Abstract
We aimed to assess the associations between caffeine and its metabolites and sex steroid hormones among children (aged 6–11 years) and adolescents (aged 12–19 years) using data from the U.S. National Health and Nutrition Examination Survey (NHANCES) conducted in 2013–2014. A total of 579 individuals aged 6–19 years with available data on urinary caffeine and its metabolites, as well as serum hormones [total testosterone (TT), estradiol (E2), sex hormone–binding globulin (SHBG)], were included. Additionally, the free androgen index (FAI) was calculated as TT/SHBG, and the ratio of TT to E2 (TT/E2) was estimated. Puberty status was defined based on hormone levels (TT ≥ 30 ng/dL in males and E2 ≥ 20 pg/ml in females for high steroid hormone levels; otherwise considered prepuberty). Linear regression, weighted quantile sum (WQS) regression, and Q-gcomp analyses were performed to estimate the associations of individual chemicals or chemical mixtures with sex hormones. Linear regression analyses indicated inverse associations between 12 and 15 caffeine metabolites with SHBG levels in male children and prepubertal boys, respectively. Furthermore, WQS regression demonstrated that caffeine mixtures were inversely associated with E2 and TT levels in male adolescents and prepubertal boys. Similar results were observed with Q-gcomp analysis. Exposure to caffeine and its metabolites, either individually or as a mixture, was inversely associated with SHBG levels in male children and prepubertal boys. Additionally, caffeine mixtures were associated with decreased levels of E2 and TT in male adolescents and prepubertal boys.
Similar content being viewed by others
Introduction
Caffeine (1,3,7-trimethylxanthine, C8H10N4O2) stands as one of the most popular and extensively consumed psychoactive substances worldwide. Global coffee consumption is estimated to reach approximately 1 million tons annually1. Caffeine pervades a diverse range of sources including foods, energy drinks, soft drinks, dietary supplements, and pharmaceuticals2,3. Predominantly, it is worth noting that different populations have varied sources of caffeine intake from food. For adults, the majority of caffeine intake is from coffee and tea, while for children, caffeinated soft drinks are the main sources of caffeine4. On the other hand, about 73% of American children consume caffeinated products daily5, which contains caffeine ranging from 50 to 500 mg (equivalent to 5 cups of coffee)6. After being ingested, caffeine is rapidly absorbed by the stomach and small intestine within 45 min, and subsequently metabolized in the liver7. Caffeine typically has an average elimination half-life of 5 h, and almost all of it undergoes metabolism in the body, with only 3% or less being excreted unchanged in urine8. Many studies indicated that caffeine has beneficial health effects including antioxidative properties, anti-inflammatory activities, improved mood and wakefulness, enhanced physical performance, and protection against diseases such as cardiovascular disease, obesity, and diabetes mellitus9,10. Despite these benefits, there are some negative effects of caffeine consumption were also observed like restlessness, insomnia, dehydration, addiction and migraine11. For instance, insomnia is one of the most reported side effects of caffeine on sleep. A previous randomized controlled trial showed that the additional caffeine supplementation impaired the sleep quality of athletes12. Moreover, another study indicated that decaffeinated green tea remarkably improved sleep quality and reduced stress and fatigue13. Therefore, caffeine has dual effects on human health. Special attention should be given to the effects of caffeine on specific conditions.
Sex steroid hormones were regulated by the hypothalamic–pituitary–gonadal (HPG) axis, which plays a pivotal role in physical development and initiation of puberty14. When puberty initiates, sex hormones contribute to the maturation of sexual organs (testes and ovaries) and the maintenance of bone, muscle, and endocrine balance15. Several studies indicated that abnormal levels of sex hormones increased the risk of fertility disorders by altering the structure of the testis and ovaries, spermatogenesis and sperm quality, oogenesis, and follicle maturation16,17. Some previous animal studies have shown that caffeine can interfere with the endocrine system by affecting sex hormones. In peripubertal male rats, researchers observed negative influences on the histological parameters of the testes and reduced responsiveness to testosterone in Leydig cells among animals fed high or low doses of caffeine18,19. As for peripubertal female rats, researchers found that exposure to high caffeine levels increases estradiol production in the ovary and delays vaginal opening, thereby interfering with sexual maturation20. Another study showed that caffeine upregulates sex hormone-binding globulin (SHBG) by increasing adiponectin in the SHBG transgenic mice21. However, few epidemiological studies have assessed the association between caffeine and sex hormones. An American study showed an inverse association between caffeine and testosterone in adult male22. Moreover, previous studies have reported that coffee and caffeine consumption are positively associated with SHBG in postmenopausal women and healthy non-diabetic women23,24.
Little is known about the associations between caffeine and sex steroid hormones in children and adolescents. Compared to adults, children and adolescents are more sensitive to the side effects of caffeine due to their immature physical development. Considering that sex hormones are crucial for the initiation of puberty and subsequent sexual maturation, it is essential to comprehend the connections between caffeine, whether as individual components or as a mixture and sex hormones in children and adolescents. Therefore, in this study, we aimed to assess the association of urinary caffeine and its 14 metabolites with serum sex steroid hormones in participants aged 6–19 years old in the U.S. National Health and Nutrition Examination Study (NHANES), and to further investigate potential mixture effects.
Material and methods
Study design and population
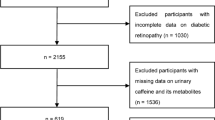
NHANES is a nationwide cross-sectional survey using multistate complex sampling strategies conducted biennially by the National Center for Health Statistics (NCHS), a department of the Centers for Disease Control and Prevention (CDC). The project involves in-person interviews conducted at participants’ homes, as well as standardized health examinations in the mobile examination center (MEC), where the blood and urinary samples were collected. All procedures and contents underwent approval by the NCHS Ethics Review Board, and written informed consent was obtained from all participants before their involvement. In our study, publicly available data from the 2013–2014 NHANES were utilized. A total of 579 participants aged 6–19 years with available data on urinary caffeine, caffeine metabolites, serum total testosterone (TT), estradiol (E2), and sex hormone binding globulin (SHBG) were selected. We reviewed participants’ health records and identified only one individual with juvenile diabetes among the included sample. Given the very low prevalence, no additional exclusions based on health conditions were applied. The participant selection process is outlined in Fig. S.1 in the Supplementary Material.
Ethics declarations
This study was based on publicly available data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014, conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). The NHANES study protocol was reviewed and approved by the NCHS Research Ethics Review Committee, and all participants provided written informed consent. As the current study involved secondary analysis of de-identified public use data, additional ethical approval was not required. Data use complied with the NHANES Data Release and Access Policy and the NCHS Data Use Agreement, ensuring participant confidentiality.
Measurements of caffeine metabolites
Urinary caffeine and its 14 metabolites were measured by NHANES using spot urine samples collected at the Mobile Examination Center (MEC) and stored under appropriate frozen conditions (-20℃). The measured metabolites included 1,3,7-trimethylxanthine (1,3,7-TMX), 1-methyluric acid (1-MU), 3-methyluric acid (3-MU), 7-methyluric acid (7-MU), 1,3-dimethyluric acid (1,3-DMU), 1,7-dimethyluric acid (1,7-DMU), 3,7-dimethyluric acid (3,7-DMU), 1,3,7-trimethyluric acid (1,3,7-TMU), 1-methylxanthine (1-MX), 3-methylxanthine (3-MX), 7-methylxanthine (7-MX), 1,3-dimethylxanthine (1,3-DMX), 1,7-dimethylxanthine (1,7-DMX), 3,7-dimethylxanthine (3,7-DMX), and 5‐acetylamino‐6‐amino‐3‐methyluracil (AAMU).
All laboratory testing was performed by certified laboratories contracted by NHANES, following standardized protocols documented in the NHANES Laboratory/Medical Technologists Procedures Manual. Specifically, caffeine and its metabolites were quantified using high-performance liquid chromatography-electrospray ionization-tandem quadrupole mass spectrometry (HPLC-ESI-MS/MS) with stable isotope-labeled internal standards. Detailed methodology is available at the official NHANES website (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CAFE_H.htm).
The lower limits of detection (LLOD) were determined by NHANES as follows: 0.05 μmol/L for 1-MU, 0.1 μmol/L for 3-MU, 0.04 μmol/L for 7-MU, 0.02 μmol/L for 1,3-DMU, 0.02 μmol/L for 1,7-DMU, 0.03 μmol/L for 3,7-DMU, 0.005 μmol/L for 1,3,7-TMU, 0.03 μmol/L for 1-MX, 0.04 μmol/L for 3-MX, 0.02 μmol/L for 7-MX, 0.01 μmol/L for 1,3-DMX, 0.006 μmol/L for 1,7-DMX, 0.004 μmol/L for 3,7-DMX, 0.003 μmol/L for 1,3,7-TMX, and 0.1 μmol/L for AAMU.
In our analyses, metabolites with a detection frequency greater than 70% were included. Values below the LLOD were substituted with LLOD/√2, in accordance with the NHANES analytic guidelines (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/CAFE_H.htm).
Measurement of sex hormone
Serum samples were processed and stored at -20℃ until shipping to the National Center for Environmental Health for testing. TT and E2 were quantified using isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS). SHBG concentrations were determined by immunoassay utilizing antibodies specific to SHBG, followed by chemiluminescence measurement of the reaction products using a photomultiplier tube. The details of the detection method were available from the official site (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/TST_H.htm). The LLODs of TT, E2 and SHBG were 0.75 ng/mL, 2.994 pg/m and 0.800 nmol/l, respectively. Following previous published studies25,26,27, we calculated the free androgen index (FAI) equal to the value of TT (ng/dL) divided by SHBG (nmol/L) and the ratio of TT to E2 (TT/E2) to indirectly assess the approximate amount of circulating free testosterone and the activity of aromatase.
Covariates
Based on previous reports28,29,30, we selected these potential confounders which were associated with both caffeine exposure and sex hormone, including age (years), race/ethnicity (Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), and family poverty-income ratio (PIR) and body mass index (BMI) category (underweight: BMI < 5th percentile, normal weight: 5th ≤ BMI < 85th percentile, overweight: 85th ≤ BMI < 95th percentile, obese: BMI ≥ 95th percentile). Furthermore, to mitigate the influence of diurnal fluctuations in hormone levels, the time of blood draw (morning, afternoon, evening) and time period of examination (November 1 through April 30, May 1 through October 31) were adjusted in the regression model as covariates. Additionally, serum cotinine levels were utilized to assess tobacco exposure, while urinary creatinine concentrations were measured in urine samples to determine the degree of dilution. Since the distribution of urinary creatinine was right-skewed, we transformed it using a natural logarithm. Consequently, to diminish the influence of tobacco exposure and urinary dilution, we adjusted for cotinine exposure status (> 0.015 ng/mL, ≤ 0.015 ng/mL) and urinary creatinine in our regression model26.
Statistical analysis
Due to substantial variations in sex hormone levels across different sexes and developmental stages, we conducted descriptive statistical analysis and performed stratified analyses based on age groups (children: 6–11 years and adolescents: 12–19 years) in both males and females. The distributions of urinary caffeine metabolites, creatinine, and sex hormones exhibited predominantly right-skewed patterns. Consequently, they underwent natural-log transformation (ln-transformation) to improve normality in both descriptive and regression analyses. Sampling weights are usually employed to produce statistically representative and unbiased estimates when analyzing survey data. However, it diminishes the accuracy of the estimates and may even introduce over-adjustment bias when variables (such as race, age, and income) used in calculating the sampling weights are further adjusted in regression analyses. So, we presented our results without using sampling weights, similar to the previously published studies31,32,33.
In our principal analysis, we applied multiple linear regression to assess the relationship between individual urinary caffeine, caffeine metabolites, and serum sex hormones. Individual caffeine and its metabolites were simultaneously modeled as both continuous variables and categorized variables (quartiles). We further conducted tests for trend (p-trend) analysis by using the median urinary chemicals concentration in each quantile as a linear variable in the regression models. It is worth noting that sex hormone concentrations are notably influenced by puberty status. Therefore, to address this concern, we further categorized participants into pubertal and prepubertal groups based on their serum sex hormone levels. Participants were grouped as a pubertal group if their levels of TT ≥ 30 ng/dL for boys or E2 ≥ 20 pg/mL for girls34,35. The remaining were classified as a prepubertal group.
Moreover, weighted quantile sum (WQS) regression was employed to investigate the mixture effects of caffeine and its metabolites on sex hormones. Briefly, WQS regression is a flexible and robust statistical method, that has been widely used to estimate the individual’s overall exposure burden value. In our study, the WQS regression model computes a weighted linear index that reflects the overall burden of all caffeine and its metabolites in the body. Each caffeine metabolite is assigned a corresponding weight, indicating its contribution to the WQS index. The WQS model was presented as follows:
where Y was continuous sex hormone with ln transformation and β0 reflects the model intercept, while β1 was the parameter estimate for the co-exposure index, wi indicates a vector of empirically estimated weights for each caffeine metabolite, and qij indicates the quantile rank assigned to each subject per variable, and β2 represents the parameter of covariable. We conducted 200 bootstrapped samples to assess the weight estimations, with the weights constrained to sum to 1 and to range between 0 and 1 to facilitate comparison. The weight assigned to each component indicated its contribution to the WQS index. WQS analyses were divided into training and validation sets (40:60 ratio), with the training dataset utilized for estimating variable weights and the validation dataset employed to assess mixture significance. Given the absence of prior evidence regarding the directional relationships between each chemical and sex hormones, two models of WQS regression analyses were conducted. One model assumed positive relationships between all components of the WQS index and sex hormones, while the other model assumed negative relationships for all components of the index.
In our secondary analysis, we conducted several additional statistical analyses to further evaluate the robustness of the findings from the primary analysis. Firstly, we reran the multiple linear regression analysis including all participants to mitigate potential bias due to limited sample size. Secondly, to validate the mixture effect of caffeine metabolites on sex hormones, we also conducted Q-gcomp as a complementary method to estimate the overall association of caffeine mixtures with sex hormones. The Q-gcomp integrates the inferential simplicity of WQS with the flexibility of g-computation. It relaxes the assumption of directional homogeneity and enables individual components within the mixtures to contribute either positively or negatively to a mixture index. The total sum of weights for all compounds is constrained to 1. The mixture slope and overall model confidence bounds were determined through 200 bootstrap iterations. Furthermore, we also repeated the multiple linear regression without adjusting for creatinine to verify the stability of the results similar as the previous study. Additionally, we calculated the E-value to evaluate the potential influence from unmeasured confounding bias36.
The results of the regression analyses were presented as coefficients along with their respective 95% confidence intervals (CIs). All the analyses were conducted in R software (version 4.1.0). WQS and Q-gcomp were implemented with R package “gWQS” (version 3.0.5) and “qgcomp” (version 2.15.2), respectively. Statistical significance was determined at the 0.05 level.
Results
Descriptive statistics
The demographic characteristics, distributions of sex hormones, and caffeine metabolites of all 579 participants, as well as those stratified by sex and age subgroups, are summarized in Table 1. The mean age of the overall participants was 12.2 years (± 3.9 years). The overall detection frequencies were 99.5% for TT, 62.7% for E2 and 100% for SHBG (Table S.1). However, the detection frequencies of E2 in children and prepubertal participants were less than 70%. Therefore, analyses for E2 and the ratio of total testosterone to estradiol (TT/E2) were not conducted in these groups (Table S.1). As for the detection of urinary caffeine and its metabolites, all of the chemicals had the detection frequency above 70% in all participants or subgroups. The overall median concentrations of caffeine and its metabolites ranged from 0.4 μg/L to 63.7 μg/L. The pairwise Spearman correlations among the caffeine and its metabolites were all statistically significant (P < 0.05), with correlation coefficients ranging from 0.37 to 0.98, indicating the need to consider the correlation among the metabolites when evaluating their individual and mixture effects (Fig. S.2).
Association of caffeine and its metabolites with sex hormone by sex-age groups
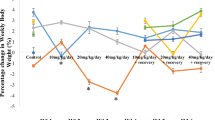
Table 2 displayed the associations between continuous caffeine metabolites and sex hormone indicators across sex-age groups. Additionally, Fig. 1 and Figs. S.3–5 illustrate the relationships between quartiles of caffeine metabolites and sex hormone indicators in the respective sex-age groups. Among male children, our study revealed an inverse association between 12 caffeine metabolites and SHBG, specifically including 1-MU, 3-MU, 7-MU, 1,3-DMU, 1,7-DMU, 3,7-DMU, 1-MX, 3-MX, 7-MX, 1,3-DMX, 1,7-DMX, 3,7-DMX, respectively (Table 2). Furthermore, the trend testing showed monotonic inverse association between 3-MU, 1,3-DMU, 1,7-DMU, 1,3,7-TMU, 1-MX, 1,3-DMX, 3,7-DMX, 1,3,7-TMX, AAMU and SHBG in male children (all P-trend < 0.05, Fig. 1). Among female children, 3,7-DMU was inversely associated with SHBG. Moreover, the trend testing indicated that 3,7-DMU associated with SHBG with a monotonic decreasing trend (Fig. S.3). In addition, positive associations between 7-MU, 3,7-DMU and FAI. However, there were no consistent trends in FAI with increasing quartiles of caffeine metabolites.
Associations of quartiles of caffeine and its metabolites with sex hormones in male children of 6–19 years old in NHANES 2013–2014. Children (aged 6–11 years) and adolescents (aged 12–19 years). Estimates were adjusted for urinary creatinine (continuous), age (continuous), race/ethnicity (categorical), BMI category, ratio of family income to poverty (continuous), cotinine (categorical), time of sample collection (categorical) and time period of examination. Analyses for E2 were not conducted in children due to the detection frequency of E2 being less than 70% in that group. The quartiles of caffeine and its metabolites were denoted as “Q1” through “Q4”, representing the 1st to 4th quartiles, respectively. In the figures, red error bars indicate statistically significant estimates (P < 0.05). The significance of the trend test was indicated as follows: * for P < 0.05, ** for P < 0.01, and *** for P < 0.001.
In adolescents, no significant association or trend between most caffeine metabolites and sex hormones was observed. In male adolescents, only 1-MX (4th vs 1st quartile) was associated with SHBG without a significant trend (Fig. S.4). Among female adolescents, 1-MU (4th vs 1st quartile), 1,3-DMU (4th vs 1st quartile) and 3,7-MX (4th vs 1st quartile) were significantly inversely associated with TT. 3,7-DMU (4th vs 1st quartile) and 3-MX (4th vs 1st quartile) presented a decreasing trend with SHBG (Fig. S.5).
Association of caffeine and its metabolites with sex hormone by sex-puberty groups
The associations and patterns across quartiles of caffeine metabolites, stratified by puberty status in both sexes, exhibited similarities to those observed in the analysis stratified by sex-age groups (Table 3, Fig. 2 and S.6–8). Some novel observations emerged within the sex-puberty groups. Among prepubertal boys, 1,3,7-TMU (2nd vs 1st quartile) and 1,3-DMX (3rd vs 1st quartile) were positively associated with FAI (Fig. 2). In prepubertal girls, 1,3-DMU (2nd vs 1st quartile) and 1,3,7-TMU (2nd vs 1st quartile) were inversely associated with TT. Additionally, considering the effect of limited sample size among different subgroups, we assessed the association between caffeine metabolites and sex hormones in all populations (Table. 2). The results revealed that 9 caffeine metabolites exhibited significant inverse associations with SHBG, namely 3-MU, 7-MU, 1,3-DMU, 1,7-DMU, 3,7-DMU, 3-MX, 1,3-DMX, 1,7-DMX, and 3,7-DMX. For pubertal boys and girls, most caffeine metabolites did not display significant associations or trends with sex hormones. Only among pubertal girls, 3-MU and 3,7-DMX were significantly inversely associated with SHBG (− 0.061, 95% CI − 0.105, − 0.017; − 0.06 95% CI − 0.115, − 0.006).
Associations of quartiles of caffeine and its metabolites with sex hormones in prepubertal boys of 6–19 years old in NHANES 2013–2014. Puberty status was defined as “pubertal” if TT ≥ 30 ng/dL in males, E2 ≥ 20 pg/ml in females, otherwise puberty status was defined as “prepubertal”. Estimates were adjusted for urinary creatinine (continuous), age (continuous), race/ethnicity (categorical), BMI category, ratio of family income to poverty (continuous), cotinine (categorical), time of sample collection (categorical) and time period of examination. Analyses for E2 were not conducted in children due to the detection frequency of E2 being less than 70% in that group. The quartiles of caffeine and its metabolites were denoted as “Q1” through “Q4”, representing the 1st to 4th quartiles, respectively. In the figures, red error bars indicate statistically significant estimates (P < 0.05). The significance of the trend test was indicated as follows: * for P < 0.05, ** for P < 0.01, and *** for P < 0.001.
Associations of the mixture of caffeine and caffeine metabolites with sex hormones
We utilized a WQS regression model to calculate the WQS index, aiming to assess the association between the mixture of caffeine and its metabolites and sex hormones. The relationships between the WQS index and sex hormones are summarized in Table 4. When stratified by sex-age groups, the WQS index demonstrated an inverse association with SHBG in male children (− 0.129, 95% CI − 0.240, − 0.018). In male adolescents, a significant inverse association between the WQS index and E2 was observed (− 0.173, 95% CI − 0.321, − 0.024). However, no significant association was detected between the WQS index and sex hormones in female children and adolescents. Upon stratification by sex and puberty status, the WQS index consistently displayed an inverse association with SHBG in the prepubertal boys (− 0.165, 95% CI − 0.277, − 0.053). Additionally, the WQS index was significantly inversely associated with TT in prepubertal boys (− 0.175, 95% CI − 0.341, − 0.010). In the pubertal girls, only a significant inverse association between the WQS index and SHBG in the negative model was observed (− 0.156, 95% CI − 0.281, − 0.031). A sensitive analysis was conducted by rerunning WQS regression models without adjusting creatine. The results are presented in Table S.3 which aligns with the findings in Table 4.
Tables S.4–5 present the estimated weights of the WQS index for the associations with sex hormones by sex-age groups and sex-puberty status groups. In general, the predominant components among the caffeine metabolites align with those demonstrating significant associations in the multiple linear regression analysis. For instance, 1,3,7-TMU was the predominant metabolite responsible for the significant inverse association with SHBG (weight = 0.170) in male children.
Table S.6 summarizes the associations between the mixture of caffeine metabolites and sex hormones by Q-gcomp regression. Consistent with the results in WQS models, the mixture of caffeine metabolites showed a significantly inverse association with SHBG in the male children and prepubertal boys (− 0.108, 95% CI − 0.204, − 0.011; − 0.145, 95% CI − 0.254, − 0.036). As for the female adolescents and pubertal girls, the mixture of caffeine metabolites was significantly inversely associated with SHBG (− 0.127, 95% CI − 0.244, − 0.010; − 0.137, 95% CI − 0.247, − 0.028).
The E value for the associations between caffeine metabolites and sex hormones
E values were calculated to evaluate the impact of potential unmeasured confounding on the relationships between caffeine, its metabolites, and sex hormones across various sex-age and sex-puberty subgroups. Overall, the E value for the caffeine metabolites that showed a significant association with sex hormones is moderate. For example, the largest E value was observed in an inverse association between 1-MU and SHBG in male children (E = 1.470). Similarly, the largest E value was noted in this association in prepubertal boys (E = 1.557). Detailed E values for each association between caffeine metabolites and sex hormone indicators were summarized in Tables S.7–8.
Discussion
Our study is the first to investigate the association between caffeine metabolites and sex hormones among participants aged 6–19 years old. We observed significant inverse associations between certain caffeine metabolites and SHBG in children and prepubertal individuals. Notably, in adolescents and pubertal individuals, our findings revealed significant inverse associations between caffeine mixture and sex steroid hormones, with some variations based on sex. Particularly, the caffeine mixture exhibited significant inverse associations with TT and E2 in males. However, our study found limited evidence supporting significant associations between caffeine mixture and sex steroid hormones in adolescent females and pubertal girls.
To date, there is limited epidemiological research exploring the effects of caffeine exposure on sex hormones. Our WQS regression analysis demonstrated a significant inverse association between caffeine mixture and TT in prepubertal boys. One cross-sectional study has indicated that caffeine and its metabolites were inversely associated with TT levels in adult males, which aligns with our study findings22. Several potential biological mechanisms may underlie this inverse association. Firstly, caffeine is a psychoactive substance that antagonizes adenosine receptors in the brain, particularly acting on all four adenosine receptor subtypes (A1, A2a, A2b, A3)37. Additionally, adenosine receptors are also present in the testes, primarily localized within the Leydig and Sertoli cells of the seminiferous tubules38. Activation of these testicular adenosine receptors is associated with the inhibition of cellular responses. Consequently, pathways involving cAMP/protein kinase, which are typically responsible for mediating testosterone production, are downregulated upon receptor activation. This downregulation may consequently lead to reduced testosterone production. Thus, it is plausible that caffeine affects testosterone production through these adenosine-dependent pathways. Secondly, caffeine has been shown in vitro to induce abnormal DNA methylation and histone acetylation of the steroidogenic factor-1 (SF-1) promoter in rat fetal adrenals39. This action results in decreased transcription of the SF-1 gene. SF-1 is a crucial transcription factor responsible for regulating genes involved in steroidogenesis and testosterone biosynthesis in males. Therefore, reduced expression of SF-1 may contribute to low testosterone levels associated with caffeine exposure. In addition, the metabolites of caffeine, such as theophylline and theobromine, have been implicated in directly affecting gonadotropin-induced steroidogenesis40. Specifically, theophylline and theobromine act as phosphodiesterase inhibitors, adenosine receptor blockers, and histone deacetylase activators directly involving testicular atrophy and impaired steroidogenesis by inhibiting precursor incorporation into RNA and protein.
On the other hand, in our study, we observed an inverse association between caffeine mixture and E2 in male adolescents. A previous epidemiological study indicated that caffe consumption was inversely associated with estrone in US health professionals which was similar to our present results41. Similarly, another study also highlighted the disruptive effect of caffeine on the E2 levels in the Astyanax altiparanae males42. The biological mechanism underlying the inverse association between caffeine mixture and E2 remains unclear. E2 is primarily converted from TT in male individuals. As discussed earlier, caffeine can reduce the TT concentration through various pathways, including antagonizing adenosine receptors, disrupting steroidogenic factor-1 promoter and toxicity of caffeine metabolites. Thus, the impaired TT levels may indirectly result in reduced E2 levels in male individuals. Moreover, animal studies have indicated that caffeine might disrupt estrogen metabolism by inhibiting aromatase43. Aromatase is the crucial enzyme responsible for converting androgens into estrogens44. The decrease in the concentration and activity of aromatase may contribute to influencing the levels of E2 in male individuals.
Regarding SHBG, the association appears to be inconsistent across previous studies. Tanja et al. explored the association between consumption of caffeinated beverages and serum SHBG in US adult men45. They observed a slight positive association between the frequency of caffeinated drinks and SHBG concentration, but there was no consistent association found for caffeine intake. Meanwhile, Frank et al. failed to observe a significant association between urinary caffeine and serum SHBG in US adults22. In our adolescent individuals, the association between urinary caffeine and serum SHBG was non-significant, consistent with the findings of previous studies. However, it is worth noting that we observed a significant inverse association between urinary caffeine and serum SHBG in children males and prepubertal boys. Several potential biases might influence the association between caffeine and SHBG. Firstly, exposure misclassification is a crucial consideration. Many studies in the past decades have evaluated caffeine exposure using information from Food-Frequency Questionnaires (FFQs)46,47. Therefore, recall bias may decrease the accuracy and precision of exposure assessment. Additionally, the study population itself is another crucial factor to consider. Sex hormone levels vary significantly between genders and age groups, with dramatic variations observed among children and adolescents during puberty. Meanwhile, caffeine is primarily metabolized in the liver. Children and adolescents might be more sensitive to caffeine exposure due to their immature liver function compared to adults. The rational mechanisms by which caffeine may affect SHBG are not clear. SHBG is primarily produced and secreted by the human liver under the influence of hormonal factors48. A recent study employing Mendelian randomization analysis showed a positive association between TT and SHBG in boys49. In our present study, we observed a negative association between caffeine exposure and TT. So, there is a reasonable hypothesis that the inverse effect of caffeine on the SHBG might be the feedback of the TT concentration in boys. Moreover, a recent study reported that caffeine exposure was significantly associated with BMI z-score and waist circumference in both boys and girls50. Traditionally, BMI has been considered a major determinant of SHBG concentrations51. Several studies have reported the inverse association between BMI and SHBG52,53,54. Therefore, the negative effect of caffeine exposure on the SHBG may be mediated by the BMI. More in vivo and vitro studies are warranted to elucidate the mechanism underlying the association between caffeine exposure and SHBG among children and adolescents.
There are several strengths to our study. Firstly, we employed HPLC-ESI-MS/MS to measure caffeine and its metabolites in urinary samples, a method that offers more precise exposure assessment and reduces the potential for recall bias compared to questionnaire-based methods. Additionally, the use of ID-LC-MS/MS for measuring sex hormones ensured accurate and reliable quantification, bolstering the credibility of our findings. Secondly, we employed diverse methodologies to estimate the impact of 15 chemical mixtures on sex hormones. WQS and Q-gcomp, two recently developed regression models for assessing the relationship between chemical mixtures and health outcomes, were utilized to account for highly correlated chemicals. Both models consistently indicated an inverse association between caffeine mixtures and SHBG levels in male children and prepubertal boys. Thirdly, we rigorously controlled for potential confounders by adjusting for various covariates based on prior research, including age, BMI status, race, time of blood draw, PIR, time period of examination, serum cotinine exposure status, and urinary creatinine. Furthermore, we calculated E values to evaluate the magnitude of the effect of unmeasured confounders on the association between caffeine and its metabolites and sex hormones.
However, our study also possesses certain limitations. Firstly, due to the cross-sectional design of the NHANCES project, we are unable to establish causal relationships between caffeine, its metabolites, and sex hormones in children and adolescents. Although there are plausible biological mechanisms that could explain the effects of caffeine on sex hormones, large prospective cohort studies are needed to provide more robust evidence. Secondly, as our measurements of caffeine exposure were directly obtained from urinary samples, we were unable to ascertain the specific sources of caffeine in this study. Different sources of caffeine, such as caffeinated drinks versus coffee, may have distinct health profiles that could potentially affect the regulation of sex hormones differently. Thirdly, our study lacked accurate data on puberty status in the study population, which inevitably limited our ability to adequately adjust for the impact of puberty status on our results. While we inferred puberty status based on levels of TT in males and E2 in females, which are indicative but not definitive markers of puberty, utilizing Tanner staging as the gold standard for classifying puberty status would have been preferable. Furthermore, we included one participant diagnosed with juvenile diabetes, the extremely low prevalence suggests minimal impact on the overall results. Nevertheless, we acknowledge that underlying health conditions could potentially influence hormone levels and should be considered in future studies. Lastly, individual variations in the metabolic capability of caffeine, particularly in relation to cytochrome P450 enzymes in the liver, were not taken into account. Several studies have highlighted how polymorphisms in P450 enzymes could modify the effects of caffeine on health55,56. Therefore, future studies should consider the metabolic capacity of individuals when investigating the relationship between caffeine and its health effects.
Conclusions
In conclusion, our study revealed significant inverse associations between specific caffeine metabolites and caffeine mixtures with SHBG levels in male children and prepubertal boys. Additionally, we found that caffeine mixtures were inversely correlated with E2 and TT levels in male adolescents and prepubertal boys. These findings contribute to a more nuanced understanding of the endocrine profile in children and adolescents. However, it’s necessary to acknowledge the limitations of this study, including cross-sectional design and small sample size. Therefore, further research is warranted to validate and expand upon our findings.
Data availability
The NHANES data set is publicly available at the National Center for Health Statistics of the CDC (https://www.cdc.gov/nchs/nhanes/index.htm).
References
Saimaiti, A. et al. Dietary sources, health benefits, and risks of caffeine. Crit. Rev. Food Sci. Nutr. 63(29), 9648–9666. https://doi.org/10.1080/10408398.2022.2074362 (2023).
Mahoney, C. R. et al. Intake of caffeine from all sources and reasons for use by college students. Clin. Nutr. 38(2), 668–675. https://doi.org/10.1016/j.clnu.2018.04.004 (2019).
Heckman, M. A., Weil, J. & Gonzalez de Mejia, E. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 75(3), R77-87. https://doi.org/10.1111/j.1750-3841.2010.01561.x (2010).
Verster, J. C. & Koenig, J. Caffeine intake and its sources: A review of national representative studies. Crit. Rev. Food Sci. Nutr. 58(8), 1250–1259. https://doi.org/10.1080/10408398.2016.1247252 (2018).
Branum, A. M., Rossen, L. M. & Schoendorf, K. C. Trends in caffeine intake among U.S. children and adolescents. Pediatrics 133(3), 386–393. https://doi.org/10.1542/peds.2013-2877 (2014).
Soos, R., Gyebrovszki, A., Toth, A., Jeges, S. & Wilhelm, M. Effects of caffeine and caffeinated beverages in children, adolescents and young adults: Short review. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph182312389 (2021).
Chen, F., Hu, Z. Y., Parker, R. B. & Laizure, S. C. Measurement of caffeine and its three primary metabolites in human plasma by HPLC-ESI-MS/MS and clinical application. Biomed. Chromatogr. 31(6), e3900. https://doi.org/10.1002/bmc.3900 (2017).
Begas, E., Kouvaras, E., Tsakalof, A., Papakosta, S. & Asprodini, E. K. In vivo evaluation of CYP1A2, CYP2A6, NAT-2 and xanthine oxidase activities in a Greek population sample by the RP-HPLC monitoring of caffeine metabolic ratios. Biomed. Chromatogr. 21(2), 190–200. https://doi.org/10.1002/bmc.736 (2007).
Zhou, D. D. et al. Antioxidant food components for the prevention and treatment of cardiovascular diseases: Effects, mechanisms, and clinical studies. Oxid. Med. Cell Longev. 2021, 6627355. https://doi.org/10.1155/2021/6627355 (2021).
Zhou, A. & Hypponen, E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: A prospective analysis of up to 347,077 individuals and 8368 cases. Am. J. Clin. Nutr. 109(3), 509–516. https://doi.org/10.1093/ajcn/nqy297 (2019).
Juliano, L. M. & Griffiths, R. R. A critical review of caffeine withdrawal: Empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology 176(1), 1–29. https://doi.org/10.1007/s00213-004-2000-x (2004).
Ramos-Campo, D. J., Perez, A., Avila-Gandia, V., Perez-Pinero, S. & Rubio-Arias, J. A. Impact of caffeine intake on 800-m running performance and sleep quality in trained runners. Nutrients 11(9), 2040. https://doi.org/10.3390/nu11092040 (2019).
Unno, K. et al. Reduced stress and improved sleep quality caused by green tea are associated with a reduced caffeine content. Nutrients 9(7), 777. https://doi.org/10.3390/nu9070777 (2017).
Hansen, A. B. et al. Diagnosis of endocrine disease: Sex steroid action in adolescence: Too much, too little; too early, too late. Eur. J. Endocrinol. 184(1), R17–R28. https://doi.org/10.1530/EJE-20-0545 (2021).
Li, J. et al. Investigation of bioeffects of G protein-coupled receptor 1 on bone turnover in male mice. J. Orthop. Translat. 10, 42–51. https://doi.org/10.1016/j.jot.2017.05.001 (2017).
Martirosyan, A. & Schneider, Y. J. Engineered nanomaterials in food: Implications for food safety and consumer health. Int. J. Environ. Res. Public Health 11(6), 5720–5750. https://doi.org/10.3390/ijerph110605720 (2014).
Tyagi, V., Scordo, M., Yoon, R. S., Liporace, F. A. & Greene, L. W. Revisiting the role of testosterone: Are we missing something?. Rev. Urol. 19(1), 16–24. https://doi.org/10.3909/riu0716 (2017).
Park, M., Choi, Y., Choi, H., Yim, J. Y. & Roh, J. High doses of caffeine during the peripubertal period in the rat impair the growth and function of the testis. Int. J. Endocrinol. 2015, 368475. https://doi.org/10.1155/2015/368475 (2015).
Bae, J., Choi, H., Choi, Y. & Roh, J. Dose- and time-related effects of caffeine on the testis in immature male rats. Exp. Anim. 66(1), 29–39. https://doi.org/10.1538/expanim.16-0060 (2017).
Kwak, Y., Choi, H., Bae, J., Choi, Y. Y. & Roh, J. Peri-pubertal high caffeine exposure increases ovarian estradiol production in immature rats. Reprod. Toxicol. 69, 43–52. https://doi.org/10.1016/j.reprotox.2017.01.007 (2017).
Brianso-Llort, L. et al. Caffeine upregulates hepatic sex hormone-binding globulin production by increasing adiponectin through AKT/FOXO1 pathway in white adipose tissue. Mol. Nutr. Food Res. 64(17), e1901253. https://doi.org/10.1002/mnfr.201901253 (2020).
Glover, F. E. et al. The association between caffeine intake and testosterone: NHANES 2013–2014. Nutr. J. 21(1), 33. https://doi.org/10.1186/s12937-022-00783-z (2022).
Goto, A. et al. Coffee and caffeine consumption in relation to sex hormone-binding globulin and risk of type 2 diabetes in postmenopausal women. Diabetes 60(1), 269–275. https://doi.org/10.2337/db10-1193 (2011).
Pihan-Le Bars, F., Gusto, G., Boutron-Ruault, M. C., Fagherazzi, G. & Bonnet, F. Cross-sectional association of coffee and caffeine consumption with sex hormone-binding globulin in healthy nondiabetic women. Clin. Endocrinol. (Oxf). 87(5), 475–483. https://doi.org/10.1111/cen.13411 (2017).
Hu, P. et al. Associations between exposure to a mixture of phenols, parabens, and phthalates and sex steroid hormones in children 6–19 years from NHANES, 2013–2016. Sci. Total Environ. 822, 153548. https://doi.org/10.1016/j.scitotenv.2022.153548 (2022).
Luo, K. et al. Associations between organophosphate esters and sex hormones among 6–19-year old children and adolescents in NHANES 2013–2014. Environ. Int. 136, 105461. https://doi.org/10.1016/j.envint.2020.105461 (2020).
Zhang, Y. et al. Independent and combined associations of urinary arsenic exposure and serum sex steroid hormones among 6–19-year old children and adolescents in NHANES 2013–2016. Sci. Total Environ. 863, 160883. https://doi.org/10.1016/j.scitotenv.2022.160883 (2023).
Wang, Y. et al. Perfluoroalkyl substances and sex hormones in postmenopausal women: NHANES 2013–2016. Environ. Int. 149, 106408. https://doi.org/10.1016/j.envint.2021.106408 (2021).
Aimuzi, R., Wang, Y., Luo, K. & Jiang, Y. Exposure to phthalates, phenols, and parabens mixture and alterations in sex steroid hormones among adolescents. Chemosphere 302, 134834. https://doi.org/10.1016/j.chemosphere.2022.134834 (2022).
Zhang, J. et al. Phthalate metabolites and sex steroid hormones in relation to obesity in US adults: NHANES 2013–2016. Front. Endocrinol. (Lausanne). 15, 1340664. https://doi.org/10.3389/fendo.2024.1340664 (2024).
Luo, K. et al. Exposure to organophosphate esters and metabolic syndrome in adults. Environ. Int. 143, 105941. https://doi.org/10.1016/j.envint.2020.105941 (2020).
Lewis, R. C. & Meeker, J. D. Biomarkers of exposure to molybdenum and other metals in relation to testosterone among men from the United States National Health and Nutrition Examination Survey 2011–2012. Fertil. Steril. 103(1), 172–178. https://doi.org/10.1016/j.fertnstert.2014.09.020 (2015).
James-Todd, T. et al. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. 120(9), 1307–1313. https://doi.org/10.1289/ehp.1104717 (2012).
Soeborg, T. et al. Sex, age, pubertal development and use of oral contraceptives in relation to serum concentrations of DHEA, DHEAS, 17alpha-hydroxyprogesterone, Delta4-androstenedione, testosterone and their ratios in children, adolescents and young adults. Clin. Chim. Acta. 437, 6–13. https://doi.org/10.1016/j.cca.2014.06.018 (2014).
Lopez-Espinosa, M. J. et al. Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ. Sci. Technol. 45(19), 8160–8166. https://doi.org/10.1021/es1038694 (2011).
VanderWeele, T. J. & Ding, P. Sensitivity analysis in observational research: Introducing the E-Value. Ann. Intern. Med. 167(4), 268–274. https://doi.org/10.7326/M16-2607 (2017).
Pereira-Figueiredo, D., Nascimento, A. A., Cunha-Rodrigues, M. C., Brito, R. & Calaza, K. C. Caffeine and its neuroprotective role in ischemic events: A mechanism dependent on adenosine receptors. Cell Mol. Neurobiol. 42(6), 1693–1725. https://doi.org/10.1007/s10571-021-01077-4 (2022).
Belardin, L. B., Brochu, K., Legare, C., Battistone, M. A. & Breton, S. Purinergic signaling in the male reproductive tract. Front. Endocrinol. (Lausanne). 13, 1049511. https://doi.org/10.3389/fendo.2022.1049511 (2022).
Ping, J. et al. Prenatal caffeine ingestion induces aberrant DNA methylation and histone acetylation of steroidogenic factor 1 and inhibits fetal adrenal steroidogenesis. Toxicology 321, 53–61. https://doi.org/10.1016/j.tox.2014.03.011 (2014).
Williams, C. D., Horner, A. K. & Catt, K. J. Effects of methylxanthines on gonadotropin-induced steroidogenesis and protein synthesis in isolated testis interstitial cells. Endocr. Res. Commun. 3(6), 343–358. https://doi.org/10.3109/07435807609073909 (1976).
Hang, D. et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am. J. Clin. Nutr. 109(3), 635–647. https://doi.org/10.1093/ajcn/nqy295 (2019).
Godoi, F. G. A. et al. Endocrine disruptive action of diclofenac and caffeine on Astyanax altiparanae males (Teleostei: Characiformes: Characidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 231, 108720. https://doi.org/10.1016/j.cbpc.2020.108720 (2020).
Simpson, E. R. Sources of estrogen and their importance. J. Steroid. Biochem. Mol. Biol. 86(3–5), 225–230. https://doi.org/10.1016/s0960-0760(03)00360-1 (2003).
Genchi, V. A. et al. Adipose tissue dysfunction and obesity-related male hypogonadism. Int. J. Mol. Sci. 23(15), 8194. https://doi.org/10.3390/ijms23158194 (2022).
Frey, T. et al. Consumption of caffeinated beverages and serum concentrations of sex steroid hormones in US men. Cancer Causes Control. 29(1), 157–166. https://doi.org/10.1007/s10552-017-0985-9 (2018).
Liu, M. et al. Tea consumption and new-onset acute kidney injury: The effects of milk or sweeteners addition and caffeine/coffee. Nutrients 15(9), 2201. https://doi.org/10.3390/nu15092201 (2023).
Henn, M. et al. Increase from low to moderate, but not high, caffeinated coffee consumption is associated with favorable changes in body fat. Clin. Nutr. 42(4), 477–485. https://doi.org/10.1016/j.clnu.2023.02.004 (2023).
Qu, X. & Donnelly, R. Sex hormone-binding globulin (SHBG) as an early biomarker and therapeutic target in polycystic ovary syndrome. Int. J. Mol. Sci. 21(21), 8191. https://doi.org/10.3390/ijms21218191 (2020).
Liao, Z., Vosberg, D. E., Pausova, Z. & Paus, T. A shifting relationship between sex hormone-binding globulin and total testosterone across puberty in boys. J. Clin. Endocrinol. Metab. 107(10), e4187–e4196. https://doi.org/10.1210/clinem/dgac484 (2022).
Yu, L. et al. Association of caffeine and caffeine metabolites with obesity among children and adolescents: National health and nutrition examination survey (NHANES) 2009–2014. Environ. Sci. Pollut. Res. Int. 29(38), 57618–57628. https://doi.org/10.1007/s11356-022-19836-1 (2022).
Simo, R., Saez-Lopez, C., Barbosa-Desongles, A., Hernandez, C. & Selva, D. M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 26(7), 376–383. https://doi.org/10.1016/j.tem.2015.05.001 (2015).
Grasa, M. D. M. et al. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur. J. Endocrinol. 176(4), 393–404. https://doi.org/10.1530/EJE-16-0834 (2017).
Veldhuis, J. D. et al. Immunologic and mass-spectrometric estimates of SHBG concentrations in healthy women. Metabolism 63(6), 783–792. https://doi.org/10.1016/j.metabol.2014.03.010 (2014).
Roberts, C. K., Croymans, D. M., Aziz, N., Butch, A. W. & Lee, C. C. Resistance training increases SHBG in overweight/obese, young men. Metabolism 62(5), 725–733. https://doi.org/10.1016/j.metabol.2012.12.004 (2013).
Bchir, F., Dogui, M., Ben Fradj, R., Arnaud, M. J. & Saguem, S. Differences in pharmacokinetic and electroencephalographic responses to caffeine in sleep-sensitive and non-sensitive subjects. C R Biol. 329(7), 512–519. https://doi.org/10.1016/j.crvi.2006.01.006 (2006).
Arnaud, M. J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb. Exp. Pharmacol. 200, 33–91. https://doi.org/10.1007/978-3-642-13443-2_3 (2011).
Acknowledgements
This research uses data from the National Health and Nutrition Examination Survey (NHANES 2013-2014). We thank the CDC, the NCHS, the National Health and Nutrition Examination Survey Questionnaire, Hyattsville, Maryland: US Department of Health and Human Services, CDC, for provid ing these data. And we thank the 2013-2014 NHANES participants and staff for their valuable contributions.
Funding
This work was supported by the National Natural Science Foundation of China, research on the Neural Mechanisms and Interventions of Parent–child Interaction in Promoting the Brain Plasticity of Children in Family Difficulties (U23A20170).
Author information
Authors and Affiliations
Contributions
S.P.L. and Z.C.Y. designed the study, conducted the data analysis, and S.P.L. drafted the manuscript. Y.Z. and N.R.W. proposed critical revisions to the manuscript. All included authors made contributions to the manuscript and approved the version submitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Yuan, Z., Zhao, Y. et al. Associations of caffeine and caffeine metabolites with sex hormones among 6–19-year-old children and adolescents. Sci Rep 15, 23052 (2025). https://doi.org/10.1038/s41598-025-06330-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06330-0