Abstract
A number of cardioprotective pharmacological agents are not effective in diabetic hearts. The role of AGGF1-EPCs therapy in diabetic ischaemia-reperfusion(I/R) injury and the underlying mechanism by which AGGF1 regulates EPCs under hyperglycemia (HG) + hypoxia/reoxygenation (H/R) stress are still unclear. We observed that the damaging effects of HG + H/R on EPCs were abolished by AGGF1. The EPCs implantation therapy successfully restores cardiac functions, inhibits ROS production and fibrosis in diabetic I/R mice. Mechanistically, AGGF1 activates the Nrf2 and induces the activation of downstream antioxidative proteins (HO1, NQO1, and CAT). These data suggest that AGGF1 protein reverses the damaging effects of HG + H/R on EPCs via the antioxidative Nrf2. AGGF1-EPCs therapy is a novel strategy for treating diabetic I/R injury.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder and global threat that affecting millions of people, and characterized by hyperglycemia, high free fatty acids, and insulin resistance1,2. Cardiovascular complications are considered to be the major cause of mortality in DM patients, accounting for approximately 80% of mortality of DM3. In DM, excessive reactive oxygen species (ROS), inflammation and fibrosis are closely linked to ischaemia-reperfusion injury (I/R)4,5,6,7. The effects of cardioprotective agents do not always extend to diabetic ischaemic hearts7. Therefore, developing new strategy for diabetic I/R is of great clinical value.
Due to their special anatomical location and direct contact with the blood system, endothelial cells (ECs) are of immense significance for the maintenance of vascular functions. The dysfunctions and damage to ECs are the major events of cardiovascular dysfunction in DM8,9. Yao et al. showed that endothelial progenitor cells (EPCs) are the progenitors of ECs and express specific markers of ECs. They can restore endothelial dysfunction and support angiogenesis10. EPCs are crucial for angiogenesis in ischemic tissues. The decreased number and impaired functions of EPCs are associated with impaired angiogenesis in patients with DM having cardiovascular complications11. The transplantation of EPCs increases the blood flow in ischemic hindlimb10. However, EPCs derived from patients with DM have limited application due to limited therapy efficacy and impaired function12. Furthermore, many cardiovascular protective drugs are less effective against cardiac ischaemia injury13. Hence, improving EPCs functions and elucidating underlying restoration mechanisms are of immense clinical value.
AGGF1 (AngioGenic factor with a G-patch domain and a Forkhead-associated domain 1) encodes an angiogenic factor and was originally discovered in Klippel-Trenaunay syndrome (KTS)14. AGGF1 is also reported to be a key regulator for the phenotype switch of vascular smooth muscle cells (VSMCs)15autophagy16endoplasmic reticulum stress17thoracic aortic aneurysms18skeletal muscle atrophy19and limb ischemia (based on AGGF1 priming of EPCs) associated with DM10. On the other hand, vascular endothelial growth factor A (VEGF-A) improves vascular permeability (decrease vascular integrity), whereas AGGF1 inhibits vascular permeability (improve vascular integrity) via VE-cadherin phosphorylation16. Additionally, AGGF1 is also reported to be an inflammation regulator in atrophy19,20. Nevertheless, the role of AGGF1-primed EPCs therapy in diabetic I/R, and the underlying mechanism by which AGGF1 regulates EPCs under HG + H/R are still unclear. Therefore, we hypothesized that AGGF1-primed EPCs alleviate the diabetic I/R injury targeting ROS pathway in diabetic hearts. The present study was designed to examine the therapeutic potential of AGGF1 on EPCs, and determine whether AGGF1-primed EPCs therapy could alleviate diabetic I/R injury.
Materials and methods
Reagents and antibodies
High-fat diet (HFD; protein: carbohydrate: fat = 20%:20%: 60%) was obtained from Jiangsu Xietong Pharmaceutical Bio-engineering (Nanjing, China). Streptozocin (STZ) was obtained from Sigma-Aldrich (MO, USA). Enzyme linked immunosorbent assay was performed according to the manufacturer’s instructions using the lactate dehydrogenase (LDH) cytotoxicity assay kit, creatine kinase-MB (CK-MB) assay kit, cardiac troponin T (cTnT) assay kit, malondialdehyde (MDA) content assay kit, and they were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Antibodies against heme oxygenase-1 (HO1), NAD(P)H dehydrogenase quinone 1 (NQO-1), catalase (CAT), Nrf2, CD31, CD34, and GAPDH were purchased from Proteintech (Wuhan, China).
Ethics statement
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Guilin Medical University (Investigation Number: GLMC-IACUC-20241079). All methods were performed in accordance with the relevant guidelines and regulations of IACUC of Guilin Medical University. All methods were carried out in accordance with ARRIVE guidelines (https://arriveguidelines.org, PLoS Bio 8(6), e1000412, 2010). Euthanasia was performed by anesthesia with intraperitoneally injection of 3% pentobarbital sodium followed by cervical vertebrae dislocation. All efforts were made to minimize the mice number and their suffering.
Animal models and treatments
Healthy C57BL/6J mice (8-week-old, male, ~ 25 g) were purchased from Guangdong Vital River Laboratory Animal Technology (Guangzhou, China). Mice were housed in cages (6 mice/cage, 12 h:12 h, light/dark cycle) and used following the 3R principles (replacement, reduction, and refinement). DM was induced with HFD and the intraperitoneal injection of 90 mg/kg of STZ. The control mice were fed a standard food (STD) and injected with physiological saline.
I/R injury models (2 h of ischemia followed by 14 days of reperfusion) were established via ligating the left anterior descending branch (LAD). The C57BL/6J mice were anesthetized, their chest cavity opened, the anterior descending branch was ligated (Left ventricle anterior wall found to be white). To determine the role of AGGF1-primed EPCs implantation, recombinant AGGF1 protein was purified. Elution buffer (negative control) either purified AGGF1 protein was used to pretreat EPCs (0.5 mg/mL, 0.5 mg/mL, 37 °C for 12 h). After pretreatment, AGGF1-primed EPCs (late EPCs, day 10, 1 × 106) were injected into C57BL/6J mice via tail vein injection10,17.
Glucose tolerance test and insulin tolerance test
The detailed method was described previously21. In brief, intraperitoneal glucose tolerance test (IPGTT) and insulin tolerance test (ITT) were performed before cardiac I/R injury to confirm the successful construction of DM. After overnight fasting, the fasting blood glucose levels were measured (tail vein blood), recorded, and further analyzed.
Characterization and treatment of bone marrow-derived EPCs
The male C57BL/6J mice (6–8 weeks) were euthanized and then immersed in 75% ethanol for 5–10 min to clean the mice. The muscles were removed gently, and the lower limb femur, tibia, and upper limb humerus were removed. Phosphate-buffered saline (PBS, 10 mL syringe) was used to slowly wash bone marrow derived mononuclear cells (MNCs) out from the bone marrow cavity. The collected bone marrow single-cell suspension was added to the histopaque-1083 separation solution (Sigma Aldrich, Volume single-cell suspension: Volume histopaque-1083 = 2:1). After centrifugation at room temperature for 20–30 min, the middle layer cells (white cloud-like) was removed using a pipette, washed 3 times with PBS, and cultured with endothelial growth factor 2-supplemented media (Lonza, Basel, Switzerland) supplemented with 15% fetal bovine serum (FBS) in dishes precoated with 0.1% gelatin as previously described10. MNCs were characterized by fluorescent staining for DiI-ac-LDL and FITC-UEA-l, immunocytochemistry for CD31 and CD34 as previously reported10,22,23. High-glucose media: 30mmol/L D-glucose, L-glucose as negative control. The hypoxia-reoxygenation (H/R) was simulated for 4 h: 2 h =(94% N2 + 5% CO2 + 1% O2):(95% air + 5% CO2).
Echocardiography
Previously, studies described the details of transthoracic echocardiography7,18Briefly, the left ventricular fractional shortening (LVFS) and the left ventricular ejection fraction (LVEF) were measured and recorded in M-mode after baseline echocardiography. LVEF and LVFS were further analyzed using GraphPad Prism 9.0 software (Version 9.5.1 (733)).
Immunohistochemistry
After reperfusion, mice hearts were collected, fixed for 48 h at room temperature in 4% buffered paraformaldehyde (PFA). Tissues were then dehydrated, embedded, and cut into sections (5–7 μm). Prepared sections were stained using H&E/Masson staining/Sirius Red staining guidelines and sealed with neutral gum. Images were captured using Nikon Eclipse Ti microscope (Tokyo, Japan) microscope and subsequently analyzed.
Western blot (WB) assay
Cells or C57BL/6J mice tissue samples were lysed (Western-IP lysis buffer and proteinase inhibitor cocktail) and subjected to WB assay. As previously reported18,24samples were first lysed. After centrifugation, the supernatant was transfered to a sterile microcentrifuge tube, mixed complely with 5×loading buffer. After boiled at 100 °C (metal bath, inverse tubes twice) for 20 min, the mixture was maintained in ice bath for 20 min and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) step (80 V, 30 min; 120 V, 100 min). The separated proteins were then transferred to polyvinylidene fluoride (PVDF) membranes (250 mA, 120 min) and further analyzed with primary antibodies, horseradish-peroxidase (HRP)-conjugated goat anti-mouse IgG (EM-35111-01, EMAR Science and Biotechnology, Beijing, China) or HRP-conjugated goat anti-rabbit IgG (EM-35110-01, EMAR Science and Biotechnology, Beijing, China). Chemiluminescence detection analysis was performed using EZ ECL PICO Western Blotting chemiluminescence substrate (AP34L024, Shanghai Life iLab Biotech Co., Ltd. China). Tanon 5200 (19T12NPFL16-809, Shanghai Tianneng Life Sciences Co., Ltd. China) was used for image acquisition and images were further processed using Adobe photoshop CS6 extended (Version 1.0.175.0, Adobe Systems Incorporated, USA) and ImageJ (Image J 1.54 g).
Tube formation analysis
The angiogenic ability of bone marrow derived EPCs was evaluated by tube formation assay in vitro. In brief, EPCs were cultured in matrigel-coated 48-well plates (200 µL/well; BD Biosciences) and cultured at 37 °C for 8–12 h to form tubes. Images of enclosed circles were captured and further calculated (total tube length) using Image J software (Image J 1.54 g)9.
Transendothelial migration analysis
Transendothelial migration (TEM) assay was used to assess the transendothelial migration of EPCs. Human umbilical vein endothelial cells (HUVECs) cells were first cultured in the insert chamber of BD Falcon transwell plate (353097, pore size = 8.0 μm). Single-cell suspension (EPCs) was added into each insert chamber gently and cultured in the incubator for 24 h. The bottom of the chamber was carefully swabbed to remove HUVECs and EPCs that had not migrated past. After fixation in methanol, the chamber was transferred to be stained with crystal violet staining solution (C0121, Beyotime) for 20 min. Images of migrated cells were captured with Nikon Eclipse Ti microscope (Tokyo, Japan) and further calculated9.
Wound healing assay
EPCs were cultured in 6-well plates to form cell monolayer (80% confluence), 200 µL tips were used to create straight scratches through the monolayer. Rinse the cells 3 times gently with phosphate buffered saline to remove detached cell debris. 36 h after the scratches created, images of scratches were captured and further analyzed.
Statistical analysis
Data were presented as mean ± standard deviation (SD). The difference between two groups of variables was compared by Student’s t-test and the comparison of multiple groups was performed by one- or two-way ANOVA with Tukey’s multiple comparison tests. p-values < 0.05 (p < 0.05, p < 0.01, p < 0.001, and p < 0.0001) were considered to be statistically significant. ns, not significant. The normality of data distribution was assessed using the Shapiro-Wilk test. GraphPad Prism 9.0 software (Version 9.5.1 (733) ) was used for statistical analysis.
Results
Characterization of MNCs
MNCs were isolated from C57BL/6J mice (male mice: no estrus cycle and their hormone levels are relatively stable) and maintained in gelatin-coated culture dishes. On day 1, MNCs appeared as rounded cells (Fig. 1A, left). On day 4 after plating, MNCs appeared as spindly peripheral cells (Fig. 1A, middle). On day 7, spindle-shaped adherent early EPCs (< 14 days) were observed (Fig. 1A, right). On day 10 in EGM-2, MNCs were used for immunostaining assay to characterize EPCs. We found that isolated MNCs were Dil-acLDL (red) and FITC-UEA-1 (green) positive. Representative images for the uptake of Dil-acLDL (red) and FITC-UEA-1 (green) are shown in Fig. 1B. The cell surface profile of MNCs was also analyzed and results suggested that MNCs were positive for both CD31 and CD34 (Fig. 1C). These findings suggest that isolated MNCs were endothelial lineage cells with the characteristics of previously described EPCs9.
EPCs isolation and characteristics. (A) Morphology of MNCs (cultured in EGM-2 and seeding on gelatin-coated dishes) isolated from C57BL/6J mice on day 1, 4, 7. Scale bar = 50 μm. (B) Representative images of Dil-acLDL (red) and FITC-UEA-1 (green) uptake. Blue, Hoechst staining of the nuclei, scale bar = 25 μm. (C) The immunofluorescence assay of cell surface profile of MNCs (CD31 and CD34), scale bar = 25 μm.
AGGF1 protein reverses impaired functions of EPCs under HG + H/R stress
To determine whether AGGF1 protein treatment can affect EPCs functions under HG + H/R stress in vitro, proliferation, tube formation analysis, TEM and scratch migration assay were performed. In H/R−, compared with control regular media (CK) cultured EPCs, we found that EPCs cultured in HG exhibited decreased proliferation (Fig. 2A), tube formation (Fig. 2B, C), TEM (Fig. 2D, E) and scratch migration (Fig. 2F, G), we got the similar data in H/R+ (Fig. 2A-G). All the HG-induced defects were reversed by AGGF1 protein treatment (HG + AGGF1). Additionally, proliferation, tube formation, TEM and scratch migration were significantly improved in the AGGF1 group EPCs (CK + AGGF1 and HG + AGGF1) compared with the CK group and HG group EPCs (Fig. 2A-G). Altogether, these results suggest that AGGF1 can counter the HG induced damage on EPCs in both H/R− and H/R+.
AGGF1 reverses HG + HR impaired angiogenic functions of EPCs. (A) Proliferation assay of EPCs using CCK8. (B–C) Tube formation assay of EPCs using matrigel (8 h after cells seeding). (D–E) Transwell assay of EPCs (24 h after cells seeding). (F–G) Wound healing assay of EPCs (36 H after cells seeding). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 4. ns, not significant.
Implantation of AGGF1-primed EPCs robustly alleviates I/R injuries in vivo
Previous studies have reported that a long-term HFD along with low-dose STZ can induce insulin resistance and glucose intolerance7. We constructed a DM model following an animal experiment protocol (Fig. 3A). We observed that HFD-fed mice showed decreased insulin sensitivity and glucose tolerance (Fig. 3B, C). These data confirmed the successful establishment of a diabetic model using HFD and STZ. Diabetic mice subjected to I/R injury exhibited severe cardiac injuries, characterized by increased levels of LDH, cardiac troponin (cTnT), and CK-MB7. Enzyme-linked immunosorbent assay results suggested that AGGF1-primed EPCs decreased the levels of LDH, cTnT, and CK-MB (Fig. 3D-F). To summarize, these results suggest that AGGF1-primed EPCs implantation effectively restored cardiac functions in diabetic I/R injury mice.
AGGF1-primed EPCs therapy markedly alleviates I/R injuries in mice hearts. (A) Overview of the T2DM model procedure. (B) IPGTT assay for C57BL/6 J mice after HFD + STZ, n = 6. (C) IPITT assay for C57BL/6 J mice after HFD + STZ, n = 6. (D–F) LDH, cTnT, and CK-MB activities in the serum (36 h post I/R). n = 6. Data are mean ± SD. *P < 0.05, ***P < 0.001, ****P < 0.0001.
AGGF1-primed EPCs suppress oxidative damage, fibrosis and inflammation in diabetic I/R mice
ROS production, fibrosis and inflammatory responses are significant contributors to cardiac I/R injuries7,25,26,27. To further explore whether AGGF1-primed EPCs implantation can combat ROS, fibrosis and inflammation, we investigated whether MDA was decreased after the AGGF1-primed EPCs implantation in diabetic mice, data suggest that MDA was significantly decreased in mice with AGGF1-primed EPCs implantation (Fig. 4A), accompanied by increased superoxide dismutase (SOD) level (Fig. 4B). Furthermore, Masson and Sirius Red staining results showed a decreased level of fibrosis in the AGGF1-primed EPCs implantation group (Fig. 4C, D). Immunohistochemistry also confirmed decreased Ly6G, MCP-1, TNF-α, IL-1βand IL-6 expression in AGGF1-primed EPCs implantation mice (Fig. 4E, F). Together, our results indicate that AGGF1-primed EPCs alleviate oxidative damage, fibrosis and inflammation in diabetic I/R mice.
AGGF1-primed EPCs implantation suppresses oxidative damage, fibrosis and inflammation in diabetic I/R mice. (A) MDA content analysis of myocardial tissue. (B) SOD content assay of myocardial tissue. (C) Masson staining (Scale bar = 1000 μm), Sirius Red staining (Scale bar = 1000 μm) and immunohistochemistry assay (Scale bar = 50 μm) of cardiac sections from diabetic I/R mice. (D) Quantification of Masson staining and Sirius Red staining. (E) Representative images of mice hearts from sham controls, I/R mice, I/R + EPCs mice, and I/R + AGGF1-EPCs mice with immunohistochemical analysis of LY6G, MCP1, TNF-α, IL-1β, and IL-6. (F) Quantification data on inflammation markers LY6G, MCP1, TNF-α, IL-1β, and IL-6. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 6.
AGGF1 improves nuclear accumulation of Nrf2 in EPCs under HG + HR
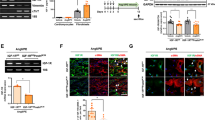
Numerous studies have demonstrated that the transcription factor Nrf2 plays an important role in the antioxidative response10,23. To further explore the underlying mechanisms by which AGGF1 decreases ROS generation in EPCs under HG + HR, we focused on the Nrf2 antioxidative pathway. Under HR stress (HR+), when compared with the control group (CK), nuclear accumulation of Nrf2 (n-Nrf2) was increased in the EPCs from CK + AGGF1 group, accompanied by the activation of downstream antioxidative proteins, including HO1, NQO1, and CAT, we got similar data for HG versus HG + AGGF1 group, (Fig. 5A-G). Additionally, we used siRNA targeting Nrf2 for WB analysis. siRNA targeting Nrf2 blocked the protective effect of AGGF1 on the antioxidative response (increase HO1, NQO1 and CAT) (Fig. 5A-G).
AGGF1 improves the nuclear localization of Nrf2 and activation of downstream antioxidative molecules. (A–C) Western blot analysis for the n-Nrf2 (nuclear Nrf2) and its downstream antioxidative proteins (HO1, NQO1, CAT) under HG + HR. siNrf2: 5’-CGAGAAGUGUUUGACUUUATT-3’ (sense); 5’-UAAAGUCAAACACUUCUCGTT-3’ (antisense), EPCs were transfected with siRNAs (synthesized by RiboBio) using a transfection reagent (Santa Cruz Biotechnology, Dallas, TX). (D–G) Quantification data on n-Nrf2, CAT, HO1and NQO1 from images in (A–C). The grouping of gels/blots cropped from different parts of the same gel. Data are mean ± SD. **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 3.
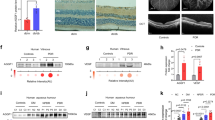
To further confirm AGGF1 promotes the functions of EPCs via Nrf2 under HG + HR, we used siNrf2 to assess the effect of siNrf2 on EPCs. Data showed that siNrf2 blocked the protective potential of AGGF1 on proliferation (Fig. 6A), angiogenesis (Fig. 6B, C), TEM (Fig. 6D, E), and scratch migration (Fig. 6F, G). A significantly increased LVEF and LVFS were observed in cardiac I/R mice after implantation of AGGF1-primed EPCs, as shown by echocardiographic analysis (Fig. 7A-C). EPCs can home to endothelial-damaged areas and restore impaired vascular functions. Here, lentivirus expressing EGFP was infected into EPCs, after EPCs implantation, mice hearts were characterized by immunostaining with an anti-GFP antibody. Our results suggested that AGGF1-primed EPCs mice hearts (EGFP labeled) showed significantly more mobilization of GFP-labeled EPCs than control mice hearts (EPCs treated with elution buffer) (Fig. 7D, E). Immunohistochemistry assay also confirmed upregulated capillary density (CD31+) in the AGGF1-primed EPCs implantation group (Fig. 7D, E). In addition, our data demonstrated AGGF1-primed siNrf2-EPCs treatment significantly attenuated the therapeutic effect compared to AGGF1-primed EPCs treatment. The pro-angiogenic effect of AGGF1-primed EPCs in I/R injured hearts was abrogated in the AGGF1-primed siNrf2-EPCs group (Fig. 7D, E). These results further suggest that AGGF1 regulates the functions of EPCs via antioxidative Nrf2. Altogether, our results suggest that AGGF1 improves EPCs function via Nrf2 under HG + HR stress.
NRF2 is required for AGGF1-primed EPCs therapy. (A) Left ventricular ejection fraction (LVEF, n = 6). (B) Left ventricular fractional shortening (LVFS, n = 6). (C) Representative images of echocardiographic analysis for mice from Sham, I/R + PBS, I/R + EPCs, I/R + AGGF1-EPCs, I/R + AGGF1-EPCs-siNRF2. (D–E) Representative images for immunostaining with an anti-GFP antibody (EPCs homing evaluation) and anti-CD31 antibody (angiogenesis evaluation), n = 6. Data are mean ± SD. *P < 0.05, **P < 0.01, ****P < 0.0001. ns, not significant.
Discussion
Previous studies have categorized mononuclear cells (MNCs)-derived EPCs into early EPCs and late outgrowth EPCs based on surface antigen expression (CD14, CD31, CD34, CD45, CD144, KDR, and c-Kit). Both early EPCs and late outgrowth EPCs contribute to endothelial restoration in response to vascular injury10,23. Our results show that isolated MNCs were positive for CD31 and CD34 on Day 7, suggesting the presence of early EPCs (Fig. 1).
Diabetes is a chronic metabolic disease that causes various cardiovascular dysfunctions, including endothelial damage and the dysfunction of EPCs1,10. Cardiac I/R is a prevalent cardiovascular event associated with oxidative damage and vascular disorders28,29. The hyperglycemic environment in DM induces the production of ROS and further exacerbates endothelial cell dysfunction10. EPCs can migrate to damaged endothelial sites, differentiate into ECs, and promote neovascularization to repair vascular damage10. EPCs implantation therapy has been widely performed to alleviate cardiac ischaemia, limb ischemia, and renal ischemia7,10,30. Moreover, emerging evidence suggests that exosomes derived from EPCs can also exert protective effects in I/R31. However, patients with DM are more susceptible to ischemic heart diseases, and current treatment options are limited7. In present study, we observed that AGGF1-primed EPCs implantation exerts robust protective potential against diabetic I/R injury in vivo. During diabetic I/R, AGGF1-primed EPCs implantation significantly improves angiogenesis-related functions, including proliferation, tube formation, TEM, and migration (Fig. 2). Additionally, AGGF1-primed EPCs implantation effectively mitigates I/R injury (LDH, cTnT, CK-MB, LVEF, and LVFS) in vivo (Figs. 3 and 7). I/R injury leads to fibrosis and inflammation, and our results suggest that AGGF1-primed EPCs can effectively attenuate fibrosis and inflammation induced by I/R injury (Fig. 4). Furthermore, our previous study found that AGGF1-primed EPCs therapy increases blood flow and motor function in hindlimb ischemia10. AGGF1 has been shown to regulate PI3K and AKT signaling pathways, which are known to modulate the Fyn-Nrf2 pathway32 and combat ROS. We focused on ROS levels in EPCs under HR + HG. Interestingly, our results show that AGGF1 increases nuclear Nrf2, and ultimately upregulates downstream antioxidative proteins (such as HO1, NQO-1and CAT) under HG + HR conditions, this was further confirmed using siNrf2 (Figs. 5 and 6). Altogether, AGGF1-primed EPCs implantation effectively alleviates diabetic I/R injury via Nrf2 in DM.
Recently, many studies have focused on elucidating the molecular mechanisms underlying angiogenesis dysfunction in DM. For instance, signaling pathways such as CXCR7/Akt/Keap-1/Nrf2 and CXCR7/Akt/GSK-3 β/Fyn/Nrf2 have been reported to regulate ECs or EPCs function in DM9,23. During I/R in DM, mitochondrial damage pathways are considered important risk factors for cardiac injury7. However, it remains unclear whether AGGF1 mediates EPCs functions via mitochondrial pathways or AGGF1 crosses talk with CXCR7 to promote antioxidative activity. Additionally, how are the ROS production and inflammatory responses in AGGF1-primed EPCs co-cultured with cardiomyocytes in vitro? These scientific issues still require further investigation.
Research has shown that fibroblast growth factor (FGF), VEGFA, platelet-derived growth factor (PDGF), granulocyte colony-stimulating factor (G-CSF), and stromal cell-derived factor 1 (SDF-1) can enhance EPCs function to promote vascular repair following injury31. However, these factors have been associated with the risks of restenosis, leakage, and other complications post-vascular injury10. AGGF1 has been shown to enhance ECs connectivity (improve vascular integrity) and inhibit vascular restenosis15,32. These results indicate that AGGF1 may be a better therapeutic candidate for diabetic I/R injury treatment associated with DM. Short peptides are known for their enhanced stability compared to full-length proteins33. Our previous research identified the RDD peptide segment as the core region of AGGF1 protein34. Therefore, investigating whether the shorter RDD peptide segment exhibits greater therapeutic potential than the full-length AGGF1 protein holds significant value in the future.
Altogether, this study revealed the therapeutic potential of AGGF1-primed EPCs in diabetic cardiac I/R injury. AGGF1 effectively boosts the EPCs functions under HG + HR and alleviates diabetic cardiac I/R injury. Mechanistically, AGGF1 improves EPCs function via antioxidative Nrf2. Our findings suggest the promising application of AGGF1-primed EPCs therapy as a novel strategy for treating diabetic cardiac I/R injury.
Conclusion
The AGGF1 protein reverses the damaging effects of HG + HR on EPCs through antioxidative Nrf2. Our findings suggest that AGGF1-primed EPCs therapy represents a novel strategy for treating diabetic cardiac I/R injury.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Bleriot, C., Dalmas, E., Ginhoux, F. & Venteclef, N. Inflammatory and immune etiology of type 2 diabetes. Trends Immunol. 44, 101–109. https://doi.org/10.1016/j.it.2022.12.004 (2023).
Menon, K. et al. Shared medical appointments May be effective for improving clinical and behavioral outcomes in type 2 diabetes: A narrative review. Front. Endocrinol. 8, 263–274. https://doi.org/10.3389/fendo.2017.00263 (2017).
Szuszkiewicz-Garcia, M. M. & Davidson, J. A. Cardiovascular disease in diabetes mellitus: Risk factors and medical therapy. Endocrinol. Metab. Clin. 43, 25–40. https://doi.org/10.1016/j.ecl.2013.09.001 (2014).
Hein, T. W. et al. Requisite roles of LOX-1, JNK, and arginase in diabetes-induced endothelial vasodilator dysfunction of Porcine coronary arterioles. J. Mol. Cell. Cardiol. 131, 82–90. https://doi.org/10.1016/j.yjmcc.2019.04.015 (2019).
J. Neumann, F. Diabetes, heart failure, and myocardial revascularization: Is there a new message from the ISCHEMIA trial? Herz 47, 442–448. https://doi.org/10.1007/s00059-022-05132-8 (2022).
Gal, R. et al. Resveratrol improves heart function by moderating inflammatory processes in patients with systolic heart failure. Antioxidants 9, 1108–1126. https://doi.org/10.3390/antiox9111108 (2020).
Xiong, Y. et al. Decreased MFN2 activates the cGAS-STING pathway in diabetic myocardial ischaemia-reperfusion by triggering the release of mitochondrial DNA. Cell. Commun. Signal. 21, 192–213. https://doi.org/10.1186/s12964-023-01216-y (2023).
Chen, Y. et al. Activating transcription factor 4 mediates hyperglycaemia-induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1 diabetes. Diabetologia 55, 2533–2545. https://doi.org/10.1007/s00125-012-2594-1 (2012).
Dai, X. et al. Elevating CXCR7 improves angiogenic function of EPCs via Akt/GSK-3β/Fyn-Mediated Nrf2 activation in diabetic limb ischemia. Circul. Res. 120, e7–e23. https://doi.org/10.1161/circresaha.117.310619 (2017).
Yao, Y. et al. Angiogenic factor AGGF1-Primed endothelial progenitor cells repair vascular defect in diabetic mice. Diabetes 68, 1635–1648. https://doi.org/10.2337/db18-1178 (2019).
Tepper, O. M. et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 106, 2781–2786. https://doi.org/10.1161/01.cir.0000039526.42991.93 (2002).
Jarajapu, Y. P. & Grant, M. B. The promise of cell-based therapies for diabetic complications: Challenges and solutions. Circul. Res. 106, 854–869. https://doi.org/10.1161/circresaha.109.213140 (2010).
Lejay, A. et al. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J. Mol. Cell. Cardiol. 91, 11–22. https://doi.org/10.1016/j.yjmcc.2015.12.020 (2016).
Tian, X. L. et al. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature 427, 640–645. https://doi.org/10.1038/nature02320 (2004).
Yao, Y. et al. Targeting AGGF1 (angiogenic factor with G patch and FHA domains 1) for blocking neointimal formation after vascular injury. J. Am. Heart Assoc. 6, e005889–e005903. https://doi.org/10.1161/JAHA.117.005889 (2017).
Lu, Q. et al. Angiogenic factor AGGF1 activates autophagy with an essential role in therapeutic angiogenesis for heart disease. PLoS Biol. 14, e1002529–e1002558. https://doi.org/10.1371/journal.pbio.1002529 (2016).
Yao, Y. et al. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 8, 133–147. https://doi.org/10.1038/s41467-017-00171-w (2017).
Da, X. et al. AGGF1 therapy inhibits thoracic aortic aneurysms by enhancing integrin α7-mediated Inhibition of TGF-β1 maturation and ERK1/2 signaling. Nat. Commun. 14, 2265–2283. https://doi.org/10.1038/s41467-023-37809-x (2023).
He, Z. et al. Protein therapy of skeletal muscle atrophy and mechanism by angiogenic factor AGGF1. J. Cachexia Sarcopenia Muscle 14, 978–991. https://doi.org/10.1002/jcsm.13179 (2023).
Zhu, Q. et al. Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-κB pathway after subarachnoid hemorrhage in rats. J. Neuroinflamm. 15, 178–190. https://doi.org/10.1186/s12974-018-1211-8 (2018).
Nagy, C. & Einwallner, E. Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). J. Vis. Exp. JoVE. https://doi.org/10.3791/56672 (2018).
Kalka, C. et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. U. S. A. 97, 3422–3427. https://doi.org/10.1073/pnas.97.7.3422 (2000).
Jiang, C. et al. Upregulating CXCR7 accelerates endothelial progenitor cell-mediated endothelial repair by activating Akt/Keap-1/Nrf2 signaling in diabetes mellitus. Stem Cell Res. Ther. 12, 264–278. https://doi.org/10.1186/s13287-021-02324-7 (2021).
Yao, Y. et al. Losartan alleviates renal fibrosis and inhibits endothelial-to-mesenchymal transition (EMT) under high-fat diet-induced hyperglycemia. Front. Pharmacol. 9, 1213–1224. https://doi.org/10.3389/fphar.2018.01213 (2018).
Stockwell, B. R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421. https://doi.org/10.1016/j.cell.2022.06.003 (2022).
Cai, W. et al. Alox15/15-HpETE aggravates myocardial Ischemia-Reperfusion injury by promoting cardiomyocyte ferroptosis. Circulation 147, 1444–1460. https://doi.org/10.1161/circulationaha.122.060257 (2023).
Jiang, L. et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics 11, 1703–1720. https://doi.org/10.7150/thno.43895 (2021).
Galaup, A. et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 125, 140–149. https://doi.org/10.1161/circulationaha.111.049072 (2012).
Halladin, N. L. Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries. Dan. Med. J. 62, B5054–B5075 (2015).
Huang, J., Kong, Y., Xie, C. & Zhou, L. Stem/progenitor cell in kidney: Characteristics, homing, coordination, and maintenance. Stem Cell. Res. Ther. 12, 197–214. https://doi.org/10.1186/s13287-021-02266-0 (2021).
Chang, H. M. et al. FGF23 ameliorates ischemia-reperfusion induced acute kidney injury via modulation of endothelial progenitor cells: Targeting SDF-1/CXCR4 signaling. Cell Death Dis. 12, 409–427. https://doi.org/10.1038/s41419-021-03693-w (2021).
Zhang, T. et al. Haploinsufficiency of Klippel-Trenaunay syndrome gene Aggf1 inhibits developmental and pathological angiogenesis by inactivating PI3K and AKT and disrupts vascular integrity by activating VE-cadherin. Hum. Mol. Genet. 25, 5094–5110. https://doi.org/10.1093/hmg/ddw273 (2016).
Fisher, E., Pavlenko, K., Vlasov, A. & Ramenskaya, G. Peptide-based therapeutics for oncology. Pharm. Med. 33, 9–20. https://doi.org/10.1007/s40290-018-0261-7 (2019).
Yu, Y. et al. Angiogenic factor AGGF1 blocks neointimal formation after vascular injury via interaction with integrin α7 on vascular smooth muscle cells. J. Biol. Chem. 298, 101759–101773. https://doi.org/10.1016/j.jbc.2022.101759 (2022).
Acknowledgements
We thank other members of Genetics and Diseases Lab for advice, help and assistance.
Funding
This study was supported by Guangxi Science and Technology Base and Talent Project (2019AC20357), the Open Project Program of Guangxi Key Laboratory of Centre of Diabetic Systems Medicine, Guilin Medical University (GKLCDSM-20220101-04), the National Natural Science Foundation of China (32260166), the Open Project Program of Guangxi Key Laboratory of Brain and Cognitive Neuroscience, Guilin Medical University (GKLBCN-202301-03), Guangxi University Young and Middle-aged Teachers Basic Scientific Research Capacity Improvement Project( 2024KY0507, 2024KY0501), College Students Innovative Entrepreneurial Training Plan Program (202210601046, 20231060104).
Author information
Authors and Affiliations
Contributions
X.L. and S.M. performed the study, analyzed the data, and drafted the manuscript; S.H., C.D., Y.L., J.L., M.W., L.W. and L.Z. contributed to the investigation and data curation; Y.L. designed the study, analyzed the data, drafted the manuscript and acquired funding. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Mu, S., Huang, S. et al. AGGF1-primed endothelial progenitor cells alleviate ischaemia-reperfusion injury in diabetic hearts. Sci Rep 15, 21803 (2025). https://doi.org/10.1038/s41598-025-06190-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06190-8