Abstract
To observe the efficacy and safety of Ripertamab in the treatment of Idiopathic Membranous Nephropathy (IMN). Clinical data from patients with IMN treated with Ripertamab or Rituximab were retrospectively collected from six tertiary hospitals in Jilin Province between January and December 2023. Patients were grouped based on treatment regimen into the Ripertamab and Rituximab groups and matched 1:1 by age and gender. Follow-ups were conducted over six months to assess baseline characteristics, laboratory parameters, and adverse reactions related to anti-CD20 monoclonal antibody therapy. A total of 112 patients with IMN were identified, including 52 treated with Ripertamab and 60 with Rituximab. After matching, 40 patients were included in each group. Baseline clinical characteristics were comparable between the groups (P > 0.05). There was no statistically significant difference in efficacy between the two groups (P > 0.05). At 6 months, the overall effectiveness rate of Ripertamab in the treatment of IMN was 65.0%, of which the partial and complete remission rates were 50.0% and 15.0%, respectively. The overall effectiveness rate of Rituximab was 60.0%, of which the partial and complete remission rates were 47.5% and 12.5%, respectively. Similarly, there were no significant differences in the incidence of infusion reactions, pulmonary infections, interstitial lung disease, HBV reactivation, neutropenia, or thrombocytopenia (P > 0.05). Ripertamab demonstrates a therapeutic efficacy comparable to Rituximab for IMN, with a similar incidence of infusion-related adverse reactions and complications.
Similar content being viewed by others
Membrane nephropathy (MN) is a common pathological form of nephrotic syndrome with a steadily increasing incidence in recent years1. While the gold standard for diagnosing idiopathic membranous nephropathy (IMN) remains the pathological examination of renal tissue using light and electron microscopy post-biopsy, recent studies have identified that approximately 80% of IMN cases are associated with antibodies targeting specific podocyte antigens, such as PLA2R and/or THSD7A2,3. From 2005 to 2018, the prevalence of IMN among primary glomerular diseases rose significantly, from 8.8–52.3%4. This growing prevalence has heightened scholarly interest in IMN.
Traditional treatment strategies for IMN have involved corticosteroids combined with immunosuppressants. However, biologics, particularly anti-CD20 monoclonal antibodies, are increasingly used in clinical practice. International multicenter randomized controlled trials have consistently demonstrated the efficacy of Rituximab in treating IMN5,6,7. Therefore, in the 2021 version of the KDIGO guidelines, Rituximab is recommended as the first-line treatment for IMN. However, adverse reactions associated with Rituximab use have also been reported8, such as infusion-related reactions (IRR), interstitial pneumonia, pulmonary infection, HBV reactivation, neutropenia, and thrombocytopenia9. Additionally, Rituximab has a slow onset of action, often necessitating adjunctive therapies with glucocorticoids or immunosuppressants10. These limitations underscore the need for safer and more effective therapeutic options.
Ripertamab, a novel anti-CD20 monoclonal antibody, represents a promising alternative. Developed by China Sino Cell Tech Engineering Co., Ltd., Ripertamab is a human-mouse chimeric antibody composed of 30% mouse-derived variable regions (Fab) and 70% human IgG1 constant regions (Fc). Both Ripertamab and Rituximab bind specifically to the transmembrane CD20 antigen. While their antigen-binding sites and variable region amino acid sequences are identical, Ripertamab’s constant region features the G1m (1,17) IgG1 allotype, which differs from Rituximab by a single amino acid at position 219 in the CH1 region (valine in Ripertamab vs. alanine in Rituximab). A phase III randomized, single-blind, multicenter trial conducted across 37 centers in China demonstrated that Ripertamab is as safe and effective as Rituximab in treating diffuse large B-cell lymphoma11. However, its efficacy and safety in IMN treatment remain unestablished. This study aims to evaluate the clinical outcomes of Ripertamab compared to Rituximab in patients with IMN, providing evidence to guide its clinical application.
Objects and methods
Study objects
Retrospective collection of clinical data from IMN patients who received treatment with Ripertamab monoclonal antibody or Rituximab at six tertiary hospitals, including The Second Norman Bethune Hospital of Jilin University, China-Japan Friendship Hospital of Jilin University, Jilin Province People’s Hospital, Jilin Province FAW General Hospital, Yanbian University Affiliated Hospital, and Jilin Central General Hospital during January 1–December 31, 2023, and were regularly followed up for 6 months. Based on the medication plan, the patients were classified into the Ripertamab group and the Rituximab group, matched 1:1 according to gender and age. We confirming that all methods were carried out in accordance with relevant guidelines and regulations. We confirming that informed consent was obtained from all subjects or their legal guardians and that all experimental protocols were approved by the ethics committee. The ethical approval number is Ethics Committee of the Second Hospital of Jilin University (2025028).
Selection criteria
The following were the patient inclusion criteria: ① patients diagnosed with IMN through renal biopsy or PLA2R antibody positivity; ② patient age ≥ 18 years; ③ eGFR ≥ 30 mL·min− 1·(1.73m2)−1; ④ urinary protein ≥ 3.5 g/day; ⑤ The main treatment regimen is Rituximab or Ripertamab (including patients who have used or combined glucocorticoids and immunosuppressants). The following are the exclusion criteria: ① combining pregnancy, stroke, liver disease, and tumors; ② incomplete clinical data available.
Data collection
The following clinical data were collected from the enrolled patients:
(1) basic information: gender, age, body mass index (BMI), history of hypertension, history of diabetes, history of past medication, current medication (anti-CD20 monoclonal antibody combined with glucocorticoid hormone or immunosuppressive agent).
(2) Risk evaluation of IMN: Clinical criteria for assessing risk of progressive loss of kidney function is based on KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases8.
(3) Routine laboratory tests: results of white blood cell count, neutrophil count, platelet count, hemoglobin, albumin, Albumin-globulin ratio, creatinine, uric acid, blood urea nitrogen, eGFR, 24-h urine protein quantification before and after treatment with anti-CD20 monoclonal antibody, as well as immunoglobulin, immune routine, lymphocyte subsets, PLA2R antibody, chest CT (only for baseline abnormalities, rechecked at 3 and 6 months).
Study outcomes
(1) Primary outcomes: Therapeutic effect: ① Complete remission: 24-h urinary protein quantification < 0.3 g/day and serum albumin level ≥ 35 g/L. ② Partial remission: 24-h urine protein quantification decreased by ≥ 50% and ≤ 3.5 g/day compared to the baseline value. ③ Total remission: complete remission and partial remission.
(2) Secondary outcomes: ① Infusion-related reaction: the general term for non-hematologic toxic reactions that occur within 24 h of infusion. ②interstitial lung disease: interstitial lung disease refers to the inflammation of the interstitial tissue of the lungs, mainly by invading the bronchial and alveolar walls, especially the connective tissue between the lobules and alveolar septa around the bronchial vessels, often presenting as necrotic lesions. The forms of drug-induced interstitial lung diseases include: non-specific interstitial pneumonia, eosinophilic pneumonia, allergic pneumonia, and pulmonary fibrosis. ③ HBV reactivation in HBV-infected patients: HBsAg-positive patients who met any of the following conditions were defined as a case of HBV reactivation: serum HBsAg conversion to positive; the serum HBV-DNA changed from undetectable to detectable. ④ Neutropenia: a syndrome characterized by a decrease in the absolute value of peripheral blood neutrophils. The absolute value of neutrophils in adult peripheral blood is < 2.0 × 109/L. ⑤ Thrombocytopenia: a syndrome characterized by a decrease in the absolute value of peripheral blood platelets. The absolute value of adult peripheral blood platelets is < 100 × 109/L.
Treatment regimens and follow up
To prevent the development of IRR, all patients were administered anti-allergic drugs such as dexamethasone, non-steroidal anti-inflammatory drugs, and promethazine injection 30 min before Ripertamab or Rituximab for IMN treatment.
The Ripertamab or Rituximab was administered using the “two-dose method”, that is, the first dose was 1000 mg, prepared using an equal amount of 0.9% sodium chloride solution, administered intravenously. Moreover, we initiated the infusion of the CD20 monoclonal antibody at a speed of 25 ml/h. If the patient did not experience significant discomfort, the speed was adjusted upwards at intervals of 30 min to 50 ml/h, 100 ml/h, 150 ml/h, and 200 ml/h. The remaining CD20 monoclonal antibody solution was infused at a speed of 200 ml/h until completion. The entire infusion process lasted no less than 5 h (if the patient had an IRR, the infusion speed was reduced appropriately. If the IRR was severe, the infusion was stopped immediately and symptomatic treatment was provided).
The second dose was administered two weeks later, with the same dose of 1000 mg as described above.
Observation endpoint: patients were followed up at 1, 3, and 6 months after starting CD20 monoclonal antibody treatment, using end-stage renal disease or death as the endpoint12.
Statistical analysis
The data analysis was conducted by using SPSS 26.0 statistical software. Quantitative data that follow a normal distribution are represented in ± s form, and a t-test was used for inter-group comparison; \(\bar {x}\)Quantitative data that did not follow a normal distribution were represented in the form of M (P25, P75), and rank-sum tests are used for inter-group comparisons. Count data was expressed in frequency or percentage, and comparison between the groups was performed using the χ2 test or Fisher’s exact probability method. P < 0.05 was considered to indicate a statistically significant difference.
Results
Baseline clinical data of ripertamab and rituximab in the treatment of IMN
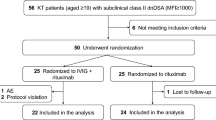
Between January 1 and December 31, 2023, 112 patients with IMN received treatment with anti-CD20 monoclonal antibodies. Of these, 52 were treated with Ripertamab and 60 with Rituximab. After matching by gender and age, 40 patients were included in each group (Ripertamab group: n = 40; Rituximab group: n = 40). Baseline clinical parameters, including serum albumin, serum creatinine, eGFR, 24-hour urine protein quantification, anti-PLA2R antibodies, B-cell percentage, and immunoglobulin G levels, showed no significant differences between the two groups (P > 0.05) (Table 1).
The efficacy of ripertamab and rituximab in the treatment of IMN 1, 3, and 6 months
Follow-up at 1, 3, and 6 months revealed no significant differences between the Ripertamab and Rituximab groups in 24-hour urine protein quantification, proportions of patients achieving 24-hour urine protein levels < 0.3 g/d or < 3.5 g/d, reductions in 24-hour urine protein levels > 50%, serum albumin, serum creatinine, eGFR, or B-cell percentage (P > 0.05) (Table 2).
The effective rates of ripertamab and rituximab in the treatment of IMN1, 3, and 6 months
At 1, 3, and 6 months, the complete remission rates, partial remission rates, and total remission rates were comparable between the Ripertamab and Rituximab groups, with no significant differences observed (P > 0.05) (Table 3).
Adverse reactions of ripertamab and rituximab in the treatment of IMN
Adverse reactions were monitored for at least six months. The incidence of IRR in the Ripertamab group was 2.5%, while that in the Rituximab group was 15%, however, this difference was not statistically significant (P = 0.481). Similarly, pulmonary infections in the Ripertamab group were not significantly different from those in the Rituximab group (20% vs. 30%, P = 0.364). Cases of interstitial lung disease, HBV reactivation, neutropenia, and thrombocytopenia were also comparable between the two groups, with no significant differences (P > 0.05; Table 4).
Discussion
This multicenter clinical study is the first to evaluate the efficacy and safety of Ripertamab in treating IMN. After matching, patients were divided into Ripertamab and Rituximab groups. The results demonstrated no significant differences in baseline clinical characteristics, efficacy, or remission rates at 1, 3, and 6 months between the two groups. Furthermore, adverse events were similarly distributed between groups. Thus, the therapeutic effectiveness and safety profiles of Ripertamab and Rituximab in IMN appear comparable.
IMN is a prevalent form of glomerular disease, with a rising incidence that now ranks it second1 in patients with primary glomerular disease. Currently, IMN is considered an autoimmune disease, and the M-type phospholipase A2 receptor (PLA2R) on the surface of podocytes is the main target antigen of IMN, which can be seen in 70 -80% of IMN patients13. Moreover, new antigens have recently been discovered, such as 7 A containing platelet reactive protein type 1 domain, neuroepidermal growth factor like 1 protein, and signal protein 3b14, further validate the rationale for B-cell depletion therapies. CD20 monoclonal antibodies, such as Rituximab, target the CD20 antigen expressed on B-cell membranes, effectively depleting B cells and reducing autoantibody production15. Ripertamab, a recombinant human-mouse chimeric anti-CD20 IgG1 monoclonal antibody, shares the same variable region and antigen-binding site as Rituximab but differs in its constant region. Specifically, Ripertamab has a valine residue at position 219 in the CH1 domain, compared to alanine in Rituximab, reflecting an adaptation to the G1m (1,17) IgG1 isotype commonly found in Asian populations11. The specification of Ripertamab is currently the most widely used CD20 monoclonal antibody (including 500 mg, 50 mg, and 10 mg). Ripertamab has demonstrated comparable pharmacokinetics, pharmacodynamics, and safety to Rituximab in phase II studies involving CD20-positive non-Hodgkin lymphoma11. In phase III trials, Ripertamab was found to be as effective as Rituximab in combination with CHOP therapy for diffuse large B-cell lymphoma and showed similar immunogenicity and safety profiles8. However, data on its use in IMN were previously unavailable, prompting this investigation. In our study, Ripertamab showed comparable outcomes to Rituximab in treating IMN over 6 months. Both groups exhibited significant improvements, with increased serum albumin and eGFR and reduced 24-hour urine protein quantification, serum creatinine, B-cell counts, and anti-PLA2R antibodies. These findings indicate that Ripertamab follows a similar therapeutic trajectory to Rituximab in IMN management. The 2021 KDIGO guidelines currently recommend Rituximab as the first-line treatment for IMN8. Therefore, Ripertamab is expected to emerge as the first-line treatment option for patients with IMN.
In terms of remission rates, our findings reveal that nearly two-thirds of patients in both the Ripertamab and Rituximab groups achieved partial or complete remission at 6 months (65% vs. 60%). This outcome aligns with several previous prospective studies7. Additionally, over 80% of patients in both groups achieved B-cell depletion (85% vs. 82.5%), although no significant correlation was observed between B-cell depletion and remission rates. Similarly, the GEMRITUX trial reported that B-cell counts do not reliably predict proteinuria remission. Instead, the depletion of anti-PLA2R antibodies has been identified as a more significant predictor of response to CD20 monoclonal antibody therapy. This discrepancy may arise due to the sustained presence of B cells in secondary lymphoid organs16. Our findings are consistent with those of Fervenza et al.5, who concluded that B-cell depletion is not a reliable predictor of treatment response. Hofstra et al.17 also reported that spontaneous remission occurred in 38% of patients with low anti-PLA2R antibody titers but was rare in those with high titers. These observations underscore the expanding role of anti-PLA2R antibodies from diagnostic and prognostic markers to therapeutic indicators. Consequently, we recommend that Ripertamab dosing in IMN treatment should be guided by PLA2R antibody levels rather than B-cell depletion rates.
CD20 monoclonal antibodies, including Ripertamab and Rituximab, can induce IRR during IMN treatment. IRR encompasses a range of non-hematologic adverse events occurring within 24 h of infusion. Common symptoms include fever and chills, often within 30 min to 2 h after the first infusion, which tend to diminish with subsequent treatments. Other symptoms may include flushing, urticaria, rash, pruritus, headache, fatigue, nausea, vomiting, bronchospasm, dyspnea, laryngeal edema, hypotension, and arrhythmias18. IRR is attributed to complement activation and cytokine release triggered by the binding of CD20 monoclonal antibodies to CD20+B cells9. To mitigate IRR risk, premedication was administered before CD20 monoclonal antibody infusion, consisting of 25 mg intramuscular promethazine, 5 mg intravenous dexamethasone, and 650 mg oral acetaminophen. In our study, the incidence of IRR in the Ripertamab group was not statistically different from that in the Rituximab group (2.5% vs. 15%, P > 0.05). This observation warrants further investigation in larger-scale studies to confirm its clinical significance.
CD20 monoclonal antibodies can cause various adverse reactions during the treatment of IMN, with pulmonary toxicity being the most common. Pulmonary toxicity may present as interstitial lung disease, pulmonary infection, alveolar hemorrhage, or adult respiratory distress syndrome, with interstitial lung disease and pulmonary infections being the most frequent manifestations19. The exact pathogenesis of CD20 monoclonal antibody-induced lung diseases remains largely unknown. Current theories suggest that CD20 monoclonal antibodies bind to CD20 + B cells and induce cytotoxicity through mechanisms such as B-cell signaling, complement activation, direct apoptosis, and antibody-dependent cytotoxicity20. Given the increased infection risk associated with CD20 monoclonal antibodies, expert guidelines recommend the co-administration of compound sulfamethoxazole to prevent opportunistic infections, particularly pulmonary infections caused by Pneumocystis carinii21. In our study, compound sulfamethoxazole was routinely administered alongside CD20 monoclonal antibody therapy to mitigate this risk. The findings showed that the incidence of interstitial lung disease and pulmonary infections within 6 months of treatment with Ripertamab was comparable to that with Rituximab, indicating similar mechanisms of pulmonary toxicity between the two agents. Besides pulmonary toxicity, other adverse reactions to CD20 monoclonal antibodies include HBV reactivation, neutropenia, and thrombocytopenia. Before initiating CD20 monoclonal antibody therapy, HBV screening was conducted for all IMN patients. Patients with active HBV infection were treated for HBV and excluded from CD20 monoclonal antibody therapy. For those with inactive HBV infection, antiviral drugs such as entecavir were co-administered with CD20 monoclonal antibodies to prevent HBV reactivation22. Before administration of CD20 monoclonal antibodies, patients at risk of experiencing hepatitis B virus reactivation were given oral entecavir for prevention. Neutropenia and thrombocytopenia were also observed in some patients after treatment with CD20 monoclonal antibodies. While preventive measures for these conditions are not feasible, routine monitoring of blood parameters is essential. In cases of severe neutropenia (neutrophil count < 0.5 × 10⁹/L) or thrombocytopenia (platelet count < 30 × 10⁹/L), CD20 monoclonal antibody treatment should be discontinued, and appropriate interventions should be initiated. These include prophylactic antibacterial and antiviral therapy for prolonged neutropenia (> 1 week) and platelet transfusions for severe thrombocytopenia23. In our study, while some patients experienced neutropenia or thrombocytopenia following Ripertamab or Rituximab treatment for IMN, no severe cases were reported. Consequently, CD20 monoclonal antibody therapy was not discontinued in any patient due to these complications.
During the 6-month follow-up period, one patient in the Ripertamab group showed poor response to the treatment and progressed to end-stage renal disease (ESRD), necessitating the initiation of regular dialysis. However, in the Rituximab group, one patient progressed to ESRD at the 6th month after treatment but did not require dialysis. The patient’s renal biopsy result was stage II membranous nephropathy. Despite treatment with prednisone, cyclophosphamide, and tacrolimus, the patient’s condition did not improve and the patient was put on Rituximab treatment. The patient’s eGFR was already 16.21 [ml·min− 1·(1.73m2)−1] (serum creatinine 350 µmol/L) at the first Rituximab treatment. After completing two doses of treatment, the patient was regularly followed up, and the eGFR gradually decreased, reaching 7.17 [ml·min− 1·(1.73m2)−1] (serum creatinine 687 µmol/L) at the 6th month after treatment. The patient received a second renal biopsy on the 7th month after treatment, which showed stage II-III membranous nephropathy with subacute tubulointerstitial nephritis. In addition, the patient underwent arteriovenous fistula surgery in preparation for regular hemodialysis. However, patient’s response to Rituximab was poor.
During the 6-month follow-up period, no patient died in the Ripertamab group, but one patient experienced chills during Rituximab treatment, and the symptoms disappeared when the infusion rate was reduced. One month after completing two doses of treatment, the patient died of severe pneumonia due to infection.
Our study has several limitations. First, it is retrospective, which may introduce inherent biases. Second, the follow-up period for assessing the efficacy and safety of Ripertamab in IMN treatment was only 6 months, limiting our ability to evaluate its long-term effects, and the clinical data were insufficient to assess the recurrence rate. Third, although this was a multicenter study, the sample size for patients receiving Ripertamab was relatively small. To address these limitations, we recommend expanding the study to include more centers and a larger patient cohort to enhance the reliability and generalizability of findings regarding the efficacy and safety of Ripertamab in IMN treatment.
In conclusion, we conducted the first multicenter clinical study on Ripertamab for IMN treatment. The results demonstrate that Ripertamab and Rituximab exhibit comparable therapeutic efficacy in IMN, with a similar incidence of complications. Based on these findings, Ripertamab has the potential to become a first-line treatment option for IMN patients.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Xin Xu, G. et al. Long-Term exposure to air pollution and increased risk of membranous nephropathy in China [J]. J. Am. Soc. Nephrol. 27 (12), 3739–3746. https://doi.org/10.1681/ASN.2016010093 (2016).
Jürgen Floege, S. J. et al. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference [J]. Kidney Int, 95(2): 268 – 80. (2019). https://doi.org/10.1016/j.kint.2018.10.018
Fernando, C., Fervenza, S., Sethi, U. & Specks Idiopathic membranous nephropathy: diagnosis and treatment [J]. Clin. J. Am. Soc. Nephrol. 3 (3), 905–919. https://doi.org/10.2215/CJN.04321007 (2008).
Lou, Y. et al. Changes in clinicopathological characteristics of patients with idiopathic membranous nephropathy: A single-center retrospective study [J]. Iran. Red Crescent Med. J. 22 (5), e97452. https://doi.org/10.5812/ircmj.97452 (2020).
Fernando, C. et al. Rituximab or cyclosporine in the treatment of membranous nephropathy [J]. N Engl. J. Med. 381 (1), 36–46. https://doi.org/10.1056/NEJMoa1814427 (2019).
Francesco Scolari, E. et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial [J]. J. Am. Soc. Nephrol. 32 (4), 972–982. https://doi.org/10.1681/ASN.2020071091 (2021).
Karine Dahan, H. et al. Rituximab for severe membranous nephropathy: A 6-Month trial with extended Follow-Up [J]. J. Am. Soc. Nephrol. 28 (1), 348–358. https://doi.org/10.1681/ASN.2016040449 (2017).
Kidney Disease KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases [J]. Kidney Int., 100(4S): S1–S276. DOI: https://doi.org/10.1016/j.kint.2021.05.021. (2021).
Freeman, C. L. et al. Role of CD20 expression and other pre-treatment risk factors in the development of infusion-related reactions in patients with CLL treated with obinutuzumab [J]. Leukemia 30 (8), 1763–1766. https://doi.org/10.1038/leu.2016.41 (2016).
The Renal Diseases Department of Peking University School of Medicine Expert Group. Expert Consensus on the Use of Rituximab in Membranous Nephropathy [J]. Chinese Journal of Internal Medicine. 61(3): 282 – 90. (2022). https://doi.org/10.3760/cma.j.cn112138-20210927-00660
Yuankai Shi, Q. Y. & Zhang, X. Efficacy and safety of Recombinant Human-Mouse chimeric Anti-CD20 monoclonal antibody (SCT400) combined with CHOP in patients with Treatment-Naïve diffuse large B-Cell lymphoma: A multicenter, randomized, Single-Blind, parallel Active-Controlled, phase III study [J]. Blood 138, 2480–2482 (2021).
Jai Radhakrishnan, Daniel, C. et al. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient [J]. Kidney Int. 82 (8), 840–856. https://doi.org/10.1038/ki.2012.280 (2012).
Liyo Kao, V. et al. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy [J]. J. Am. Soc. Nephrol. 26 (2), 291–301. https://doi.org/10.1681/ASN.2013121315 (2015).
Suzuki, T. et al. Clinical characteristics of thrombospondin type-1 domain-containing 7A-associated membranous nephropathy [J]. Ren. Fail. 42 (1), 966–968. https://doi.org/10.1080/0886022X.2020.1819318 (2020).
Gilles Salles, M. et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience [J]. Adv. Ther. 34 (10), 2232–2273. https://doi.org/10.1007/s12325-017-0612-x (2017).
Kamburova, E. G. et al. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function [J]. Am. J. Transpl. 13 (6), 132–137. https://doi.org/10.1111/ajt.12220 (2013).
Julia, M. et al. Anti–phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy [J]. Clin. J. Am. Soc. Nephrol. 6 (6), 1286–1291. https://doi.org/10.2215/CJN.07210810 (2011).
Maude Plante, L. et al. Impact of split dosing the first rituximab infusion in patients with high lymphocyte count [J]. Curr. Oncol. 28 (5), 4118–4128. https://doi.org/10.3390/curroncol28050349 (2021).
Aymen Zayen, H. et al. Rituximab-induced interstitial lung disease: case report and literature review [J]. Pharmacology, 87(5–6): 318 – 20. (2011). https://doi.org/10.1159/000327681
Mitchell, R. Smith. Rituximab (monoclonal anti-CD20 antibody): mechanism of action and resistance [J]. Oncogene, 22(47): 7359-68. (2003). https://doi.org/10.1038/sj.onc.1206939
Tio, S. W. et al. Prophylactic co-trimoxazole prescribing practice in patients who received rituximab therapy: comparison across indications and specialties [J]. Rheumatology 63 (1), 132. https://doi.org/10.1093/rheumatology/keae163.247 (2024).
Hematology Branch of Chinese Medical Association. Expert consensus on the management of patients with lymphoma complicated with HBV infection in China [J]. Chinese Journal of Hematology. 34(11): 988 – 93. (2013). https://doi.org/10.3760/cma.j.issn.0253-2727.2013.11.019
Klastersky, J. et al. Management of febrile neutropaenia: ESMO clinical practice guidelines [J]. Ann. Oncol. 27 (5), v111–v8. https://doi.org/10.1093/annonc/mdw325 (2016).
Acknowledgements
Thanks to Dr. Yingying Liu, Dr. Xueyan Zhu, Dr. Hua Jin, Dr. Xiaoxuan Zhang and Dr. Lei Wang, the five chief physicians of the cooperation center, for providing clinical data.
Funding
(1) Beijing Medical Award Foundation, Grant/Award Numbers: YXJL-2023-0475-0304. (2) China Zhongguancun Precision Medicine science and technology foundation, Grant/Award Numbers:2024-JZYX-01362. (3) Health Commission Jilin Provincial, Grant/Award Numbers: 2023LC015.
Author information
Authors and Affiliations
Contributions
X.L. and W.C. wrote the main body of the manuscript text.Y.L. and X.Z. and H.J. and X.Z. and L.W. provide data support.Y.C. and F.X. and Y.W. and Y.S. participate in data analysis.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The Second Hospital of Jilin University (2025028).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Liu, Y., Zhu, X. et al. The efficacy and safety of ripertamab in the treatment of idiopathic membranous nephropathy: a retrospective multicenter cohort study. Sci Rep 15, 20567 (2025). https://doi.org/10.1038/s41598-025-06046-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06046-1