline may be important, not just the presence of the group.
Calling rates in many cetaceans are influenced by behavior state and the presence of conspecifics. For example, minke whales (Balaenoptera acutorostrata) call more rapidly when another minke whale is nearby37 and when swimming faster40. Humpback whale (Megaptera novaeangliae) singing rates have decreased with increasing population size off Australia, suggesting shifting reproductive strategies44. Bottlenose dolphins (Tursiops truncatus)34,35 and AT1 transient killer whales19 call at higher rates when socializing, and northern resident killer whales produce more whistles, aberrant calls, and variable calls when socializing33. Gray whale (Eschrichtius robustus)53 and beluga (Delphinapterus leucas)55 calling rates may increase as the square of the number of animals present, at least in some circumstances. In this study, while we expect that some of the variation in discrete call detection rates we observed was related to behavior, we found that the acoustic presence of another resident killer whale pod did not affect call detection rate. In other words, roughly doubling group size (at least—sometimes multiple other pods were detected) had no significant effect on call detection rate per animal. Again, this may be related to the fact that we only analyzed call detection rate once the animals were already calling—whether resident killer whales were more likely to begin calling when in the presence of another vocal pod was not addressed. We were not able to use field recordings to further test for the effect of group size on call detection rate because they were often taken in the vicinity of only a portion of the matrilines photographed in large encounters (this fact did not affect the test of whether AD8 and AD16 pod detection rates were representative of the greater population, because this bias affected field recording observations in the same way regardless of which pods were present).
Application to passive acoustic group size estimation
Resident and transient killer whales exhibit specific local movement and abundance patterns in the North Pacific6,56,70,74. Therefore, estimating the number of killer whales present when they are acoustically detected is useful for understanding local ecological effects and can inform management policies such as seasonal vessel speed limits in areas of interest. The discrete call detection rates we found for the southern Alaska resident and AT1 transient populations can be applied to estimate the number of killer whales present during acoustic encounters across the spatiotemporal and social factors we tested, as these variables had no significant effect. For Gulf of Alaska transients, location-specific call detection rates are more appropriate. Call detection rates should be adjusted for SNR for residents and Gulf of Alaska transients, unless rates are averaged across a sufficient sample size with a similar SNR profile to the recordings used in this study (Table S3).
Several aspects of the call detection rates we estimated require particular attention. First, we focused on call detection rates, or calls per whale per minute detected on the hydrophones, which is lower than the call production rate, which reflects the true number of calls produced by the animals. Call detection rates can be applied to estimate group size only when the probability of detection is expected to be similar (e.g., by accounting for the effects of SNR) and a sufficiently large sample size is available to mitigate the effects of changing probability of detection among observations. Second, in this study we focused on call detection rates during vocal bouts. Periods when animals may have been silent but within the detection ranges of the hydrophones were not addressed. Therefore, these rates can be used to estimate the number of killer whales present when at least some animals are vocalizing, but not across silent periods. If these call detection rates were applied to estimate the number of animals present in recordings with silent periods longer than five minutes (the length of most autonomous recordings used in this study), the number estimated would be biased low, especially for transients, which vocalize less often16,75.
For all populations, it is also likely that some members of the group were outside the range of the hydrophone in some recordings. Resident killer whales in particular sometimes spread out up to several kilometers, especially while foraging. However, killer whale discrete calls can propagate 10–16 km in low noise conditions and 100 m water depth73, which are comparable to the conditions we analyzed, and variation in the number of animals recorded is captured within our overall variance estimate. Our call detection rate estimates are likely lower than if we had been able to determine when all animals were within detection range, and some faint calls were also likely missed. Including the linear effect of SNR on call detection rate (Table S2) can help account for potential missed calls at low SNR levels, at least above the threshold we established that calls must be visually and aurally distinguishable in the recordings.
As this study shows, call detection rates may vary between populations of the same subspecies. We detected more Gulf of Alaska transient calls within a recording, but AT1 transients called with a shorter inter-call interval. This finding aligns with previous work showing that AT1 transients produce consistent call sequences19. Before extrapolating the rates we estimated to other killer whale populations, especially in minimally studied areas such as the Bering and Chukchi seas76, a comparison with additional populations using similar methods is recommended. Acoustic data, long-term photo-ID catalogues, and vessel-based fieldwork records exist for other Noth Pacific killer whale populations that could support such a comparison. These include the southern residents25,77; northern residents25,77; eastern Kamchatka residents78; and West Coast transients16,31.
Conclusion
In this study, we found distinct call detection rates for three killer whale populations of the resident and transient subspecies in an area of high killer whale use in the Gulf of Alaska. The rates we estimated were variable but mostly stable across the spatiotemporal and social variables we tested—with the exception of the effect of location on Gulf of Alaska transient call detection rate. Variability within acoustic encounters generally exceeded variability among encounters, and may reflect factors such as animals entering and leaving the detection area as well as the actual number of calls produced. Our focus on vocally active periods revealed novel insight into killer whale communication—notably that at least some transient populations call at a higher rate per animal than residents, once they are vocalizing. These call detection rates can also enable passive acoustic group size estimation year-round and across diverse areas, thereby supporting a greater understanding of each of these populations’ top-down impacts and appropriate conservation and management strategies.
Data availability
Data used in this study is accessible on OBIS-SEAMAP at https://seamap.env.duke.edu/dataset/2158.
References
Ford, J. K. et al. Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 76, 1456–1471 (1998).
Hoelzel, A. R., Dahlheim, M. & Stern, S. J. Low genetic variation among killer whales (Orcinus orca) in the eastern North Pacific and genetic differentiation between foraging specialists. J. Hered. 89, 121–128 (1998).
Dahlheim, M. E. et al. Eastern temperate North Pacific offshore killer whales (Orcinus orca): occurrence, movements, and insights into feeding ecology. Mar. Mamm. Sci. 24, 719–729 (2008).
Ford, J. et al. Shark predation and tooth wear in a population of northeastern Pacific killer whales. Aquat. Biol. 11, 213–224 (2011).
Yurk, H., Barrett-Lennard, L., Ford, J. K. B. & Matkin, C. O. Cultural transmission within maternal lineages: vocal clans in resident killer whales in southern Alaska. Anim. Behav. 63, 1103–1119 (2002).
Saulitis, E., Matkin, C., Barrett-Lennard, L., Heise, K. & Ellis, G. Foraging strategies of sympatric killer Whale (Orcinus orca) populations in Prince William Sound, Alaska. Mar. Mamm. Sci. 16, 94–109 (2000).
Ford, J. K. B. Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Can. J. Zool. 69, 1454–1483 (1991).
Ford, J. K. B., Stredulinsky, E. H., Ellis, G. M., Durban, J. W. & Pilkington, J. F. Offshore killer whales in Canadian Pacific waters: distribution, seasonality, foraging ecology, population status and potential for recovery. DFO Can. Sci. Advisory Secretariat Res. Doc. 2014/088, 55 p. (2014). https://www.dfo-mpo.gc.ca/csas-sccs/publications/resdocs-docrech/2014/2014_088-eng.html
Morin, P. A. et al. Revised taxonomy of Eastern North Pacific killer whales (Orcinus orca): Bigg’s and resident ecotypes deserve species status. R Soc. Open. Sci. 11, 231368 (2024).
Society for Marine Mammalogy Committee on Taxonomy. List of Marine Mammal Species and Subspecies (2024). https://marinemammalscience.org/science-and-publications/list-marine-mammal-species-subspecies/
Forney, K. A. & Wade, P. R. Worldwide distribution and abundance of killer whales. in Whales, Whaling and Ocean Ecosystems (eds Estes, J. A., DeMaster, D. P., Doak, D. F., Williams, T. M. & Brownell, R. L.) 145–162 (University of California Press, Oakland, CA, USA, 2006).
Matkin, C. O., Testa, J. W., Ellis, G. M. & Saulitis, E. L. Life history and population dynamics of southern Alaska resident killer whales (Orcinus orca). Mar. Mamm. Sci. 30, 460–479 (2014).
Zerbini, A. N. et al. Estimating abundance of killer whales in the nearshore waters of the Gulf of Alaska and Aleutian Islands using line-transect sampling. Mar. Biol. 150, 1033–1045 (2007).
Matkin, C. O. et al. Contrasting abundance and residency patterns of two sympatric populations of transient killer whales (Orcinus orca) in the northern Gulf of Alaska. Fish. Bull. 110, 143–155 (2012).
Matkin, C. O., Saulitis, E. L., Ellis, G. M., Olesiuk, P. & Rice, S. D. Ongoing population-level impacts on killer whales Orcinus orca following the Exxon Valdez oil spill in Prince William sound, Alaska. Mar. Ecol. Prog Ser. 356, 269–281 (2008).
Deecke, V. B., Ford, J. K. B. & Slater, P. J. B. The vocal behaviour of mammal-eating killer whales: communicating with costly calls. Anim. Behav. 69, 395–405 (2005).
Barrett-Lennard, L. G., Ford, J. K. B. & Heise, K. A. The mixed blessing of echolocation: differences in sonar use by fish-eating and mammal-eating killer whales. Anim. Behav. 51, 553–565 (1996).
Castellote, M., Small, R. J., Stafford, K. M., Whiting, A. & Frost, K. J. Beluga (D. leucas), harbor porpoise (P. phocoena), and killer whale (O. orca) acoustic presence in Kotzebue Sound, Alaska: silence speaks volumes. Front. Remote Sens. 3, 940247 (2022).
Saulitis, E. L., Matkin, C. O. & Fay, F. H. Vocal repertoire and acoustic behavior of the isolated AT1 killer whale subpopulation in southern Alaska. Can. J. Zool. 83, 1015–1029 (2005).
Riesch, R. & Deecke, V. B. Whistle communication in mammal-eating killer whales (Orcinus orca): further evidence for acoustic divergence between ecotypes. Behav. Ecol. Sociobiol. 65, 1377–1387 (2011).
Deecke, V. B., Slater, P. J. B. & Ford, J. K. B. Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420, 171–173 (2002).
Ford, J. K. B. Killer whales: behavior, social organization, and ecology of the oceans’ apex predators. in Ethology and Behavioral Ecology of Odontocetes (ed Würsig, B. G.) 239–259 (Springer Nature, Cham, Switzerland, 2019).
Wright, B. M., Stredulinsky, E. H., Ellis, G. M. & Ford, J. K. B. Kin-directed food sharing promotes lifetime natal philopatry of both sexes in a population of fish-eating killer whales, Orcinus orca. Anim. Behav. 115, 81–95 (2016).
Bigg, M. A., Olesiuk, P. F., Ellis, G. M., Ford, J. K. B. & Balcomb, K. C. I. Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington state. Rep. Int. Whaling Comm. 12, 383–405 (1990).
Ford, J. K. B., Ellis, G. M. & Balcomb, K. C. I. Killer whales: the natural history and genealogy of Orcinus Orca in British Columbia and Washington (University of British Columbia Press, Vancouver, BC, Canada, 2000).
Matkin, C. O., Ellis, G. M., Saulitis, E. L., Barrett-Lennard, L. G. & Matkin, D. R. Killer whales of Southern Alaska (North Gulf Oceanic Society, Homer, AK, USA, 1999).
Danishevskaya, A. Y. et al. Crowd intelligence can discern between repertoires of killer whale ecotypes. Bioacoustics 29, 15–27 (2020).
Yurk, H., Filatova, O., Matkin, C. O., Barrett-Lennard, L. G. & Brittain, M. Sequential habitat use by two resident killer whale (Orcinus orca) clans in Resurrection Bay, Alaska, as determined by remote acoustic monitoring. Aquat. Mamm. 36, 67–78 (2010).
Baird, R. W. & Dill, L. M. Ecological and social determinants of group size in transient killer whales. Behav. Ecol. 7, 408–416 (1996).
Baird, R. W. & Whitehead, H. Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105 (2000).
Ford, J. K. B. & Ellis, G. M. Transients: mammal-hunting killer whales of British Columbia, Washington, and southeastern Alaska (University of British Columbia Press, Vancouver, BC, Canada, 1999).
Sharpe, D. L., Castellote, M., Wade, P. R. & Cornick, L. A. Call types of Bigg’s killer whales (Orcinus orca) in western Alaska: using vocal dialects to assess population structure. Bioacoustics 28, 74–99 (2019).
Ford, J. K. B. Acoustic behaviour of resident killer whales (Orcinus orca) off Vancouver Island, British Columbia. Can. J. Zool. 67, 727–745 (1989).
Quick, N. J. & Janik, V. M. Whistle rates of wild bottlenose dolphins (Tursiops truncatus): influences of group size and behavior. J. Comp. Psychol. 122, 305–311 (2008).
Luís, A. R., Couchinho, M. N. & Dos Santos, M. E. Signature whistles in wild bottlenose dolphins: long-term stability and emission rates. Acta Ethol. 19, 113–122 (2016).
Guazzo, R. A. et al. Migrating eastern North Pacific gray whale call and blow rates estimated from acoustic recordings, infrared camera video, and visual sightings. Sci. Rep. 9, 12617 (2019).
Martin, C. R. et al. North Pacific minke whales call rapidly when calling conspecifics are nearby. Front. Mar. Sci. 9, 897298 (2022).
Helble, T. A. et al. Fin whale song patterns shift over time in the central North Pacific. Front. Mar. Sci. 7, 587110 (2020).
Blackwell, S. B. et al. Effects of airgun sounds on bowhead whale calling rates: evidence for two behavioral thresholds. PLoS ONE. 10, e0125720 (2015).
Helble, T. A. et al. Minke whales change their swimming behavior with respect to their calling behavior, nearby conspecifics, and the environment in the central North Pacific. Front. Mar. Sci. 10, 1148987 (2023).
Hofmeyr-Juritz, L. & Best, P. Acoustic behaviour of southern right whales in relation to numbers of whales present in Walker Bay, South Africa. Afr. J. Mar. Sci. 33, 415–427 (2011).
Holt, M. M., Noren, D. P., Veirs, V., Emmons, C. K. & Veirs, S. Speaking up: killer whales (Orcinus orca) increase their call amplitude in response to vessel noise. J. Acoust. Soc. Am. 125, EL27–32 (2009).
Noad, M. J., Dunlop, R. A. & Mack, A. K. Changes in humpback whale singing behavior with abundance: implications for the development of acoustic surveys of cetaceans. J. Acoust. Soc. Am. 142, 1611 (2017).
Dunlop, R. & Frere, C. Post-whaling shift in mating tactics in male humpback whales. Commun. Biol. 6, 162 (2023).
Marques, T. A., Thomas, L., Ward, J., DiMarzio, N. & Tyack, P. L. Estimating cetacean population density using fixed passive acoustic sensors: an example with Blainville’s beaked whales. J. Acoust. Soc. Am. 125, 1982–1994 (2009).
Warren, V. E. et al. Spatio-temporal variation in click production rates of beaked whales: implications for passive acoustic density estimation. J. Acoust. Soc. Am. 141, 1962–1974 (2017).
Guazzo, R. A. et al. Migratory behavior of eastern North Pacific gray whales tracked using a hydrophone array. PLoS One. 12, e0185585 (2017).
Marques, T. A., Munger, L., Thomas, L., Wiggins, S. & Hildebrand, J. A. Estimating North Pacific right whale Eubalaena japonica density using passive acoustic cue counting. Endanger. Species Res. 13, 163–172 (2011).
Blackwell, S. B. et al. Estimating acoustic cue rates in bowhead whales, Balaena mysticetus, during their fall migration through the Alaskan Beaufort Sea. J. Acoust. Soc. Am. 149, 3611 (2021).
Parks, S. E. et al. Sound production behavior of individual North Atlantic right whales: implications for passive acoustic monitoring. Endanger. Species Res. 15, 63–76 (2011).
Stimpert, A. K. et al. Sound production and associated behavior of tagged fin whales (Balaenoptera physalus) in the Southern California Bight. Anim. Biotelemetry. 3, 23 (2015).
Guazzo, R. A., Stevenson, D. L., Edell, M. K., Gagnon, G. J. & Helble, T. A. A decade of change and stability for fin whale song in the North Atlantic. Front. Mar. Sci. 11, 1278068 (2024).
Ponce, D., Thode, A. M., Guerra, M., Urbán, R., Swartz, S. & J. & Relationship between visual counts and call detection rates of gray whales (Eschrichtius robustus) in Laguna San Ignacio, Mexico. J. Acoust. Soc. Am. 131, 2700–2713 (2012).
Marques, T. A. et al. Estimating animal population density using passive acoustics. Biol. Rev. 88, 287–309 (2013).
Simard, Y. et al. Estimating whale density from their whistling activity: example with St. Lawrence beluga. Appl. Acoust. 71, 1081–1086 (2010).
Olsen, D. W., Matkin, C. O., Andrews, R. D. & Atkinson, S. Seasonal and pod-specific differences in core use areas by resident killer whales in the Northern Gulf of Alaska. Deep Sea Res. Part. II: Top. Stud. Oceanogr. 147, 196–202 (2018).
Matkin, C. O., Matkin, D. R., Ellis, G. M., Saulitis, E. & McSweeney, D. Movements of resident killer whales in southeastern Alaska and Prince William Sound, Alaska. Mar. Mamm. Sci. 13, 469–475 (1997).
Pante, E. & Simon-Bouhet, B. marmap: A package for importing, plotting and analyzing bathymetric and topographic data in R. PLoS ONE. 8, e73051 (2013).
R Core Team. R: A language and environment for statistical computing. (2023).
NOAA National Centers for Environmental Information ETOPO 2022 15 Arc-Second global relief model. NOAA Natl. Centers Environ. Inform. https://doi.org/10.25921/FD45-GT74 (2022).
Gillespie, D. et al. PAMGuard: Semiautomated, open source software for real-time acoustic detection and localization of cetaceans. J. Acoust. Soc. Am. 125, 2547 (2009).
Myers, H. J., Olsen, D. W., Matkin, C. O., Horstmann, L. A. & Konar, B. Passive acoustic monitoring of killer whales (Orcinus orca) reveals year-round distribution and residency patterns in the Gulf of Alaska. Sci. Rep. 11, 20284 (2021).
NOAA National Centers for Coastal Ocean Science, Kasitsna Bay Laboratory. State of Kachemak Bay. 1–14 (2021). https://cdn.coastalscience.noaa.gov/projects-attachments/369/KachemakBay_StateoftheBay2021.pdf
Dugan, P. J. The Raven-X software package: A scalable high-performance computing framework for the analysis of large bioacoustics sound archives. Presentation at the Detection, Classification, Localization, and Density Estimation Workshop, Paris, France. (2018).
K. Lisa Yang Center for Conservation Bioacoustics. Raven Pro: Interactive sound analysis software. (2023).
North Gulf Oceanic Society. Photo-ID catalogue: Salmon-specialist residents. (2023). https://www.whalesalaska.org/salmon-specialist-residents
Kelley, D. & Richard, C. oce: Analysis of oceanographic data. (2023).
K. Lisa Yang Center for Conservation Bioacoustics. Signal-to-noise ratio (SNR) - NIST Quick method. (2022). https://ravensoundsoftware.com/knowledge-base/signal-to-noise-ratio-snr-nist-quick-method/
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R Stat. Soc. Ser. B Stat. Methodol. 73, 3–36 (2011).
Olsen, D. W., Matkin, C. O., Mueter, F. J. & Atkinson, S. Social behavior increases in multipod aggregations of southern Alaska resident killer whales (Orcinus orca). Mar. Mamm. Sci. 36, 1150–1159 (2020).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48 (2015).
Helble, T. A., D’Spain, G. L., Campbell, G. S. & Hildebrand, J. A. Calibrating passive acoustic monitoring: correcting humpback whale call detections for site-specific and time-dependent environmental characteristics. J. Acoust. Soc. Am. 134, EL400 (2013).
Miller, P. J. O. Diversity in sound pressure levels and estimated active space of resident killer whale vocalizations. J. Comp. Physiol. Neuroethol Sens. Neural Behav. Physiol. 192, 449–459 (2006).
Riera, A., Pilkington, J. F., Ford, J. K. B., Stredulinsky, E. H. & Chapman, N. R. Passive acoustic monitoring off Vancouver Island reveals extensive use by at-risk resident killer whale (Orcinus orca) populations. Endanger. Species Res. 39, 221–234 (2019).
Riera, A., Ford, J. K. & Chapman, N.R. Effects of different analysis techniques and recording duty cycles on passive acoustic monitoring of killer whales. J. Acoust. Soc. Am. 134, 2393–2404 (2013).
Stafford, K. M. Increasing detections of killer whales (Orcinus orca), in the Pacific Arctic. Mar. Mamm. Sci. 35, 696–706 (2019).
Ford, J. K. B. A catalogue of underwater calls produced by killer whales (Orcinus orca) in British Columbia. Can. Data Rep. Fisheries Aquat. Sci. No. 633, 165 p. (1987).
Filatova, O. A., Fedutin, I. D., Burdin, A. M. & Hoyt, E. The structure of the discrete call repertoire of killer whales Orcinus orca from southeast Kamchatka. Bioacoustics 16, 261–280 (2007).
Acknowledgements
Thank you to Sara Kirlin and Gabriela Alongi for support with data analysis and to John Ford, Tyler Helble, and two anonymous reviewers for feedback that improved this manuscript. Graeme Ellis completed photo-identification. We are grateful to Rob Campbell, David Janka, and Katrin Iken for assistance recovering hydrophones. Thank you to the numerous tour boat, water taxi, and fishing charter captains and deck hands who reported sightings and contributed photographs. Funding was provided by the North Gulf Oceanic Society, Exxon Valdez Oil Spill Trustee Council Gulf Watch Alaska Project 19120114-N, a National Defense Science and Engineering Graduate Fellowship from the Office of Naval Research, the National Science Foundation under award #OIA-1757348 and the State of Alaska, and a Coastal Marine Institute Graduate Student Funding Opportunity award. Passive acoustic monitoring and vessel surveys took place under National Marine Fisheries Service research permit #20341, primary permit holder Craig Matkin. The University of Alaska Fairbanks Institutional Animal Care and Use Committee approved the observational protocol (#1492735) under which this research was conducted.
Author information
Authors and Affiliations
Contributions
H.J.M. contributed Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original draft, Funding acquisition. D.W.O contributed Conceptualization, Investigation, Writing – Review & editing. B.H.K. contributed Writing – Review & editing, Supervision. A.W. contributed Data curation. K.B. contributed Data curation. P.D. contributed Software, Writing – Review & editing. F.M. contributed Formal analysis, Writing – Review & editing. R.A.G. contributed Writing – Review & editing. L.A.H. contributed Writing – Review & editing. C.O.M. contributed Investigation, Resources, Writing – Review & editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Myers, H.J., Olsen, D.W., Konar, B.H. et al. Killer whale call detection rates vary among subspecies and populations in the North Pacific. Sci Rep 15, 21072 (2025). https://doi.org/10.1038/s41598-025-06041-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06041-6