Abstract
We investigated eyes with pathological myopic (PM) with punctate inner choroidopathy (PIC), a rare inflammatory disease, and focused on the association between PIC lesions and lacquer cracks (LCs). We retrospectively reviewed 36 myopic eyes diagnosed with PIC from 2001 to 2020 and followed for at least 3 years. Clinical characteristics were compared between the PM and non-PM eyes and between the PIC lesions with/without a coincident LC course. Factors associated with the final best-corrected visual acuity (BCVA) and PIC lesion area also were investigated. PM eyes were significantly older (p = 0.0440), had higher refractive error (p < 0.0001), a greater increase in the number of PIC lesions (p < 0.0001), poorer final BCVA (p = 0.0010), and more macular atrophy (p = 0.0252) than non-PM eyes. There were significant differences between PIC lesions with/without coincident LCs in age (p = 0.0034), refractive error (p = 0.0072), presence of PM (p < 0.0001), baseline and final BCVA (p = 0.0294 and p = 0.0373, respectively), and PIC lesion area % change/year (p = 0.0076). The final BCVA was associated with macular atrophy (p = 0.0006); the PIC lesion area % change/year was associated with a change in the number of PIC lesions (p = 0.0130) and whether they coincided with the LC course (p = 0.0491). PIC in PM eyes has a poor prognosis and could be subtyped according to the relationship with LCs or not.
Similar content being viewed by others
Punctate inner choroidopathy (PIC) is a relatively rare inflammatory condition that commonly affects young female patients with myopia1,2. Characteristic ophthalmoscopic findings are a small, well-defined, yellow-gray spot in the macular area without intraocular inflammation1,2,3,4. The degree of myopia is highest in all inflammatory chorioretinopathies, and Essex et al. reported a mean equivalent spherical value of −4.6 diopters (D) with a range of −14 to + 4 D3,5. The characteristic optical coherence tomography (OCT) findings include an elevated retinal pigment epithelium (RPE) caused by hyperreflective material, accompanied by a disrupted photoreceptor ellipsoid zone and interdigitation zone, and/or choroidal hyper-transmission below the lesions1,6,7,8. These lesions eventually result in focal choroidal excavation9. In the long term, PIC lesions may develop chorioretinal atrophy resembling myopic patchy atrophy and be complicated by macular neovascularization (MNV) and subretinal fibrosis1,3,10,11,12. The precise disease pathogenesis remains uncertain.
Pathological myopia (PM), a condition in which excessive elongation of the axial length (AL) associated with myopia results in structural changes in the posterior segment of the eye, is the primary cause of visual impairment worldwide and is distinctly different from high myopia (HM)13. Lacquer cracks (LCs) are fine, irregular, yellow lines in the macular area13,14,15,16 that are considered to be healed mechanical breaks in the RPE, Bruch’s membrane, and the choriocapillaris complex13,14,15,16. LCs are defined as the presence of a plus lesion in the classification of myopic maculopathy (META-Analysis for Pathologic Myopia; META-PM classification) proposed by Ohno-Matsui et al.13,17. The frequency rates of LCs have been reported to range from 5 to 15% of cases of HM. Indocyanine green angiography (ICGA) is most useful for diagnosing LCs, with linear hypofluorescence depicted in the late phase13,16,18,19. Fluorescein angiography (FA) shows linear hyperfluorescence13,19. OCT shows continuity or discontinuity of the RPE and Bruch membrane complex, choroidal thinning, and acoustic shadows in the choroid13,16,19,20.
Because PIC is a relatively rare disease, many aspects of the disease are not well clarified, including its pathogenesis and long-term prognosis. Hady et al. analyzed 500 PM eyes with patchy atrophy, found PIC lesions in 55 eyes and reported that 7 eyes had PIC lesions at the edge of a LC or along a LC. However, their analysis may have been a slight underestimation of the LCs because it was evaluated by fundus photography11. Ramtohul et al. recently performed a cluster analysis of 102 eyes with PIC and reported two subtypes: one is older and more myopic, and the other is younger and associated with pachychoroid21. We considered the possibility that the pathogenesis of PIC may differ between PM and non-PM, and focused on the relationship between LCs, a characteristic finding in PM, and PIC lesions. In this report, we analyzed the clinical features and long-term prognosis of PIC in PM, and also examined the relationship between LCs and PIC lesions in detail using angiography to investigate the possibility of classification according to the relationship with LC.
Results
The characteristics of the 36 eyes of 27 cases are summarized in Table 1. The mean patient age was 56.8 ± 13.4 years (range, 26–78 years), and 22 patients (81%) were women. The mean AL, excluding the six eyes for which measurements could not be obtained, was 28.6 ± 2.6 mm (range, 23.81–34.61 mm). The mean refractive error, excluding the seven eyes with an intraocular lens, was −10.4 ± 5.5 diopters (D) (range, −0.25–−18.25 D). Twenty-nine of the 36 eyes had HM, including 4 eyes with M0 or M1, 22 eyes with M2, 3 eyes with M3, and 0 eyes with M4. Twenty-seven eyes (75%) developed myopic MNV during the study, including the date of the first PIC diagnosis. The mean follow-up period was 86.8 ± 52.5 months (range, 30–248 months). During the follow-up, six eyes underwent cataract surgery, 24 eyes received anti-vascular endothelial growth factor (VEGF) therapy with either bevacizumab (Avastin, Genentech Inc., South San Francisco, CA, USA), ranibizumab (Lucentis, Genentech Inc.), or aflibercept (Eylea, Regeneron, Tarrytown, NY, USA), and six eyes received sub-Tenon triamcinolone acetonide injection (STTA).
Differences in clinical characteristics between PM and non-PM eyes
Twenty-five eyes had PM eyes (69%) and 11 did not PM eyes (31%). Table 2 shows a comparison of the clinical characteristics between PM and non-PM eyes. Eyes with PM were significantly older (p = 0.0440), had a higher refractive error (p < 0.0001), longer AL (p = 0.0008), poorer final logarithm of the minimum angle of resolution (logMAR) BCVA (p = 0.0010), a greater increase in the number of PIC lesions (p < 0.0001), more LCs (p < 0.0001), more coincidence of PIC lesion and LC course (p < 0.0001), and more macular atrophy (p = 0.0252) than those with non-PM eyes. There were no significant differences in gender (p = 0.8715), baseline logMAR BCVA (p = 0.0783), baseline PIC lesion number (p = 0.9862), PIC lesion area % change/year (p = 0.2831), presence of MNV (p = 0.2963) or follow-up period (p = 0.6304).
Characteristics of eyes with PIC lesions coincident with LCs
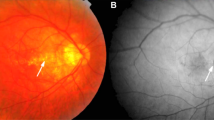
LCs were observed in 19 of 32 eyes (59%), excluding one eye that was difficult to determine due to macular atrophy from the 33 eyes for which ICGA could be performed during the follow-up. Of the cases with LCs, 16 eyes (84%) had PIC lesions coincident with the course of LCs (Fig. 1). Table 3 shows a comparison of the findings that differed in clinical characteristics between those with and without coincident PIC lesions and LC courses. Eyes with PIC lesions and LCs were significantly older (p = 0.0034), had higher refractive error (p = 0.0072), more pathological myopia (p < 0.0001), poorer baseline (p = 0.0294) and final logMAR BCVA (p = 0.0373), and higher PIC lesion area % change/year (p = 0.0076) than those without PIC lesions. No significant differences were seen in gender (p = 0.6264), AL (p = 0.1625), baseline PIC lesion number (p = 0.7327), change in PIC lesion number (p = 0.1556), presence of MNV (p = 1.0000), macular atrophy (p = 0.2642), or follow-up period (p = 0.3653).
PIC lesions coincident or not coincident with the course of LCs. (A) Fundus photograph of the right eye of a 66-year-old woman with an AL of 29.57 mm. Multiple PIC lesions can be seen in the macular area. (B) A FA image shows hyperfluorescence at the PIC lesion. (C) An ICGA image shows a PIC lesion with hypofluorescence on the course or at the edge of the LCs exhibiting line hypofluorescence. (D) A fundus photograph of the right eye of a 26-year-old woman with an equivalent spherical value of. −9 diopters. Multiple PIC lesions are visible in the macular area. (E) A FA image shows hyperfluorescence at the PIC lesions. (F) ICGA shows circular hyperfluorescence indicating a PIC lesion, whereas no LCs are seen.
Table 4 shows the results of the comparison of clinical characteristics between those with and without coincident PIC lesions and LC courses in the PM eyes only. Eyes with coincident PIC lesions and LC courses were significantly older (p = 0.0204). There were no significant differences in gender (p = 0.2956), refractive error (p = 0.9024), AL (p = 0.2021), baseline (p = 0.1833) and final logMAR BCVA (p = 0.5071), baseline PIC lesion number (p = 0.6175), presence of increased PIC lesion number (p = 0.0910), PIC lesion area % change/year (p = 0.9304), presence of MNV (p = 0.2956) or macular atrophy (p = 5254), or follow-up period (p = 0.1163).
Visual outcomes and factors affecting VA
The mean baseline best-corrected visual acuities (BCVA) in all eyes were 0.19 ± 0.29 logMAR unit and the mean final BCVA was 0.35 ± 0.55 logMAR unit. No significant difference was seen between the baseline and final BCVAs (p = 0.0951). When the eyes were divided into those with and without macular atrophy, the final BCVA was significantly lower than the baseline BCVA in those with macular atrophy and no significant difference was seen in those without (p = 0.0020 and p = 0.3758, respectively) (Fig. 2).
Factors significantly associated with the final VA were age (p = 0.0044), presence of PM (p = 0.0038), baseline BCVA (p = 0.0015), change in number of PIC lesions (p = 0.0150), presence of MNV (p = 0.0006), presence of LCs (p = 0.0160), whether PIC lesions and the course of LCs coincided (p = 0.0332), and presence of macular atrophy (p < 0.0001). Multivariate analysis showed that macular atrophy (p = 0.0006) was associated significantly with the final VA (Table 5).
Progression of atrophy during long-term follow-up
Excluding the two eyes with severe diffuse atrophy that prevented measurement of the area, the PIC lesion area increased in 23 eyes (68%), remained unchanged in 11 eyes (32%), and did not decrease in any of the 34 cases. The mean PIC lesion area was 0.50 ± 0.7 disk area (DA) at baseline and 2.8 ± 4.4 DA at the last observation, with a mean PIC lesion area % change/year of 17.0 ± 30.6% (range, 0–118%). The mean PIC lesion area at the last observation increased significantly compared to baseline (p < 0.0001). Factors associated significantly with the PIC lesion area % change/year were the refractive error (p = 0.0166), the presence of PM (p < 0.0001), the change in the number of PIC lesions (p < 0.0001), the presence of LCs (p = 0.0010), and coincident PIC lesions and LC course (p = 0.0051). Multivariate analysis showed that the change in the number of PIC lesions (p = 0.0130) and whether PIC lesions and the course of LCs coincided (p = 0.0491) were associated significantly with the PIC lesion area % change/year (Table 6).
No eyes had macular atrophy at baseline, and during the follow-up period, 13 eyes (36%) developed macular atrophy, all of which resulted from MNV. Sixteen eyes (44%) progressed from PIC lesions to myopic patchy atrophy (Fig. 3).
A case of progression from PIC lesions to patchy atrophy. A fundus photograph of the left eye of a 75-year-old woman with an AL of 29.52 mm. (A) A fundus photograph shows multiple PIC lesions consisting of small dots in the macular area. There is myopic MNV at the fovea. (B) Fundus photograph shows a chorioretinal atrophy with an enlarged PIC lesion resulting in patchy atrophy. Scarred myopic MNV and MNV-related macular atrophy are seen at the fovea.
Discussion
This study evaluated the clinical characteristics and long-term prognosis of PIC in PM eyes and examined whether whether PIC can be classified according to the relationship between LC and PIC lesions. The results showed that PIC in PM eyes had significantly more PIC lesions during follow-up, more macular atrophy, and poorer final BCVA compared to PIC in non-PM eyes. About half of the total cases had PIC lesions consistent with the course of LCs, and there were significant differences in age, number of PM cases, baseline and final BCVA, and change in PIC lesion area % change/year between the cases that were consistent and those that were not.
Although PIC occurs in myopic eyes, there have been few reports on PIC in PM eyes. Hady et al. examined the differences in clinical features with and without PIC lesions in PM eyes with patchy atrophy; however, they did not address prognosis11; Chen et al. followed 45 PIC eyes for 5 years and reported no difference in the baseline and final VAs, and that PIC lesion enlargement was associated with AL and the baseline PIC lesion number12. Previous studies have reported that the visual prognosis of PIC is associated with the occurrence of MNV2. In the present report, we found that PIC in the PM eye caused significantly more macular atrophy, poorer visual prognosis, and an increased number of PIC lesions than PIC in the non-PM eye, whereas there was no significant difference in the presence of MNV or the PIC lesion area % change/year. In the analysis of all cases, final BCVA was also correlated with the presence of PM, presence of MNV, macular atrophy, and whether PIC lesions were consistent with the course of LC in univariate analysis, and only with the presence of macular atrophy in multivariate analysis. In PM eyes, a higher rate of macular atrophy occurs when MNV develops than in non-PM eyes, and Fang et al. observed 810 HM (n = 289) and PM (n = 521) eyes for 18 years and reported that PM was associated with significantly poorer VA than HM and that the factors correlated with the VA were the development of MNV and MNV-related macular atrophy and macular atrophy enlargement22,23. In the present report, PM eyes had inferior final BCVAs compared to non-PM eyes, and the presence of macular atrophy was a factor related to the final BCVA, which is consistent with the report by Fang et al.22.
LCs are defined as plus lesions of myopic maculopathy and when observed on fundus photographs as yellowish linear lesions in the macula are thought to represent healed mechanical disruption of the RPE and Bruch’s membrane and choriocapillaris complex13,14,15,16,17. Few reports have been published on the relationship between PIC and LCs; Hady et al. reported that fundus photographs showed yellowish linear lesions indicative of LCs in seven of 55 eyes with PIC and that the PIC lesions were present along the course of the LCs in all cases11. We confirmed the presence of LCs in 19 (59%) of 32 eyes in which angiography could be performed. The reason for the difference in the detection rates between their report and ours is the examination equipment; previous reports have shown that fundus photographs have a lower detection rate than angiographic images16.
Ramtohul et al. reported the possibility of PIC subgroups21. Their cluster analysis identified two categories of PICs, i.e., one associated with myopia and two with pachychoroid. In the current report, we classified PICs according to whether or not they coincided with the course of LCs and reported that the two groups had different clinical characteristics. The pachychoroid group reported by Ramtohul et al. had several features in common with our group that were not consistent with the course of the LCs, such as patients being younger, more cases of normal or low myopia, and more cases without LCs; however, theirs differed in that mainly single PIC lesions were present, whereas our cases had multiple PIC lesions. Furthermore, unfortunately, the choroidal thickness was not measured in the current study, because baseline swept-source (SS)-OCT images were unavailable for all patients, making it difficult to assess the exact extent of overlap between their pachychoroid-focused classification and our LC-focused classification. Nevertheless, when the above is considered together, it can be inferred that there are more than two subgroups of PICs.
Essex et al.3 followed 74 eyes with PIC for 4.5 years and reported that the BCVA decreased from 0.12 logMAR unit at baseline to 0.24 logMAR unit at the final examination. Of these, 22% of the patients who developed MNV had a decrease in BCVA from the baseline 0.12 logMAR unit to 0.70 logMAR unit3. In contrast, Chen et al. observed 45 eyes with PIC for 5 years and reported no significant difference in BCVA, with 0.47 logMAR unit at baseline and 0.37 logMAR unit at the last visit, even though 29 eyes (64%) had MNV12. In the current report, the mean BCVA was 0.19 ± 0.29 logMAR unit and the mean final BCVA was 0.35 ± 0.55 logMAR unit. No significant difference was seen between the baseline and final VAs in all patients (p = 0.0951), but those with macular atrophy had a significantly worse final VA than the baseline VA, while those without macular atrophy had no significant difference (p = 0.0020 and p = 0.3758, respectively). The incidence of MNV in our report was 75%, similar to that reported by Chen et al.; however, the visual outcomes were similar to those reported by Essex et al.3 The reason for this can be attributed to the difference in the patient population and the observation period. Prospective long-term follow-up studies with a larger number of patients are needed to accurately analyze the visual prognosis.
In the current report, the PIC lesions were unchanged or enlarged in all patients, with a mean PIC lesion area % change/year of 17.0 ± 30.6%. Hua et al.24 measured and compared the progression rate of laser photocoagulation scars and PIC lesions using retinal autofluorescence. The results showed that the mean progression rates of PIC lesions and photocoagulation scars were 3.735 mm2/year and 0.127 mm2/year, respectively, and that the progression rate of PIC lesions was significantly higher than that of photocoagulation scars24. Chen et al. measured the PIC lesion size on fundus photographs in 45 eyes with PIC and reported that the PIC lesions enlarged significantly over a 5-year period, with a progression rate of 0.69 mm2/year12. In the current report, we determined the mean PIC lesion area % change/year and therefore a direct comparison with the previously reported progression rates was impossible. However, since the progression rates of PIC lesions differed in the reports of Hua et al. and Chen et al. as well, it is possible that the progression rate may differ depending on the included cases and the measurement method. Chen et al. also reported that the progression rate was significantly correlated with AL and the baseline number of PIC lesions12. In our analysis, a mean PIC lesion area % change/year was correlated with the increase in the number of PIC lesions and the coincidence of PIC lesion and the course of LCs. This discrepancy in results may be due to the fact that our cases were older and that Chen et al. did not investigate the association with LCs. In general, it is clear that PIC lesions expand over time, requiring careful follow-up and anti-inflammatory therapy in cases involving high inflammation with an increasing number of PIC lesions or a high number of PIC lesions at baseline.
The relationship between PIC and the patchy atrophy of pathological myopia has been suggested in several studies. Xu et al. in their report on the clinical features of LCs reported two patterns of progression from LCs to patchy atrophy16. One is a type in which the LCs remain widened, and the other is a type in which patchy atrophy develops and expands. Hady et al. reported that 11% of patients with patchy atrophy had active PIC and, of these, 45.5% had PIC lesions before the patchy atrophy developed11. In the current report, 44% of PIC lesions progressed to patchy atrophy, which is comparable to that reported by Hady et al. The mechanism of patchy atrophy has been implicated in Bruch’s membrane defects, and it has been reported that Bruch’s membrane defects are also observed in PIC lesions11,25,26; however, there currently is no definitive way to distinguish between an enlarged PIC lesion and patchy atrophy, and it is unclear whether they are equivalent or not. Long-term follow-up of a large number of cases should be reported in the future.
The current study had several limitations. First, the sample size was relatively small and the study was retrospective and conducted at one center. Second, the possibility cannot be ruled out that the inclusion of cases that received STTA, VEGF treatment, and other treatments during the follow-up period may have affected the results. Third, it is difficult to completely distinguish between enlarged PIC lesions and patchy atrophy or macular atrophy, which may affect the area measurement. Fourth, because this report was retrospective, the OCT equipment and timing of imaging were not standardized; thus, the choroidal thickness was not analyzed. However, analysis of the choroidal thickness is important and requires further study. Fifth, ICGA was not always performed at baseline because this was a retrospective study and ICGA is an invasive examination. Therefore, there may be a difference in the time of onset of LCs and PIC lesions. However, since this study focused on the relationship between the location of the LC course and PIC lesions, the analysis should not be affected. Finally, the area measurements were performed multiple times, which may cause intra-rater error.
In conclusion, we investigated the long-term course of PIC in PM eyes and the association between PIC and LCs. PIC in PM eyes had an unfavorable long-term prognosis. Clinical characteristics, including final visual acuity and PIC lesion enlargement, differed depending on whether the PIC lesion coincided with the LC course. When treating PIC, it is recommended to confirm if the eye has PM, the presence or absence of LCs, and their relationship to the PIC lesions.
Methods
This was a retrospective, observational study based on the medical records of patients treated at Osaka University Hospital, Osaka, Japan. We reviewed the medical records of 36 eyes and 27 cases diagnosed with PIC between November 2001 and December 2020. No patients had been treated previously for PIC and its related MNVs.
The inclusion criteria were at least one eye diagnosed with PIC and a follow-up of more than 3 years. Diagnosis of PIC was based on the clinical findings, including single or multiple whitish yellow chorioretinal lesions at the posterior pole without intraocular inflammation or infection. The exclusion criteria included ocular turbidity such as severe cataract, severe vitreous opacity, and/or severe hemorrhage that caused blurring of the fundus and angiography images; and a history of previous treatments including STTA, anti-VEGF therapy, and pars plana vitrectomy except cataract surgery. Eligible and non-eligible cases were determined based on slit-lamp biomicroscopy, fundus photographs, and OCT and angiographic findings. The research adhered to the tenets of the Declaration of Helsinki. The institutional review board of Osaka University Hospital approved this retrospective study. Informed patient consent was obtained in the form of an opt-out option on the website.
Clinical examinations
At each follow-up visit, the patients underwent a complete ophthalmic examination, which included measurement of the BCVA and AL, slit-lamp biomicroscopy, dilated funduscopy, color fundus photography (TRC-50DX, Topcon Corporation, Tokyo, Japan), OCT (HRA + OCT Spectralis; Heidelberg Engineering, Heidelberg, Germany), SS-OCT DRI OCT-1 Atlantis (Topcon Corporation), and/or SS-OCT DRI OCT-1 Triton (Topcon Corporation). Except for three eyes that could not be evaluated due to allergy to the angiographic dye, 33 eyes underwent at least one FA and ICGA procedure using the Heidelberg Retina Angiograph + OCT (Heidelberg, Germany) and a fundus camera (TRC-50DX, Topcon Corporation) during the follow-up period. The decimal BCVA was measured using the Landolt chart and was expressed in logMAR units. The initial and final PIC lesion numbers were counted only in the posterior pole area between the upper and lower arcade vessels on the fundus camera. Multimodal imaging, including OCT, FA, and ICGA, was used to detect MNV and LCs. ICGA images, which are considered to have the highest detection rate for LCs, were used primarily as references for the FA and OCT images13,16,19. The definition of PM was based on the META-PM study group classification, which divides myopic maculopathy into five categories: 0, no maculopathy; 1, tessellated fundus; 2, diffuse atrophy; 3, patchy atrophy; and 4, macular atrophy with three plus lesions that included LCs and myopic MNV. PM was defined as category 2 or higher with the presence of a plus sign or posterior staphyloma.
Measurement of PIC lesion size
All PIC lesions seen on fundus photographs in the posterior pole between the temporal arcade vessels were measured manually at the initial and final time points using ImageJ software (US National Institutes of Health, Bethesda, MD, USA). The papillary area of the optic nerve then was measured in the same manner and the DA of the PIC lesion was calculated. When MNV was present, MNV and MNV-related macular atrophy were excluded from the measurement. Measurements were obtained twice on separate days, and the mean value was used for analysis.
Statistical analysis
Statistical analysis was performed using JMP Pro software version 17.1.0 (SAS Institute, Cary, NC, USA). Qualitative data were compared using the Fisher’s exact test. The Wilcoxon t-test was used for nonparametric numerical data. Univariate and multiple linear regression analyses were performed to determine the associations of PIC lesion area % change/year and the final BCVA with ocular and systemic factors. P < 0.05 was considered significant.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Watzke, R. C. et al. Punctate inner choroidopathy. Am. J. Ophthalmol. 98, 572–584. https://doi.org/10.1016/0002-9394(84)90243-5 (1984).
Ahnood, D. et al. Punctate inner choroidopathy: A review. Surv. Ophthalmol. 62, 113–126. https://doi.org/10.1016/j.survophthal.2016.10.003 (2017).
Essex, R. W. et al. Punctate inner choroidopathy: clinical features and outcomes. Arch. Ophthalmol. 128, 982–987. https://doi.org/10.1001/archophthalmol.2010.157 (2010).
Amer, R. & Lois, N. Punctate inner choroidopathy. Surv. Ophthalmol. 56, 36–53. https://doi.org/10.1016/j.survophthal.2010.03.009 (2011).
Reddy, C. V. et al. Enlarged blind spots in chorioretinal inflammatory disorders. Ophthalmology 103, 606–617. https://doi.org/10.1016/s0161-6420(96)30645-3 (1996).
Channa, R. et al. Characterization of macular lesions in punctate inner choroidopathy with spectral domain optical coherence tomography. J. Ophthalmic Inflamm. Infect. 2, 113–120. https://doi.org/10.1007/s12348-011-0054-6 (2012).
Spaide, R. F. et al. Redefining multifocal choroiditis and panuveitis and punctate inner choroidopathy through multimodal imaging. Retina 33, 1315–1324. https://doi.org/10.1097/IAE.0b013e318286cc77 (2013).
Zhang, X. et al. Spectral-domain optical coherence tomographic findings at each stage of punctate inner choroidopathy. Ophthalmology 120, 2678–2683. https://doi.org/10.1016/j.ophtha.2013.05.012 (2013). Epub 2013 Jun 12.
Kim, H. et al. Focal choroidal excavation in multifocal choroiditis and punctate inner choroidopathy. Ophthalmology 122, 1534–1535. https://doi.org/10.1016/j.ophtha.2015.01.012 (2015). Epub 2015 Feb 14.
Brown, J., Folk, J. C. & Kimura, C. V. R. Visual prognosis of multifocal choroiditis, punctate inner choroidopathy, and the diffuse subretinal fibrosis syndrome. Ophthalmology 103, 1100–1105. https://doi.org/10.1016/s0161-6420(96)30561-7 (1996).
Hady, S. K. et al. Prevalence and characteristics of multifocal choroiditis/punctate inner choroidopathy in pathologic myopia eyes with patchy atrophy. Retina 42, 669–678. https://doi.org/10.1097/IAE.0000000000003383 (2022).
Chen, Y. C., Chen, Y. L. & Chen, S. N. Chorioretinal atrophy in punctate inner choroidopathy/multifocal choroiditis: a five-year follow-up study. Ocul Immunol. Inflamm. 30, 270–275. https://doi.org/10.1080/09273948.2020.1869269 (2022).
Ohno-Matsui, K. et al. IMI pathologic myopia. Invest Ophthalmol. Vis. Sci 62, 5. https://doi.org/10.1167/iovs.62.5.5
Klein, R. M. & Curtin, B. J. Lacquer crack lesions in pathologic myopia. Am. J. Ophthalmol. 79, 386–392. https://doi.org/10.1016/0002-9394(75)90611-x (1975).
Grossniklaus, H. E. & Green, W. R. Pathologic findings in pathologic myopia. Retina 12, 127–133. https://doi.org/10.1097/00006982-199212020-00009 (1992).
Xu, X. et al. Clinical features of lacquer cracks in eyes with pathologic myopia. Retina 39, 1265–1277. https://doi.org/10.1097/IAE.0000000000002168 (2019).
Ohno-Matsui, K. et al. International photographic classification and grading system for myopic maculopathy. Am. J. Ophthalmol. 159, 877–883. https://doi.org/10.1016/j.ajo.2015.01.022 (2015). Epub 2015 Jan 26.
Ohno-Matsui, K. et al. Indocyanine green angiographic findings of lacquer cracks in pathologic myopia. Jpn J. Ophthalmol. 42, 293–299. https://doi.org/10.1016/s0021-5155(98)00008 (1998).
Liu, C. F. et al. Multimodal imaging including spectral-domain optical coherence tomography and confocal near-infrared reflectance for characterization of lacquer cracks in highly myopic eyes. Eye (Lond). 28, 1437–1445. https://doi.org/10.1038/eye.2014.221 (2014). Epub 2014 Sep 19.
Wang, N. K. et al. Choroidal thickness and biometric markers for the screening of lacquer cracks in patients with high myopia. PLoS One. 8, e53660. https://doi.org/10.1371/journal.pone.0053660 (2013). Epub 2013 Jan 22.
Ramtohul, P. et al. Punctate inner pachychoroidopathy demographic and clinical features of inner choroidal inflammation in eyes with pachychoroid disease. Retina 43, 1960–1970. https://doi.org/10.1097/IAE.0000000000003926 (2023).
Fang, Y. et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology 125, 863–877. https://doi.org/10.1016/j.ophtha.2017.12.005 (2018). Epub 2018 Jan 19.
Yoshida, T. et al. Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 110, 1297–1305. https://doi.org/10.1016/S0161-6420(03)00461-5 (2003).
Hua, R., Liu, L. & Chen, L. Evaluation of the progression rate of atrophy lesions in punctate inner choroidopathy (PIC) based on autofluorescence analysis. Photodiagnosis Photodyn Ther. 11, 565–569. https://doi.org/10.1016/j.pdpdt.2014.07.002 (2014).
Ruiz-Moreno, J. M. et al. Evolution of macular Bruch membrane defects of patchy chorioretinal atrophy in pathologic myopia based on a recent classification system. Ophthalmologica 244, 309–314 (2021). 10.1159/000516526. Epub 2021 May 3.
Ohno-Matsui, K., Jonas, J. B. & Spaide, R. F. Macular Bruch membrane holes in highly myopic patchy chorioretinal atrophy. Am. J. Ophthalmol. 166, 22–28. https://doi.org/10.1016/j.ajo.2016.03.019 (2016).
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
K.S. designed the study, the main conceptual ideas, and the proof outline. K.S., M.S., S.F., S.G., C.H, Y.F., K.N., K.M., and S.S. collected the data. K.S. aided in interpreting the results and worked on the manuscript. T.M., K.N. supervised the project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sayanagi, K., Suzue, M., Fujimoto, S. et al. Long-term prognosis of punctate inner choroidopathy lesions in pathologic myopia and their relationship to lacquer cracks. Sci Rep 15, 21689 (2025). https://doi.org/10.1038/s41598-025-06165-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06165-9