Abstract
Environmental pollution, a pressing global concern, is primarily caused by the release of harmful gases. These gases, such as carbon monoxide (CO), carbon dioxide (CO2), ammonia (NH3), nitrogen oxides (NO, NO2), and sulphur dioxide (SO2), significantly contribute to climate change, environmental degradation, and adverse health effects. To address this issue, the development of advanced materials is important. In this study, we have theoretically discovered a novel Ga6N6 nanoring of high formation energy. After adsorbing these gases onto the surface of the nanoring using GGA-PBE functionals within density functional theory (DFT), we have investigated the adsorption energy, charge density difference, energy gap, projected density of states (PDOS), total density of states (TDOS), and global index parameters. The strong binding between the nanoring and NO, NO2, and SO2 gas molecules is revealed through adsorption energies of -1.75 eV, -2.04 eV, and − 1.01 eV, respectively. Besides, CO2 gas dissociates on the active side of the nanoring. Further, it is observed that the interactions between CO and NH3 with nanoring are weaker, suggesting that Ga6N6 nanoring may be well-suited for detecting these gases. The Ga6N6 nanoring exhibits potential for storing or removing NO, NO2, and SO2 gas molecules from a specific environment, as its high adsorption energy and longer recovery time allows it to effectively bind and retain these molecules, making it a promising candidate for environmental remediation applications.
Similar content being viewed by others
Introduction
In recent years, burning of fossil fuels for the generation of electricity and increasing industrial works emit various hazardous gases which weaken the surroundings and worsen health-care1,2. Gases like carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxide (NO), nitrogen dioxide (NO2), sulphur dioxide (SO2), ammonia (NH3) etc. are mainly responsible for degradation in the atmosphere3. The primary contributors to acid rain, particularly NO2 and SO2, cause significant harm to property and crops, resulting in substantial losses for humanity and posing one of the greatest environmental threats4. Highly pungent smelled NH3 gas is frequently used in various industries that create mouth, throat, skin, lungs, eyes problems and have a bad effect among animals5,6,7,8. Consequently, there is a demand for an effective gas sensor that not only has the capacity to detect unsafe gas molecules in a specific environment but also possesses the ability to eradicate harmful gases, thereby significantly contributing to societal enhancement5.
Though 2D materials and other substances exhibit adsorption properties3research on clusters has increased for certain specific materials due to their electronic and geometrical stability, as well as their catalytic behaviour9. These characteristics make them applicable in various fields, including hydrogen storage10gas sensing11,12,13,14the formation of super atoms as building blocks for materials15,16forming metallic glasses17,18. Because of cluster’s (0D materials) wide range of tailoring properties regarding shape, size and chemical composition, binary metallic clusters are widely popular among others19,20,21. For instance, aluminum and transition metal doped aluminum22,23carbon nanotube (CNT)24,25,26B12N12 and fullerene27,28 are widely investigated for adsorption of gases. However, the use of pristine CNT is limited as it has low participation and poor sensitivity towards CO, NO and NH3 gas molecules owing to the minimal impact on electrical conductance29,30. The low sensitivity of specific gases, including H2, CO2, NO, NO2, CO, and NH₃, toward pristine graphene demonstrates its inability to effectively detect these gases. Despite extensive research utilizing graphene nanoribbons and graphene quantum dots to identify their presence, the results have shown minimal improvement, as the interaction of gas particles with their pristine forms remains insufficient for the development of reliable nano sensors31,32,33,34.
These limitation of using pristine carbon-based nanomaterials make the opportunity for the researchers to explore both theoretically and experimentally on different materials including the group III-based cluster35 and group III-based nitrides materials like boron nitride and aluminium nitride as they have the property like electron affinity and outstanding physical and chemical attributes36,37,38. Similar to graphene, the pristine h-AlN monolayer exhibits weak interactions with CO and NO gases. However, unlike carbon nanotubes, h-AlN demonstrates strong interaction or chemisorption with NH₃ gas molecules39. B6N6 and B12N12 structure have weak interaction towards NO and CO gas molecules40. On the other hand, Al6N6 theoretically can be a good sensor for both CO, NO and NH3 gases. Similar to these group-III based nitrides, metal oxides nanoclusters are also useful in gas sensing application41,42. Removal of toxic gases is also possible by using these nanoclusters39,43. These previous studies show that the demand of nanoclusters is increasing within the realm of gas detection and toxic gas removal applications39,42,43.
After synchronized 0 dimensional (0D) ring type structure (polynic cyclo (18) carbon or C18 nanocluster), scientists work on the polynic cyclo (12) Carbon or C12 ring and these carbon allotropes gain huge attention in the field of material science44,45. Though some of the studies have already done on polyynic cyclo (12) carbon and similar to this C12 analogues structure X6N6 (X is group III atom) ring for hydrogen storage application, detail investigation of these ring in the field of material science is still missing46,47. Besides, X6N6 (X = B/Al) cluster is studied for gas sensing and toxic gas removal applications; however, Ga6N6 structure did not gain much traction in these field. A study suggests that the ring shaped Ga6N6 structure is possible as it has minimum energy level48. Unlike B6N6 and Al6N6 structures, Ga6N6 is not studied much for the gas sensing and gas removal application. Moreover, GaN 2D monolayer has photo detecting capacity, better electronic structure and opto-electronic properties. Inspiring from the above fact, we study the prospect of a Ga6N6 nanoring for the toxic gas sensing and removal application.
In this study, we explore the significance of the Ga6N6 structure and its potential application for use in gas removal device in the future. We examine the adsorption energy, partial density of states (PDOS), charge transfer using density functional theory (DFT). We believe that this theoretical study will facilitate the use of Ga6N6 nanocluster as a toxic gas adsorber.
Computational methods
Here, we have executed the first-principles based density functional theory (DFT) calculations using the SIESTA code49 to estimate the structural and electronic properties of Ga6N6 nanoring. We also employed Perdew-Burke-Ernzerhof (PBE)50 approach for the treatment of exchange correlation (XC) effect and generalised gradient approximation (GGA)51. Plane wave with kinetic energy cut-off 450 Ry and double zeta polarisation (DZP)51 basis set with an energy shift of 0.01 Ry has been used in the Kohn-Sham equation. To integrate over the Brillouin zone, a k-grid of 10 × 10 × 10 is considered to meet the convergence. The formation energy (\(\:{E}_{Form}\)) of the present structure is calculated by the following equation52:
where, \(\:{E}_{{Ga}_{6}{N}_{6}}\) is the total energy of Ga6N6 nanoring, \(\:{E}_{Ga}\) (or \(\:{E}_{N}\)) is total energy of single Ga (or N) atom.
After relaxation of the structures without given any geometric constraint and specified atomic force (0.01 eV/Å), we examined the sensing potential of different gasses in the structure using the equations53,
where, \(\:{E}_{\text{n}\text{a}\text{n}\text{o}\text{r}\text{i}\text{n}\text{g}+\text{g}\text{a}\text{s}}\) is the total energy of the nanoring with the gas molecules absorbed on, whereas \(\:{E}_{\text{n}\text{a}\text{n}\text{o}\text{r}\text{i}\text{n}\text{g}}\) and \(\:{E}_{\text{g}\text{a}\text{s}\:}\)are the total energy of nanoring without the gas molecules and the isolated gas molecules, respectively. From the definition of adsorption energy if the result is negative that means a chemical adsorption is happened and it is thermodynamically favourable for molecular gas sensing. Initially the distance between the gas molecule and the ring was set to (2.0–4 Å).
From the base of activated complex theory, recovery time (τ), a crucial parameter for gas sensing, is defined as54
Here, ω is the attempt frequency (∼1012s−1), \(\:{E}_{\text{a}\text{d}\text{s}}\) is the adsorption energy, KB is the Boltzmann constant and T is the temperature in Kelvin. Attempt frequency refers to the rate at which molecules strive to overcome an energy barrier and shift from one state to another.
To estimate the charge transfer (Q), we use Bader atomic population analysis method55. The negative value of Q means that electron conveyance from cluster to gas molecules and vice versa. The adsorbed and isolated gases charge transfer \(\:{\Delta\:}\rho\:\) which is written about DDEC method is56,
where, \(\:{\rho\:}_{nanoring+gas}\) is the charge density of gas with nanoring; \(\:{\rho\:}_{nanoring}\) is the charge density of nanoring and \(\:{\rho\:}_{gas}\) is the charge density of gas only which is visualised using VESTA software57.
Results and discussion
Ga6N6 Nanoring optimization
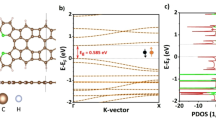
The optimized structure of Ga6N6 nanocluster is shown in the Fig. 1 where the bond length of Ga-N is 2.01Å and the angle between Ga-N-Ga is 105.35° and N-Ga-N is 165.46° respectively. However, the previous study for Ga-N pristine structure has shown that the bond length of hexagonal Ga-N was between 1.76 Å to 2.40 Å48,58where most of the studies are based on 2D materials. The previous studies for different shapes of Ga6N6 nanoring have shown that the bond length between Ga-N was also the same and it was 1.76 Å to 2.40 Å48. The formation energy for the optimized structure is calculated by using Eq. (1) and the result is -4.12 eV/atom which shows that the structure is stable in nature. From the Fig. 1(c) (PDOS), it is shown that in the energy level − 6.35 eV and − 1.45 eV the contribution of N-2p and Ga-4p is high, making strong superposition results in a strong polar covalent bond between Ga and N.
In addition, the evaluation of the thermal stability of the Ga6N6 nanoring is conducted using an ab initio molecular dynamics (AIMD) simulation at 298 K for 10 ps, employing a time interval of 1 fs. Figure 2a,b show the picture of AIMD simulation (before and after) with top and side view of the nanoring in the graph of energy variation with simulation time. The structure experiences a change in shape; nevertheless, any bond of the structure has not broken which indicate the thermal stability of the structure.
Further, the isolated CO, CO2, NO, NO2, SO2 and NH3 gas molecules are optimized using the same frame. The bond lengths of CO, CO2, NO, NO2, SO2 and NH3 gas molecules are observed as 1.15Å, 1.18Å, 1.18Å, 1.22Å, 1.50Å and 1.03Å respectively whereas the bond angle for CO2 (O-C-O), NO2 (O-N-O), SO2 (O-S-O) and NH3 (H-N-H) gas molecules are 179.99°, 132.60°, 118.90° and 106.20° respectively.
Interactions of Ga6N6 Nanoring with toxic gases
Adsorption energy calculation and recovery time
We have examined different toxic gas molecules that interact with Ga6N6 nanocluster. For the interaction of CO, CO2, NO, NO2, SO2 and NH3 gas molecules with Ga6N6 nanocluster, adsorption energies are calculated by using a number of orientations of Ga and N atoms with these gas molecules positioned on the edge of the nanoring and the slightly above or below the hollow side of the nanoring. Minimum energy configuration in adsorbing gas molecules with the cluster is considered in our cases. The adsorption energy is calculated by using the Eq. (2) and the pictorial view of the adsorption energies of different gas molecules with the cluster Ga6N6 nanoring is depicted in Fig 3. The adsorption energies obtained from edge-side interactions with the nanoring are -0.25 eV, +2.09 eV, -1.75 eV, -0.77 eV, -0.53 eV, and -0.19 eV for CO, CO2, NO, NO2, SO2, and NH3 gas molecules, respectively. In contrast, when these gases are positioned near the centre of the nanoring, the observed adsorption energies are -0.41 eV, -0.36 eV, -0.54 eV, -2.04 eV, -1.01 eV, and -0.46 eV for CO, CO2, NO, NO2, SO2, and NH3 gas molecules, respectively. Among the various positions of gases on the nanoring, the adsorption energy is highest for CO, NO2, SO2, and NH3 gas molecules, keeping these gases near the centre of the ring. In contrast, for CO2 and NO gas molecules, the highest adsorption energy (in terms of absolute value) occurs at the edge of the nanoring. The adsorption of CO2 gas on the nanoring leads to its dissociation into CO, which may exhibit positive energy. Therefore, we have depicted the highest absorption energy (in terms of magnitude) in this article.
In some cases, bond length and angle of Ga6N6 nanoring has changed after the adsorption. In CO adsorption, the minimum separation distance between the C atom of CO and the Ga atom of the cluster is 3.47 Å. Though after the adsorption of CO gas in the nanoring the bond length has almost remained the same and the value is 2.01Å, the bond angle between Ga-N-Ga has changed to 105.56° from 105.35°. Similarly, for the sensing of CO2, SO2 and NH3 gas molecules with the Ga6N6 nanoring, bond length of Ga-N has remained stable after adsorption of these gases and bond angle Ga-N-Ga has changed significantly (for CO2, Ga-N-Ga bond angle has changed to 105.49° from 105.35°, for SO2, Ga-N-Ga bond angle has changed to 105.23° from 105.35° and for NH3, Ga-N-Ga bond angle has changed to 105.51° from 105.35°). However, the adsorption of NO and NO2 gas molecules on the Ga6N6 nanoring results in significant modifications to both the bond length and bond angle of the nanoring. In the case of NO, the Ga-N bond length increases from 2.01 Å to 2.14 Å, while the Ga-N-Ga bond angle near the adsorption site changes from 105.35° to 105.38°; similarly, for NO2 adsorption, the Ga-N bond length shifts from 2.01 Å to 2.02 Å, and the Ga-N-Ga bond angle near the adsorption site is altered from 105.35° to 106.31°, indicating strong interactions between the gas molecules and the Ga6N6 nanoring. A schematic picture of these gas adsorption with the nanoring is shown in Fig. 3. The calculated highest (absolute value) absorption energy for CO, CO2, NO, NO2, SO2 and NH3 gas molecules with the Ga6N6 nanoring are − 0.41 eV, 2.09 eV, -1.75 eV, -2.04 eV, -1.01 eV and − 0.46 eV respectively. Adsorption is generally classified into two types: physisorption and chemisorption. Adsorption is influenced by multiple factors, where in physisorption, the adsorption energy is higher than − 0.21 eV/atom, whereas in chemisorption, it is lower than − 0.42 eV/atom (considering negative values), along with additional dependencies on molecule-surface distance, electronic structure analysis, and bonding nature59. According to the definition of two types of adsorption12in our study the adsorption of CO, CO2, and NH3 (Fig. 3a,b,f) gas molecules with the Ga6N6 nanoring is treated as physisorption or weak interaction because the interaction between these gasses with the pristine have negligible Eads (although the adsorption energy of NH3 gas is significant for chemisorption, the electronic properties- such as band gap and orbital hybridization remain absent after adsorption), as well as large adsorption distance between gas molecules and Ga6N6 nanoring. However, the interaction between NO, NO2 and SO2 (Fig. 3c–e) gas molecules with nanoring is strong interaction or chemisorption because of high Eads and smaller adsorption bond length. For instance, during NO adsorption on the nanoring, the N-N bond length- where one nitrogen atom originates from the nanoring and the other from the NO gas molecule- is 1.25Å60. Moreover, for NO2 and SO2 adsorption on nanoring—the bond length between Ga-O (Ga from nanoring and O from the gas molecule) are 2.49 Å and 2.64 Å respectively. An interesting phenomenon occurs during the absorption of CO2 gas molecule on the nanoring, where positive absorption energy is observed due to dissociation, as the original CO2 bond breaks and the newly formed bond has a short length of 1.28 Å. We use PBE functional which predicts higher level accuracy in calculating adsorption energies61.
We have compared the results of our adsorption energy (Eads) of the hazardous gases with other results. In the case of CO gas adsorption in Ga6N6 nanoring, the value of adsorption energy is − 0.41 eV, whereas adsorption of CO with the small nanoring C12, B6N6 and Al6N6, the adsorption energies are − 0.95 eV, -0.16 eV and − 1.21 eV respectively12. For CO2 adsorption on pristine Ga6N6, the absorption energy is 2.09 eV, generated due to dissociation; however, on the Ag₅ nanoring, the adsorption energy is only − 0.27 eV14. We have obtained − 1.75 eV adsorption energy in the case of NO gas adsorption on Ga6N6 cluster which is better result compared to the NO gas absorption on C12, B6N6 and Al6N6 nanoring valued − 0.97 eV, -0.14 eV and − 0.37 eV respectively12. Ga6N6 nanoring absorbed NO2 gas molecules tightly as the adsorption energy is higher valued − 2.04 eV compared to the adsorption energy of B9N9 nanoring with NO2 gas molecule is only − 0.15 eV13. The adsorption energy of SO2 gas molecule on the nanoring Ga6N6 is -1.01 eV which is very close to the adsorption energy of SO2 on Graphene − 1.17 eV38. In our study, Ga6N6 cluster showed comparatively better affinity towards gases than other absorbers except NH3 gas molecules absorption where the value of adsorption energy is -0.46 eV which is low compared to the absorption of NH3 gas molecules on C12 (-0.97 eV)12.
Further, the recovery time (τ) is pivotal in the application of sensing56 as it indicates how fast a gas becomes assimilated onto the surface of nanoring. In the case of our study (Ga6N6 nanoring), the recovery time τ is summarized in Table 1, which is calculated by using Eq. (3) at room temperature (298 K). From all the value of recovery time, we observed that the recovery time of NO and NO2 is very long owing to the strong adsorption capacity of the Ga6N6 nanoring towards these gases. However, the lower value of recovery time from the adsorption of CO and NH3 gases with the Ga6N6 nanoring predicts that Ga6N6 might be used as a gas detection process of these gases. In the case of SO2 gas interaction with the nanoring, the recovery time is on the scale of (106 s) which reveals that it can be used as sensor (if the changes in electrical properties are measurable). For the high recovery time of the interaction of Ga6N6 with NO, NO2 and SO2 indicates that the nanoring can be used as absorber for these gases, whereas in the case of CO and NH3 interaction with the nanoring, the recovery time is in the µs (10− 6 s) level, which indicates that Ga6N6 nanoring might be a good choice of reversible sensors of these (CO and NH3) gases.
Variation in charge concentration and charge transfer
Charge transfer and Iso-surface of (a) CO, (b) CO2, (c) NO, (d) NO2, (e) SO2 and (f) NH3 (top views) gas molecules after the interaction with pristine Ga6N6 nanoring is pictured where red and green color represent accumulation and depletion of charges, respectively. For plotting, we keep the iso value 0.15e/Å3.
By using atomic population analysis, the electron relocation process is studied by Hirshfeld. In our study, the value of transferred charge is negative. The negative value of the charge in gas molecules indicates that these gas molecules are receiving electrons (accumulation) from the pristine Ga6N6 and these electrons stay in the gas molecules which makes interaction. In Fig. 4, red colour is used for the indication of the electron density for charge accumulation and the green colour is applied for the indication of electron deficiency or charge depletion. For different gas molecules adsorption, the arrow indicates the direction of electron transfer and also the net value of the electron that transferred from the ring to the gas molecules (Fig. 4) which is obtained by the Eq. (4). From this, it can be said that charge is accumulated at the negative charge region (shown as red colour) and charge is depleted in the positive charge region (shown as green colour) due to the reformation of the effective interface dipoles and polarization of electrons. Redistribution of electrons at the interface region creates adsorption energy (Eads). Among the six gases, the gas molecules NO, NO2 and SO2 have higher charge transfer between the adsorbing Ga6N6 nanoring and gas molecules are responsible for high adsorption energy. On the other hand, the redistribution of charges and interaction of VBs orbital is high for CO2 gas molecules (from the Fig. 4) due to dissociation of CO2 gas molecules in the active side of the nanoring. On the other hand, due to the relatively low charge transfer in CO and NH3 gases, their interaction with the nanoring exhibits moderate adsorption energy.
Electronic properties
Frontier molecular orbital theory analysis
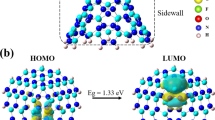
The frontier orbitals- the highly occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) - analysis is one of the leading characteristics to investigate chemical stability because these orbitals are mainly taking part in chemical reactions. The energy level difference of HOMO and LUMO is treated as the energy gap of the molecular orbitals that unwavering the probability of charge movement in the absorption system comprised of the surface of Ga6N6 nanoring and gases is expressed as12
Using the above equation our calculated result of \(\:{E}_{g}\) for the Ga6N6 nanoring is 1.73 eV where the HOMO and LUMO energy value is -5.07 eV and − 3.34 eV respectively. We verify energy gap in Fig. 5a using band structure calculation. The Fermi energy EF (-4.32 eV), HOMO and LUMO energy are depicted in the Fig. 5b which are indicated by black, blue and green dash line respectively and Furthermore, Using the Heyd-Scuseria-Ernzrhof (HSE) functional HSE06 calculation we obtain the energy gap 2.82 eV and the Fermi energy − 3.97 eV. However, we use PBE density functional for calculating data and picturing graphs.
This value of energy gap represents that the material is semiconductor in nature. This indicates the material has a promising character to adsorb gas on its surface. After adsorption of different gases on the surface of the nanoring, the changes of HOMO-LUMO gap and Fermi energy shift analysis are vital in gas adsorption techniques62. After adsorption of CO, CO2, NO, NO2, SO2 and NH3 gas molecules the DOS has changed which is shown Fig. 6 and from the analysis of the changes of DOS it can be predicted that the interaction of gases and nanoring has taken place.
The changes Fermi level energy (EF), HOMO, LUMO (indicated by black, blue and green dash lines) and energy gap (\(\:{E}_{g}\)) are noted down in the Table 2. For the proportion of band gap of GaN material and energy gap for the cluster Ga6N6 nanoring, GaN outnumber from our Ga6N6 nanoring almost 2 to 1 - the band gap of GaN material is 3.55 eV63while energy gap of our Ga6N6 nanoring is 1.73 eV. After absorption of the gases on the surface of nanoring, the energy gap and Fermi level energy have changed which indicate that there is superposition of orbitals and make some interactions. In the case of NO2 adsorption on the surface of nanoring the energy gap reduced 25.43% which indicate a strong interaction as the electrons easily come from valance band to conduction band. Following the adsorption of CO and NH₃ gases, the system experiences a slight reduction in its energy gap, indicating a modest increase in electrical activity.
Conversely, the adsorption of NO and SO2 gases onto the nanoring surface leads to an increase in the energy gap, indicating enhanced system stability following the adsorption process62. For the dissociation of CO2 gas on the nanoring, the energy gap also increases.
Global indices parameter analysis
Globally reactivity parameters widely use in density functional theory commonly known as global indices are chemical potential (µ), softness (S), hardness (η) and electrophilicity (ω) are crucial to analyze the responsiveness of a molecular system or cluster12. These parameters have been listed in Table 3 after calculating using the following Eq.
The chemical potential (µ), softness (S), hardness (η) and electrophilicity (ω) of pristine Ga6N6 are observed as 4.21 eV, 0.57 eV, 0.87 eV and 10.19 eV respectively. Upon adsorption of gases, the HOMO-LUMO positions of the system undergo significant changes, leading to notable variations in these parameters. Chemical potential change is positive for the gases CO and NO2 reveals that more gases adsorption on the surface require energy, while for the adsorption of CO2, NO and NH3 the chemical potential is negative indicates further adsorption of these gases on the surface of nanoring happen spontaneously. In the case of SO2 gas adsorption on the surface of the absorbent the chemical potential changes remain the same. The hardness (η) reveals the chemical stability whereas softness divulges the high chemical reactivity and favorable interaction and they have an inverse relationship showed in the expression12. From the Table 3, it is observed that after adsorption of the gases system Ga6N6@ NO is hardest in nature, while system Ga6N6@NO2 becomes more active. Electrophilicity (ω) reflects the charge redistribution of the absorbent after adsorption12. The more redistribution of charges, the greater the change in electrophilicity of the system. The electrophilicity changes is highest for Ga6N6@NO2 system, followed by Ga6N6@CO system, whereas the lowest value is for Ga6N6@ NO system, followed by Ga6N6@NH3, Ga6N6@CO2 and Ga6N6@SO2 system, which reveals the charge redistribution of these system. The complete picture of adsorption cannot be captured by a single parameter; the variations in all these quantities contribute to improved adsorption. Based on Table 3 and the preceding discussion, NO2 is identified as the most active gas for adsorption on the nanoring surface.
Partial density of States analysis
To explicate the gas adsorption behavior of Ga6N6 nanoring towards CO, CO2, NO, NO2, SO2 and NH3 gas molecules, projected density of states-PDOS for clusters with absorbed gas molecules is examined due to the participation of orbitals in the reaction. Because of the adsorption of CO, CO2, NO, NO2, SO2 and NH3 gas molecules with the pristine Ga6N6 nanoring, superposition of orbitals has changed compare to the Ga6N6 nanoring. We plotted PDOS graph for CO, NO2, SO2 and NH3 gas molecules adsorbed near center of the ring and in the case of CO2 and NO gas molecules adsorption we have chosen edge side adsorption because of highest adsorption energy for these orientations. It is clearly observed that after the adsorption of the gas molecules with the Ga6N6 nanoring the Fermi level changes its position relative to the original position in the nanoring showed in Figs. 1c and 7. In the Fig. 1c of Ga6N6 nanoring in HOMO side N-2p orbital contribution is high meaning that HOMO is mostly N character while in LUMO side Ga-4p dominates the density of electrons that is LUMO is mostly Ga character. This further supports the idea that the distribution of HOMO-LUMO concentration plays a crucial role in two distinct molecular orbitals. In a chemical reaction, the higher electron density in the N-2p orbital on the HOMO side facilitates electron donation, while the lower electron density in the N-2p orbital on the LUMO side indicates a strong attraction for electrons. As a result, the system’s conductivity may depend on the energy gap between these orbitals62. After adsorption of different gas molecules on the nanoring, the contribution of the newly added orbitals injected electrons near the Fermi level shows the adsorption of gases onto the surface of the nanoring. After adsorption, these newly emerged molecules orbitals energy concentration near the Fermi level is highest in NO2 gas adsorption, followed by NO, SO2, CO and NH3 - the high concentration of the energy level of orbitals carries charge and injected into the material makes strong interaction. In addition, in the case of CO2 dissociation, newly emerged molecular orbital concentration is high near Fermi level.
From the Fig. 7a, it is shown that C-2p orbital has peaks in the energy level − 3.57 eV (2.07 states/eV), -1.06 eV (0.80states/eV) and 8.86 eV (1.25 states/eV) whereas O-2p has a peak at energy level − 3.57 eV (0.85 states/eV). In the energy level − 3.57 eV (near Fermi energy level) both N and Ga atoms have a small significant PDOS valued 1.07(states/eV) and 1.36(states/eV) of Ga-4p and N-2p orbitals respectively have created weak interaction that make weak covalent bond. Similarly, for CO2 dissociation on the Ga6N6 nanoring Fig. 7b, the interrelation of the C-2p and O-2p orbital with the Ga-4p and N-2p is comparatively high and superposition on energy level − 2.07 eV (near the Fermi level), PDOS contribution of Ga-4p, N-2p, C-2p and O-2p is 4.12 states/eV, 2.59 states/eV, 0.91 states/eV and 0.45 states/eV respectively which contributes interaction among the orbitals creates covalent bond. However, in the case of NO adsorption on the Ga6N6 nanoring (Fig. 7c), the interaction among the O-2p orbital, Ga-4p and N-2p orbitals is notably strong, with significant orbital overlap occurring at energy levels − 4.86 eV and − 2.80 eV, close to the Fermi level. At -4.86 eV, the projected density of states (PDOS) contributions is 0.55 states/eV for Ga-4p, 1.6 states/eV for N-2p, and 0.40 states/eV for O-2p. Similarly, at -2.80 eV, the PDOS contribution increases to 2.16 states/eV for Ga-4p, 3.87 states/eV for N-2p, and 0.46 states/eV for O-2p. This substantial orbital interaction leads to the formation of a strong covalent bond, resulting in higher adsorption energy – second highest adsorption energy in our study. Likewise, upon the adsorption of NO2 gas molecules on the nanoring (as shown in Fig. 7d), there is a significant alteration in the projected density of states (PDOS) for the Ga-4p, N-2p, and O-2p orbitals near the Fermi level. This shift leads to a reduction in the energy gap, suggesting that NO2 exhibits the strongest adsorption among the gases analysed in this study. Furthermore, the adsorption PDOS of SO2 on the nanoring (as depicted in Fig. 7e) exhibits a trend similar to that of NO. Nevertheless, the HOMO-LUMO gap remains relatively large, and there is no significant contribution from the S-3p orbital near the Fermi level. In stark contrast, however, In the case of NH3 absorption with the pristine Ga6N6 nanoring from the Fig. 7f, we have observed that the emerging orbitals H-1s and N-2p almost annihilate the pervious energy level near the Fermi level creates a smaller interaction among the gas with the Ga6N6 nanoring that we have studied.
We selected the Ga6N6 nanoring due to its relatively low cost, high thermodynamic stability, and intrinsic semiconducting properties. It has exhibited strong interactions with toxic NOx gases, demonstrating the potential to remove these harmful substances from a specific environment. However, fabrication of this nanoring is highly difficult right now due to its size and chemical structure. We anticipate that this 0D material will be synthesized in the future, enabling its practical application, though further research on the nanoring is still required.
Conclusions
The structural, electronic and sensing properties of pristine nanoring (Ga6N6) materials towards hazardous gas molecules (CO, CO2, NO, NO2, SO2 and NH3) are examined in this work using DFT theory. The pristine Ga6N6 nanoring is found to be thermodynamically and thermally stable. In most of the absorption cases (adsorption of NO, NO2 and SO2), the adsorption distances are shorter and have high absorption energies indicating the good chemisorption capability of the cluster for these gases. However, for CO and NH₃, the relatively large absorption distances and low absorption energies indicate that these processes correspond to physisorption. Moreover, the charge transfer mechanism, HOMO-LUMO and PDOS analyze justify this result as more charges are transferred in the case of NO and NO2 absorption. Because of overlapping of electron densities between gas molecules and nanocluster, NO shows a strong interaction. Whereas, for CO and NH3 gases the overlapping is not so strong. The higher recovery time and strong interaction of NO, NO2 and SO2 gas molecules with the pristine Ga6N6 nanoring predict that this substance can serve as a gas absorber or eliminator of gases within a particular setting, while very negligible recovery time and physisorption of CO and NH3 gas molecules with the cluster indicates the limitation of the use of Ga6N6 nanocluster for corresponding gas absorber, yet it may be use as gas sensor of this gases. Furthermore, the energy gap of the Ga6N6 nanoring (1.73 eV) is smaller compared to its bulk structure (3.55 eV), which makes it promising for optoelectronic applications. Further investigation is necessary for the synthesis and practical utilization of this nanoring.
Data availability
The datasets originated and/ or analyzed during the current study are not publicly available due to privacy or other restrictions; however, it will be made available by corresponding authors on reasonable request.
References
Williams, A. Role of fossil fuels in electricity generation and their environmental impact. IEE Proc. Part. A: Sci. Meas. Technol. 140, 8–12. https://doi.org/10.1049/IP-A-3.1993.0003/CITE/REFWORKS (1993).
Barea, E., Montoro, C. & Navarro, J. A. R. Toxic gas removal – metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 43, 5419–5430. https://doi.org/10.1039/C3CS60475F (2014).
Cortés-Arriagada, D., Villegas-Escobar, N. & Ortega, D. E. Fe-doped graphene nanosheet as an adsorption platform of harmful gas molecules (CO, CO2, SO2 and H2S), and the co-adsorption in O2 environments. Appl. Surf. Sci. 427, 227–236. https://doi.org/10.1016/J.APSUSC.2017.08.216 (2018).
Grennfelt, P. et al. Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 49, 849–864. https://doi.org/10.1007/S13280-019-01244-4/FIGURES/8 (2020).
Upadhyay, D., Roondhe, B., Pratap, A. & Jha, P. K. Two-dimensional delafossite Cobalt oxyhydroxide as a toxic gas sensor. Appl. Surf. Sci. 476, 198–204. https://doi.org/10.1016/J.APSUSC.2019.01.057 (2019).
Yu, Z., Li, Y., Yu, X. & Chen, F. Computational study of Borophene with line defects as sensors for nitrogen-containing gas molecules. ACS Appl. Nano Mater. 3, 9961–9968 (2020).
Pandey, D., Kamal, C., Dutt, R. & Chakrabarti, A. Improved gas adsorption on functionalized aluminene surface: A first-principles study. Appl. Surf. Sci. 531, 147364. https://doi.org/10.1016/J.APSUSC.2020.147364 (2020).
Vadalkar, S. et al. An Ab-initio Study of the C18 nanocluster for Hazardous Gas Sensor Application. ChemistrySelect 7, e202103874. https://doi.org/10.1002/SLCT.202103874 (2022).
Kovalenko, M., Bovgyra, O., Dzikovskyi, V. & Bovhyra, R. A DFT study for adsorption of CO and H2 on Pt-doped ZnO nanocluster. SN Appl. Sci. 2, 1–9. https://doi.org/10.1007/S42452-020-2591-9/FIGURES/5 (2020).
Charkin, O. P., Klimenko, N. M. & Charkin, D. O. DFT modeling of successive hydrogenated subnano-size aluminum clusters. Chem. Phys. 522, 112–122. https://doi.org/10.1016/J.CHEMPHYS.2019.02.007 (2019).
Hu, X., Gui, Y., Zhu, S. & Chen, X. First-principles study of the adsorption behavior and sensing properties of C2H4 and C2H6 molecules on (CuO/TiO2)n (n = 1–3) cluster modified MoTe2 monolayer. Surf. Interfaces. 31, 102003. https://doi.org/10.1016/J.SURFIN.2022.102003 (2022).
Patel, S., Patel, P., Chodvadiya, D., Som, N. N. & Jha, P. K. Adsorption performance of C12, B6N6 and Al6N6 nanoclusters towards hazardous gas molecules: A DFT investigation for gas sensing and removal application. J. Mol. Liq. 352, 118702. https://doi.org/10.1016/J.MOLLIQ.2022.118702 (2022).
Panchal, J., Gauswami, A., Chodvadiya, D., Jadeja, H. & Jha, P. K. Adsorption performance of CO, NO and NH3 hazardous gas molecules over B9N9 and Al9N9 nanorings: acumen from density functional theory. Mater. Chem. Phys. 311, 128565. https://doi.org/10.1016/J.MATCHEMPHYS.2023.128565 (2024).
Alotaibi, M., Alotaibi, T., Alshammari, M. & Ismael, A. K. The structural and electronic properties of the Ag5 atomic quantum cluster interacting with CO2, CH4, and H2O molecules. Cryst. (Basel). 13, 1691. https://doi.org/10.3390/CRYST13121691/S1 (2023).
Leskiw, B. D. & Castleman, A. W. The interplay between the electronic structure and reactivity of aluminum clusters: model systems as Building blocks for cluster assembled materials. Chem. Phys. Lett. 316, 31–36. https://doi.org/10.1016/S0009-2614(99)01295-6 (2000).
Sengupta, T., Das, S. & Pal, S. Transition metal doped aluminum clusters: an account of spin. J. Phys. Chem. C. 120, 10027–10040 (2016).
Kartouzian, A. Cluster-assembled metallic glasses, Nanoscale Research Letters 8:1 8 (2013) 1–4. (2013). https://doi.org/10.1186/1556-276X-8-339
Reyes-Retana, J. A. & Naumis, G. G. Ab initio study of Si doping effects in Pd–Ni–P bulk metallic glass. J. Non Cryst. Solids. 409, 49–53. https://doi.org/10.1016/J.JNONCRYSOL.2014.11.011 (2015).
Chen, F. & Johnston, R. L. Energetic, electronic, and thermal effects on structural properties of Ag - Au nanoalloys. ACS Nano. 2, 165–175 (2008).
Pittaway, F. et al. Theoretical studies of palladium-gold nanoclusters: Pd-Au clusters with up to 50 atoms. J. Phys. Chem. C. 113, 9141–9152 (2009).
Taran, S., Garip, A. K., Arslan, H. & Ferrando, R. Symmetry breaking and morphological instabilities in core-shell metallic nanoparticles. J. Phys.: Condens. Matter. 27, 013003. https://doi.org/10.1088/0953-8984/27/1/013003 (2014).
Tamafo Fouégué, A. D., Tendongmo, H., Sakué Ngankam, E. & Abdoul Ntieche, R. Investigating the X-aminopyridine (X = 2 and 3) molecules sensing by Al12N12 and B12N12 fullerene-like nanocages: DFT, QTAIM, RDG and TD-DFT insights. J. Biomol. Struct. Dyn. 41, 9721–9731. https://doi.org/10.1080/07391102.2022.2146199 (2023).
Xiong, J., Xiong, B. & Mustafa, R. W. Density functional theory study of Al-doped MoS2–O–H nanoribbons for their potentials use in dangerous gas sensing applications. Phys. B Condens. Matter. 673, 415484. https://doi.org/10.1016/J.PHYSB.2023.415484 (2024).
Chen, Y., Han, J. & Yang, X. DFT simulation of structure stability and nitrogen oxide adsorption for nitrogen and oxygen co-modified carbon nanotubes. Surf. Interfaces. 42, 103498. https://doi.org/10.1016/J.SURFIN.2023.103498 (2023).
Yuksel, N., Kose, A. & Fellah, M. F. Formaldehyde adsorption and sensing: A density functional theory study on Pd4 nanocluster decorated CNT structure. Periodica Polytech. Chem. Eng. 67, 83–93. https://doi.org/10.3311/PPCH.20522 (2023).
Kakil, S. A. & Abdullah, H. Y. Pt-and Pd-doped carbon nanotubes with different diameters as CO sensors: A comparative DFT study. Diam. Relat. Mater. 139, 110311. https://doi.org/10.1016/J.DIAMOND.2023.110311 (2023).
Zoua, V. P., Fouegue, A. D. T., Bouba, M. O., Ntieche, R. A. & Abdoul, W. Adsorption of juglone on pure and boron-doped C24 fullerene-like nano-cage: A density functional theory investigation. Comput. Theor. Chem. 1222, 114077. https://doi.org/10.1016/J.COMPTC.2023.114077 (2023).
Hussain, S., Chen, H., Zhang, Z. & Zheng, H. Vibrational spectra and chemical imaging of cyclo[18]carbon by tip enhanced Raman spectroscopy. Chem. Commun. 56, 2336–2339. https://doi.org/10.1039/C9CC09130K (2020).
Buasaeng, P., Rakrai, W., Wanno, B. & Tabtimsai, C. DFT investigation of NH3, PH3, and AsH3 adsorptions on Sc-, Ti-, V-, and Cr-doped single-walled carbon nanotubes. Appl. Surf. Sci. 400, 506–514. https://doi.org/10.1016/J.APSUSC.2016.12.215 (2017).
Jia, X., An, L. & Chen, T. Adsorption of nitrogen oxides on Al-doped carbon nanotubes: the first principles study. Adsorption 26, 587–595. https://doi.org/10.1007/S10450-020-00218-3/FIGURES/5 (2020).
Montejo-Alvaro, F., Oliva, J., Herrera-Trejo, M., Hdz-García, H. M. & Mtz-Enriquez, A. I. DFT study of small gas molecules adsorbed on undoped and N-, Si-, B-, and Al-doped graphene quantum Dots. Theor. Chem. Acc. 138, 1–15. https://doi.org/10.1007/S00214-019-2428-Z/FIGURES/8 (2019).
Kumar, S., Meenakshi, H. & Sharma Effect of gas adsorption on graphene nanoribbons: A density functional theory. Mater. Today Proc. 4, 10441–10445. https://doi.org/10.1016/J.MATPR.2017.06.396 (2017).
Kumar, S., Malhotra, M. & Sharma, H. Adsorption of gas molecules on ultra-thin pristine and doped graphene nanoribbons. Mater. Res. Express. 5, 105007. https://doi.org/10.1088/2053-1591/AADAA8 (2018).
Lu, Z. et al. Detection of gas molecules on single Mn Adatom adsorbed graphyne: a DFT-D study. J. Phys. D Appl. Phys. 51, 065109. https://doi.org/10.1088/1361-6463/AAA3B3 (2018).
Hossain, M. A. et al. An Ab initio study of the B35 Boron nanocluster for application as atmospheric gas (NO,NO2,N2O,NH3) sensor. Chem. Phys. Lett. 754, 137701. https://doi.org/10.1016/J.CPLETT.2020.137701 (2020).
Ahmadi, A., Beheshtian, J. & Hadipour, N. L. Interaction of NH3 with aluminum nitride nanotube: electrostatic vs. covalent. Phys. E Low Dimens Syst. Nanostruct. 43, 1717–1719. https://doi.org/10.1016/J.PHYSE.2011.05.029 (2011).
Rastegar, S. F., Peyghan, A. A., Ghenaatian, H. R. & Hadipour, N. L. NO2 detection by nanosized AlN sheet in the presence of NH3: DFT studies. Appl. Surf. Sci. 274, 217–220. https://doi.org/10.1016/J.APSUSC.2013.03.019 (2013).
Zhang, H. P. et al. Band structure of graphene modulated by Ti or N dopants and applications in gas sensoring. J. Mol. Graph Model. 61, 224–230. https://doi.org/10.1016/J.JMGM.2015.08.004 (2015).
Wang, Y. et al. A first-principles study of gas adsorption on monolayer AlN sheet. Vacuum 147, 18–23. https://doi.org/10.1016/J.VACUUM.2017.10.014 (2018).
Ammar, H. Y., Badran, H. M. & Eid, K. M. TM-doped B12N12 nano-cage (TM = Mn, Fe) as a sensor for CO, NO, and NH3 gases: A DFT and TD-DFT study. Mater. Today Commun. 25, 101681. https://doi.org/10.1016/J.MTCOMM.2020.101681 (2020).
Bhati, V. S., Kumar, M. & Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: a review. J. Mater. Chem. C Mater. 9, 8776–8808. https://doi.org/10.1039/D1TC01857D (2021).
Majhi, S. M., Mirzaei, A., Kim, H. W. & Kim, S. S. Reduced Graphene Oxide (rGO)-Loaded Metal-Oxide Nanofiber Gas Sensors: An Overview. Sensors 21, 1352–1352. https://doi.org/10.3390/S21041352 (2021).
Baraiya, B. A., Mankad, V. & Jha, P. K. Degrading CO poisoning over foreign atom seized Ag12M icosahedral bimetallic clusters. AIP Conf. Proc. 2265 https://doi.org/10.1063/5.0016620/725438 (2020).
Charistos, N. D. & Muñoz-Castro, A. Induced magnetic field in sp-hybridized carbon rings: analysis of double aromaticity and antiaromaticity in cyclo[2 N ]carbon allotropes. Phys. Chem. Chem. Phys. 22, 9240–9249. https://doi.org/10.1039/D0CP01252A (2020).
Kaiser, K. et al. An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. Science 365 (2019), 1299–1301. https://doi.org/10.1126/SCIENCE.AAY1914/SUPPL_FILE/AAY1914_KAISER_SM.PDF (1979).
Desales, L. A. et al. Isidro ortega, modelling Carbyne C12-ring calcium decorated for hydrogen storage. Revista Mexicana De Física. 64, 634–641. https://doi.org/10.31349/REVMEXFIS.64.634 (2018).
Allangawi, A., Shanaah, H. H., Mahmood, T. & Ayub, K. Investigation of the cyclo[12]carbon Nanoring and respective analogues (Al6N6 and B6N6) as support for the single atom catalysis of the hydrogen evolution reaction. Mater. Sci. Semicond. Process. 162, 107544. https://doi.org/10.1016/J.MSSP.2023.107544 (2023).
Song, B., Cao, P. L. & Li, B. X. Theoretical study of the structure of a Ga6N6 cluster. Phys. Lett. A. 315, 308–312. https://doi.org/10.1016/S0375-9601(03)01035-1 (2003).
Soler, J. M. et al. The SIESTA method for Ab initio order-N materials simulation. J. Phys.: Condens. Matter. 14, 2745. https://doi.org/10.1088/0953-8984/14/11/302 (2002).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865. https://doi.org/10.1103/PhysRevLett.77.3865 (1996).
Shukla, R. & Zala, V. Modulation of electronic bandgaps and subsequent implications on SQ efficiencies via strain engineering in ultrathin SnX (X = S, Se) nanowires. J. Mater. Chem. C (2022). https://pubs.rsc.org/en/content/articlehtml/2022/tc/d2tc03400j (accessed June 5, 2024).
Bhattacharyya, K., Pratik, S. M. & Datta, A. Controlled pore sizes in monolayer C2N act as ultrasensitive probes for detection of gaseous pollutants (HF, HCN, and H2S). J. Phys. Chem. C. 122, 2248–2258 (2018).
Chen, J., Zhou, Q., Jia, L., Cui, X. & Zeng, W. The gas-sensing mechanism of Pt3 cluster doped SnS2 monolayer for SF6 decomposition: A DFT study. Appl. Surf. Sci. 597, 153693. https://doi.org/10.1016/J.APSUSC.2022.153693 (2022).
Truhlar, D. G., Garrett, B. C. & Theory, V. T. S. Acc. Chem. Res. 13 440–448. https://doi.org/10.1021/AR50156A002/ASSET/AR50156A002.FP.PNG_V03. (1980).
Bader, R. F. W. & Nguyen-Dang, T. T. Quantum theory of atoms in Molecules–Dalton revisited. Adv. Quantum Chem. 14, 63–124. https://doi.org/10.1016/S0065-3276(08)60326-3 (1981).
Raval, D., Gupta, S. K. & Gajjar, P. N. Detection of H2S, HF and H2 pollutant gases on the surface of penta-PdAs2 monolayer using DFT approach. Sci. Rep.. 13, 1. https://doi.org/10.1038/s41598-023-27563-x (2023).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. Urn:Issn:0021-8898 41, 653–658. https://doi.org/10.1107/S0021889808012016 (2008).
Cui, Z. et al. Adsorption of CO, NH3, NO, and NO2 on pristine and defective g-GaN: improved gas sensing and functionalization. Appl. Surf. Sci. 530, 147275. https://doi.org/10.1016/J.APSUSC.2020.147275 (2020).
Kokalj, A. Corrosion inhibitors: physisorbed or chemisorbed? Corros. Sci. 196, 109939. https://doi.org/10.1016/J.CORSCI.2021.109939 (2022).
Muthuperiyanayagam, A., Nabi, A. G., Zhao, Q., Aman-Ur-Rehman, N. & Di Tommaso, D. Adsorption, activation, and conversion of carbon dioxide on small copper–tin nanoclusters. Phys. Chem. Chem. Phys. 25, 13429–13441. https://doi.org/10.1039/D3CP00477E (2023).
Araujo, R. B., Rodrigues, G. L. S., dos Santos, E. C. & Pettersson, L. G. M. Adsorption energies on transition metal surfaces: towards an accurate and balanced description. Nat. Commun.. 13, 1. https://doi.org/10.1038/s41467-022-34507-y (2022).
Gong, W., Liu, J. & Gui, Y. Adsorption of greenhouse decomposition products on Ag2O–SnS2 and CuO–SnS2 surfaces. ACS Omega. 7, 21043–21051. https://doi.org/10.1021/acsomega.2c01828 (2022).
Czelej, K. et al. Atomistic origins of various luminescent centers and n-Type conductivity in gan: exploring the point defects induced by cr, mn, and O through an Ab initio. Chem. Mater. 36, 6392–6409. https://doi.org/10.1021/acs.chemmater.4c00178 (2024).
Acknowledgements
The computer facility developed under DST-FIST Level-I (2006, 2014) programmes of Department of Science and Technology, Government of India, New Delhi, India and support under DRS-SAP-I (2010), DRS-SAP-II (2018) of University Grants Commission, New Delhi, India are highly acknowledged. Moreover, Rajib K. Sutradhar thanks to the Govt. of India for having Indian Council for Cultural Relations (ICCR) PhD fellowship award (no YG7057573425054/SJS2022): And also, thanks to Ministry of Education Bangladesh, Dhaka 1000, Bangladesh and Directorate of Secondary and Higher Education, Dhaka 1000, Bangladesh for providing necessary leaves and helping hands. Sanjeev K. Gupta is thankful to Anusandhan National Research Foundation (ANRF), India, for the financial support (Grant No. CRG/2022/007329).
Author information
Authors and Affiliations
Contributions
R.K.S., V.B.Z. and R.S.S. were associated with the DFT calculations and wrote the manuscript. S.K.G. and P.N.G. helped to improve the scientific content of the article. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sutradhar, R.K., Zala, V.B., Shukla, R.S. et al. DFT-based insights into novel Ga6N6 nanoring for pollutant gas adsorption: energetics and electronic modulations. Sci Rep 15, 20982 (2025). https://doi.org/10.1038/s41598-025-06067-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06067-w