Abstract
Osteoarthritis (OAI) and osteoarthrosis (OAO) are subsets of degenerative joint diseases affecting the temporomandibular joint (TMJ), distinguished by the presence of joint pain. However, clinical diagnosis based on pain alone is challenging. This study aimed to differentiate OAI from OAO using imaging modalities, including single-photon emission computed tomography (SPECT-CT) and magnetic resonance imaging (MRI). Data from 65 patients (98 joints) who underwent MRI and SPECT-CT between January 2017 and December 2022 at Gangnam Severance Hospital were retrospectively analyzed. Patients were divided into the OAI and OAO groups based on the presence of TMJ pain and bony changes on MRI. Univariate and multivariate binary logistic regression analyses were performed to identify significant risk factors for two diseases. The maximum standardized uptake value (SUVmax) on SPECT-CT and disc shape on MRI were significantly different between the groups. Combining these two variables provided the highest diagnostic accuracy (area under the curve [AUC] = 0.889) compared with SUVmax or disc shape alone (AUC = 0.846 and AUC = 0.752, respectively). There was significant difference in AUC between disc shape and SUVmax + disc shape (p = 0.023). These findings suggest that the combination of SPECT-CT and MRI findings improves the differentiation between OAI and OAO.
Similar content being viewed by others
Introduction
Osteoarthritis (OAI) and osteoarthrosis (OAO) are subsets of degenerative joint diseases (DJD) affecting the temporomandibular joint (TMJ) that are distinguished by the presence of joint pain. However, the sensitivity and specificity of clinical criteria alone for diagnosing TMJ OAI are low1,2. In 2014, a group comprising several prominent researchers published improved diagnostic criteria for the temporomandibular joint (DC/TMD) based on the existing research diagnostic criteria for temporomandibular disorder3. However, precise diagnostic criteria for distinguishing OAI from OAO have not been established.
DJD is diagnosed based on morphological changes such as flattening, sclerosis, erosion, or osteophyte formation in the mandibular condyle or the presence of subcortical bone cysts on imaging studies such as computed tomography (CT), cone-beam CT, and magnetic resonance imaging (MRI)4,5,6 Among patients with DJD findings on imaging studies, those with and without arthralgia, are diagnosed with OAI and OAO, respectively3.
Wahaj et al.5 reported that bone marrow edema observed on MRI is helpful for diagnosing OA. However, other researchers have reported that cone-beam CT is an effective examination method that is cost-effective compared to CT and is more appropriate than MRI for evaluating bony changes7,8. Thus, identifying a single imaging study that is effective for TMJ OA diagnosis is difficult. Recently, efforts have been made to diagnose TMJ-OA using nuclear medicine9,10. Seo et al. (2016)11 reported that the use of the maximum standardized uptake value (SUVmax) in single-photon emission computed tomography (SPECT)-CT can be helpful in the diagnosis of arthralgic TMJ.
This study aimed to evaluate the usefulness of SPECT-CT and MRI for differentiating between OAI and OAO.
Materials and methods
Participants
Patients with chief complaints of pain or discomfort in the TMJ area who visited the Department of Oral and Maxillofacial Surgery, Gangnam Severance Hospital, Yonsei University (Seoul, Korea) between January 2017 and December 2022 were included in this retrospective study. MRI and SPECT-CT were performed at the Department of Radiology and Nuclear Medicine of the same hospital after clinical evaluation. OAO and OAI were diagnosed based on previous studies, including the DC/TMD classification and the clinical features of Wilkes classification stage IV or stage V3,12,13,14. The OAI and OAO groups included patients with bony changes on MRI with and without TMJ pain based on palpation of joint, respectively. That is, if there was pain during joint palpation, it was diagnosed as OAI, and if there was no pain during joint palpation or referral pain during masticatory muscle palpation, it was diagnosed as OAO.
The exclusion criteria were as follows: (1) interval between MRI and SPECT-CT > 6 months; (2) history of radiation therapy to the head and neck; (3) diagnosis of rheumatoid arthritis; (4) history of maxillofacial trauma or surgery, including the TMJ; and 4) bone changes on MRI without pain or with pain in the masticatory muscles rather than the joint area.
This study was approved by the Institutional Review Board of Gangnam Severance Hospital (approval no. #3-2024-0273), and the requirement for written informed consent was waived. This study complied with the tenets of the Declaration of Helsinki.
MRI Parameters.
TMJ MRI was performed using a 3.0-T Magnetom scanner (Achieva; Philips Medical Systems, Best, The Netherlands) with 3-inch surface coils. MRI conditions were identical to those in our previous study15. Briefly, for T1-weighted imaging, the following parameters were used: repetition time, 450 ms; echo time, 20 ms; slice thickness, 3 mm; field of view, 120 mm; and acquisition matrix size, 240 × 240. The parameters for T2-weighted imaging were as follows: repetition time, 2900 ms; and echo time, 90 ms.
MRI Evaluation.
The disc morphology, relationship between the disc and condyle, bone marrow signal, fluid collection, and joint space were analyzed using MRI based on a previous study15. MRI findings were interpreted by the consensus of two oral and maxillofacial surgeons (J.Y. Kim and J.K. Huh) with 15 and 30 years of experience, respectively.
Disc shape
The disc shape on closed-mouth images was categorized as biconcave, folded, flattened, eyeglass, or amorphous (Fig. 1). A “biconcave” disk was defined as a disk with structure and position within normal limits (WNL). Discs with a cap or cup shape, (∩- or ∪-shaped) without shortening in length on the anterior band, intermediate zone, and posterior band, were classified as a “folded”. Discs with a biplanar shape and loss of voluminous configuration of the anterior band, posterior band, or both were classified as “flattened”. Discs with anteroposterior shortening were classified as having an “eyeglass” shape. Deformed discs in which the configuration could not be distinguished were classified as “amorphous.” The higher category was recorded for discs with anterolateral or anteromedial displacement and those with more than two shapes16,17.
Relationship between the disc and condyle
The positional relationship between the disc and condyle was classified into the following three types (Fig. 2): normal, anterior disc displacement with reduction, and anterior disc displacement without reduction.
Bone marrow signal
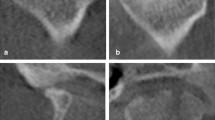
Bone marrow signal (BMS) was classified as “WNL” or “Low” compared to that of the ipsilateral ramus or body of the mandible based on the signal intensity on T1-weighted images. (Fig. 3)
BMS15 (a) The signal intensity of the condyle is similar that of the ramus, indicating normal BMS; (b) the asterisk indicates low BMS. BMS Bone marrow signal.
Joint effusion
Joint effusion was evaluated on T2-weighted images. Based on previous studies, it was classified into three types according to the amount of fluid collected (Fig. 4): G0, no fluid collection or only a thin line of high signal intensity;18 G1, fluid collection within the boundary of the disc; G2, moderate to large amount of fluid collection beyond the disc boundary or capsular expansion15,19.
Joint space
The joint space was classified into three categories: “normal,” “narrowed,” and “bone-to-bone contact.” (Fig. 5) Narrowing was defined as insufficient space for the disc between the condyle and fossa. Bone-to-bone contact was defined as complete or almost complete contact between the cortices of the condyle and fossa with no space between them on a closed-mouth image.
Joint space15. (a) Normal; (b) Narrowed (white arrow); (c) Bone to bone contact (white arrowhead). The dotted and solid yellow lines indicate the condyle and temporal bone, respectively.
SPECT-CT parameters
The parametes of SPECT-CT was based on our previous study20. SPECT-CT was conducted using the Symbia Intevo16 system (Siemens Healthineers, Erlangen, Germany). The scan covered the area from the top of the head to the lung apex, performed 3 h after administering 740 MBq of 99mTc-hydroxydiphosphonate. The imaging parameters included a 3° rotation per step, 20 s of acquisition time per projection, and a 512 × 512 matrix size. For the CT scan, low-dose images were acquired with settings of 110 kV and 120 mA, incorporating adaptive dose modulation. The reconstructed images were processed using xSPECT Quant and xSPECT Bone software (Siemens Healthineers, Erlangen, Germany) for high-resolution analysis and quantification20.
For each participant, regions of interest (ROIs) were manually delineated on the CT component of fused SPECT/CT images to quantify radiotracer uptake in TMJ. A spherical ROI of a 30-mm diameter was placed at the center of the mandibular condyle, carefully adjusted on axial and coronal CT images to include all relevant anatomical structures of the TMJ. The ROI was optimized to sufficiently cover the area with the highest radiotracer uptake while avoiding surrounding non-target tissues. The standardized uptake value (SUV) was calculated as follows: SUV = (decay-corrected activity [kBq/mL]) / (injected activity [kBq] / body weight [g]). SUVmax was defined as the highest voxel value within each ROI. Two nuclear medicine physicians (J.H. Lee and Y.H. Ryu) independently measured SUVmax for both left and right TMJs using the open-source software LIFEx (version 7.4.6; LIFEx, RRID: SCR_025284, www.lifexsoft.org21. Any discrepancies between the observers were resolved by consensus through a joint review of the images. Half of the patients were randomly selected to calculate inter-observer agreement.
Statistical analysis
The data were analyzed using SAS ver 9.4 (SAS Institute, Cary, NC, USA), SPSS (version 27.0; SPSS Inc., Chicago, IL, USA) and the R Statistical Package version 4.4.2 (Institute for Statistics and Mathematics, Vienna, Austria; www.R-project.org). Intraclass correlation coefficient was analyzed. Cut-off value for SUVmax was calculated using the Youden Index, and the receiver operating characteristic (ROC) curves were constructed, and the areas under the curves (AUC), sensitivity, specificity, accuracy, PPV, and NPV was calculated. Number of joints between OAI and OAO based on SUVmax cut-off value was analyzed using chi-square test. Univariate and multivariate binary logistic regression analyses were performed to identify the risk factors for OAI and OAO. Multivariate regression analysis was conducted in a stepwise manner. AUC were compared with multiple comparison by Bonferroni correction. Statistical significance was set at p < 0.05.
Results
This study included 65 patients (98 joints). The OAI and OAO groups included 62 joints (51 patients; 5 men and 46 women; age, 45.96 ± 17.35 years) and 36 joints (28 patients; 2 men and 26 women; age, 45.00 ± 17.32 years), respectively. Sixteen patients were diagnosed with OAI on one side and OAO on the other; therefore, they were included in both groups. Table 1 presents a comparison of the clinical characteristics between the groups. The inter-observer agreement for SUVmax measurements was excellent, showing an intraclass correlation coefficient of 0.973 (95% CI: 0.956–0.983).
The optimal cut-off based on SUVmax to distinguish OAI and OAO was 5.64. The AUC was 0.850 (95% confidence internal (CI) 0.770–0.920) with sensitivity, 0.66 (95% CI 0.54–0.78); specificity, 0.94 (95% CI 0.87–1.00); accuracy, 0.77 (95% CI 0.68–0.85); positive predictive value (PPV), 0.95 (0.89-1.00); and negative predictive value (NPV), 0.62 (95% CI 0.49–0.75). There thirty-four out of 36 (94.44%) whose SUVmax was under 5.64 in OAO group, whereas 41 out of 62 (66.13%) patients whose SUVmax was over 5.64 in OAI group (p < 0.001). They are summarized in Table 2.
In the univariate logistic regression analyses, all variables except BMS showed significant differences between the OAO and OAI groups. In multivariate logistic regression analysis (R2 = 0.562), SUVmax and disc shape were risk factors for OAI but not for OAO. Particularly, a 1-point increase in SUVmax increased the risk of OAI by 2.11 times. (Table 3)
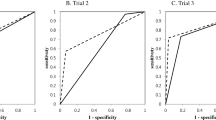
The AUC for the disc shape was the lowest (0.752; range, 0.651–0.854), and the AUC for SUVmax was 0.846 (range, 0.771–0.921). However, the AUC was the highest (0.889; range, 0.825–0.952) when SUVmax and disc shape were considered simultaneously (SUVmax + disc shape). AUCs were not significantly different between disc shape and SUVmax (p = 0.391) and between SUVmax and SUVmax + disc shape (p = 0.125). However, there was significant difference in AUC between disc shape and SUVmax + disc shape (p = 0.023). (Fig. 6)
Discussion
In the DC/TMD published in 2014, OAO and OAI were classified separately as subsets of DJD3. The two diseases are distinguished by the presence of arthralgia; nonetheless, some studies use the two terms interchangeably, and the distinction is ambiguous even in the field of orthopedics22. Furthermore, a complaint of pain can cause confusion in diagnosis.
In this study, disc shape alone had the lowest predictive power (0.752; range, 0.651–0.854), whereas SUVmax had a slightly higher power (0.846; range, 0.771–0.921). The highest predictive power (0.889; range, 0.825–0.952) was achieved when SUVmax and disc shape were considered simultaneously. Nuclear imaging study itself is a good modality for diagnosis of osteoarthritis20,23. Therefore, when considering the AUC value, it is thought that using the SUVmax value in SPECT-CT would be helpful to distinguish between OAI and OAO. The optimal SUVmax cut-off for differentiating OAI and OAO was 5.64. In particular, 34 out of 36 joints diagnosed with OAO showed SUVmax values below 5.64. Therefore, cases diagnosed below the cut-off value are interpreted as having a higher possibility of osteoarthosis than osteoarthritis. In addition, we suppose that a combination of SUVmax on SPECT-CT and disc shape on MRI is more effective than a single imaging test. This is consistent with the results of Hussain et al. (2008)8 that using multiple tests comprehensively, rather than a single imaging test, is more accurate for diagnosing erosion and osteophytes.
Several studies have investigated the relationship between TMJ pain and MRI findings. Although some studies have reported a correlation between MRI features and TMJ pain24,25others have shown that MRI signs of OAI do not always coincide with the cause of pain26,27. Higuchi et al. (2020)28 reported a significant correlation between reduced anterior disc displacement and joint effusion, and TMJ pain, which is similar to our results. However, MRI alone has several limitations in diagnosing TMJ-OA. Particularly, differentiating between OAI and OAO, which are diagnosed based on pain, is difficult. Toshima et al.26 analyzed several characteristics of patients with OAO with bone changes on MRI. In this study, TMJ pain was not significantly correlated with bone changes. Thus, determining the presence of TMJ pain based on bone changes alone was difficult, and other tests were necessary.
Nuclear medicine can be used to evaluate active lesions relatively early because it reflects the bone metabolism. Among various nuclear medicine examinations, SPECT-CT using 99mTc methylene diphosphonate is useful for evaluating the local osteometabolic activity29. In addition, it is helpful for diagnosing arthralgic condyles11. In particular, if it exceeds 5.64 and the disc shape is amophous, the possibility of osteoarthrisis is much higher. However, the extent of bony changes on imaging studies and pain are not necessarily correlated30. Therefore, we believe that a more objective examination is needed to distinguish OAI from OAO and that using multiple imaging modalities in combination is necessary.
This study had several limitations. First, since this study aimed to investigate SPECT-CT and MRI characteristics, clinical symptoms such as limited mouth opening and their correlation with imaging findings were not considered. Second, the classification was based on the history of pain. This is an inevitable choice because pain is the clinical diagnostic criterion for differentiating between OAO and OAI; however, pain can be subjective and pain in the joint area can originate from the surrounding soft tissues or masticatory muscles31. Third, age and sex were not considered during SPECT-CT evaluation. More accurate results can be obtained if these details were investigated in more patients. Forth, the duration and severity were not considered. According to the study of Jung32the degree of bone marrow sclerosis varies depending on the duration of osteoarthritis. Accordingly, it is assumed that the SUV value of SPECT-CT may also be affected. Finally, the 95% confidence interval is wide in logistic regression analysis. This is thought to be because the number of joints included in some items is somewhat small. If the total number of joints increases, this phenomenon may be reduced, but this phenomenon has also been observed in some other papers comparing pathological and normal conditions15. Therefore, care must be taken in interpretation.
In conclusion, we suppose considering SUVmax on SPECT-CT is helpful for differentiating OAI from OAO. In addition, combination of both SUVmax on SPECT-CT and the disc shape on MRI would be more effective than using a single imaging method. Future studies using various examination modalities are warranted.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Delpachitra, S. & Dimitroulis, G. Osteoarthritis of the temporomandibular joint: a review of aetiology and pathogenesis. Br. J. Oral Maxillofac. Surg. 60, 387–396 (2022).
Kopp, S. Degenerative and inflammatory temporomandibular joint disorders: clinical perspectives. Temporomandibular Disorders Relat. Pain Conditions. 4, 119–131 (1995).
Schiffman, E. et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network** and orofacial pain special interest groupdagger. J. Oral Facial Pain Headache. 28, 6–27 (2014).
Li, L., Shi, H., Xie, H. & Wang, L. MRI assessment and histopathologic evaluation of subchondral bone remodeling in temporomandibular joint osteoarthritis: a retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 126, 355–362 (2018).
Wahaj, A., Hafeez, K. & Zafar, M. S. Association of bone marrow edema with temporomandibular joint (TMJ) osteoarthritis and internal derangements. CRANIO®35, 4–9 (2017).
Shi, J. et al. Association of condylar bone quality with TMJ osteoarthritis. J. Dent. Res. 96, 888–894 (2017).
Kiliç, S., Kiliç, N., Güven, F. & Sümbüllü, M. Is magnetic resonance imaging or cone beam computed tomography alone adequate for the radiological diagnosis of symptomatic temporomandibular joint osteoarthritis? A retrospective study. Int. J. Oral Maxillofac. Surg. 52, 1197–1204 (2023).
Hussain, A., Packota, G., Major, P. & Flores-Mir, C. Role of different imaging modalities in assessment of temporomandibular joint erosions and osteophytes: a systematic review. Dentomaxillofacial Radiol. 37, 63–71 (2008).
Jeon, S. H. et al. The clinical effectiveness of fused image of single-photon emission CT and facial CT for the evaluation of degenerative change of mandibular condylar head. Maxillofacial Plast. Reconstr. Surg. 45, 33 (2023).
Munakata, K. et al. The use of SPECT/CT to assess resorptive activity in mandibular condyles. Int. J. Oral Maxillofac. Surg. 51, 942–948 (2022).
Suh, M. S., Lee, W. W., Kim, Y. K., Yun, P. Y. & Kim, S. E. Maximum standardized uptake value of (99m)Tc hydroxymethylene diphosphonate SPECT/CT for the evaluation of temporomandibular joint disorder. Radiology 280, 890–896 (2016).
Kim, J. H. et al. Effectiveness of bone scans in the diagnosis of osteoarthritis of the temporomandibular joint. Dentomaxillofac Radiol. 41, 224–229 (2012).
Wilkes, C. H. Internal derangements of the temporomandibular joint. Pathological variations. Arch. Otolaryngol. Head Neck Surg. 115, 469–477 (1989).
Dimitroulis, G. The prevalence of osteoarthrosis in cases of advanced internal derangement of the temporomandibular joint: a clinical, surgical and histological study. Int. J. Oral Maxillofac. Surg. 34, 345–349 (2005).
Kim, J. Y., Jeon, K. J., Kim, M. G., Park, K. H. & Huh, J. K. A nomogram for classification of temporomandibular joint disk perforation based on magnetic resonance imaging. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 125, 682–692 (2018).
Huh, J. K., Kim, H. G. & Ko, J. Y. Magnetic resonance imaging of temporomandibular joint synovial fluid collection and disk morphology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 95, 665–671 (2003).
Paesani, D., Westesson, P. L., Hatala, M. P., Tallents, R. H. & Brooks, S. L. Accuracy of clinical diagnosis for TMJ internal derangement and arthrosis. Oral Surg. Oral Med. Oral Pathol. 73, 360–363 (1992).
Westesson, P. & Brooks, S. Temporomandibular joint: relationship between MR evidence of effusion and the presence of pain and disk displacement. AJR Am. J. Roentgenol. 159, 559–563 (1992).
KIM, H. G. et al. A retrospective study on the relationship between MRI evidence of temporomandibular joint effusion and clinico-surgical findings. J. Korean Assoc. Oral Maxillofacial Surgeons, 133–140 (1999).
Kim, J. Y. et al. Diagnostic criteria for temporomandibular joint osteoarthritis using standardized uptake value in single-photon emission computed tomography–computed tomography. Sci. Rep. 14, 31569 (2024).
Nioche, C. et al. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 78, 4786–4789 (2018).
Al-Ani, Z. & Temporomandibular Joint Osteoarthrosis A review of clinical aspects and management. Prim. Dent. J. 10, 132–140 (2021).
Lee, Y. H., Hong, I. K. & Chun, Y. H. Prediction of painful temporomandibular joint osteoarthritis in juvenile patients using bone scintigraphy. Clin. Experimental Dent. Res. 5, 225–235 (2019).
Ogura, I. Magnetic resonance imaging characteristics of temporomandibular joint pain during opening and biting in patients with disc displacement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology. 102, 669–672 (2006).
Takahara, N. et al. Association of temporomandibular joint pain according to magnetic resonance imaging findings in temporomandibular disorder patients. J. Oral Maxillofac. Surg. 75, 1848–1855 (2017).
Toshima, H. & Ogura, I. Characteristics of patients with temporomandibular joint osteoarthrosis on magnetic resonance imaging. J. Med. Imaging Radiat. Oncol. 64, 615–619 (2020).
Orhan, K., Seki, U. & Rozylo-Kalinowska, I. Diagnostic accuracy of magnetic resonance imaging and clinical signs of temporomandibular joint disorders: a 10-year research update review. Oral Radiol. 33, 81–91 (2017).
Higuchi, K. et al. Relationship between temporomandibular joint pain and magnetic resonance imaging findings in patients with temporomandibular joint disorders. Int. J. Oral Maxillofac. Surg. 49, 230–236 (2020).
Coutinho, A., Fenyo-Pereira, M., Dib, L. L. & Lima, E. N. The role of SPECT/CT with 99mTc-MDP image fusion to diagnose temporomandibular dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 101, 224–230 (2006).
Lamot, U., Strojan, P. & Popovič, K. Š. Magnetic resonance imaging of temporomandibular joint dysfunction-correlation with clinical symptoms, age, and gender. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 116, 258–263 (2013).
Okeson, J. P. Management of temporomandibular disorders and occlusion 8th edition (Elsevier, 2013).
Jung, J. H. Hounsfield unit and maximum standardized uptake value of temporomandibular joint osteoarthritis in SPECT/CT in Thesis(Master’s thesis) -- Yonsei University, Graduate School Department of Dentistry,. 2 (Graduate School, Yonsei University, 2023).
Acknowledgements
We thank Soyoung Jeon, PhD (biostatistician), and Eun-Ju Lee, MS (biostatistician) of the Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Republic of Korea, for their help with the statistical analyses.
Author information
Authors and Affiliations
Contributions
J.-Y. Kim and C. Lee wrote the original draft; J.-Y. Kim, J.-H. Lee, and J.-K. Huh designed the research; J.-Y. Kim, C. Lee, M.-J. Kang, J.-H. Lee, and Y.H. Ryu collected and analyzed the data. H.-S. Kim, J.-H. Lee and J.-K. Huh revised the manuscript and edited the manuscript; All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the institutional review board of Yonsei University Gangnam Severance Hospital approved this retrospective study (IRB No. 3-2024-0273), and waiver of written informed consent for this retrospective study. This study was also conducted according to the principles of the Declaration of Helsinki for research on humans.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, JY., Lee, C., Lee, JH. et al. Comparison of the maximum standardized uptake value and magnetic resonance imaging characteristics between temporomandibular joint osteoarthrosis and osteoarthritis. Sci Rep 15, 23542 (2025). https://doi.org/10.1038/s41598-025-06045-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06045-2