Abstract
The rising incidence of cognitive disorders has become a significant challenge in global public health. The ratio of Gamma-glutamyl transferase (GGT) to high-density lipoprotein (HDL), associated with insulin resistance, has an unclear relationship with cognitive function. Our cross-sectional study utilized data from the NHANES database, employing the digit symbol substitution test (DSST) to assess cognitive ability. We conducted weighted linear regression, weighted mediation analysis, dose-response relationships, and subgroup analyses to investigate the link between the GGT/HDL ratio and DSST scores, with a particular focus on the mediating role of diabetes. The study included 2750 participants. After adjusting for covariates, we found that a higher GGT/HDL ratio was significantly correlated with lower DSST scores (β = −0.06, 95% CI −0.08 to −0.04, P < 0.001, P for non-linear = 0.169), and diabetes mediated the relationship between the GGT/HDL ratio and DSST scores, accounting for 13.8% of the total effect (P = 0.034). These findings suggest that in the U.S. population aged 60 and above, an increased GGT/HDL ratio is associated with an increased risk of cognitive decline, with diabetes playing a mediating role in this association.
Similar content being viewed by others
Introduction
With the growth of the global population and the acceleration of the aging process, the incidence of dementia is continuously increasing1and cognitive decline is a hallmark feature of dementia2. It is reported that in 2023, the total expenditure on healthcare, long-term care, and end-of-life care services for dementia patients aged 65 and above in the United States is projected to be around 345 billion dollars3placing a significant burden on society. Cognitive health has become one of the focal issues in the field of public health. Due to the current lack of effective treatments for dementia, the early identification of modifiable risk factors is crucial for the prevention and delay of cognitive decline.
Cognitive impairment manifests in diverse forms, with Alzheimer’s disease (AD) being the primary presentation. Its pathogenesis is complex and is currently thought to be associated with the abnormal deposition of pathological proteins such as amyloid-beta (Aβ) and tau, chronic neuroinflammation, oxidative stress, and other factors4,5,6. Recent reports suggest that the interplay between metabolic dysfunction and inflammation is pivotal to the onset and progression of cognitive disorders like AD7. Diabetes mellitus (DM) and other metabolic dysregulation disorders are recognized as significant risk factors for neuroinflammation and the development of neurodegenerative conditions7,8,9,10. Additionally, research has identified cognitive impairment as an emerging complication associated with DM11.The liver’s key role in managing blood glucose levels and inflammatory processes cannot be overlooked. Recent research has highlighted the liver’s capacity to eliminate around 60% of Aβ proteins associated with AD, indicating a dual mechanism: direct degradation by hepatocytes and indirect modulation of plasma lipoprotein levels and Aβ-linked cholesterol metabolism. These findings emphasize the liver-brain axis and the importance of hepatic metabolic markers in identifying potential etiologies of cognitive impairments12,13.
Gamma-glutamyl transpeptidase (GGT), an enzyme found extensively in various human tissues and involved in a multitude of metabolic pathways, is recognized as an indicator of liver damage and mild chronic inflammation14. It has been hypothesized in research that increased GGT levels might be biomarkers of oxidative stress associated with cognitive decline15. High-density lipoproteins (HDL), a sophisticated and multifunctional lipoprotein, is capable of modulating reverse cholesterol transport and exhibits properties such as endothelial protection, anti-inflammatory, and antioxidant effects16. Evidence from animal models indicates that HDL can prevent memory deterioration, neuroinflammation, and cerebral amyloid angiopathy, and numerous observational studies suggest an inverse relationship between HDL and neurodegenerative conditions like AD17,18,19. However, some genetic studies and meta-analyses have questioned this causal link20,21,22.
The ratio of GGT to HDL has emerged as a new metric, demonstrating superior predictive power for the onset of non-alcoholic fatty liver disease (NAFLD) and T2DM23,24. Additionally, this ratio correlates positively with the risk of metabolic syndrome (MetS) in individuals with T2DM25.
Considering the association between GGT and HDL with cognitive decline, exploring the association between the GGT/HDL ratio and cognitive function is of significant importance, as there is a dearth of research on this subject. Consequently, the aim of this study is to investigate the relationship between the GGT/HDL ratio and cognitive function in the elderly, with the goal of identifying potentially modifiable risk factors contributing to cognitive decline.
Results
Weighted baseline demographic characteristics
Table 1 presents the demographic characteristics of the 2,750 participants after weighting, with an average age of 69.14 years (± 6.65). Females comprised 53.9% of the study population. A significant majority, approximately 80%, identified as non-Hispanic whites. Over half of the participants (62.3%) had completed at least a high school education, and 65.1% were currently married or in a cohabiting relationship. Approximately 52.3% of households had a poverty-to-income ratio (PIR) of 3 or greater. Furthermore, 72.8% of the individuals reported a habit of alcohol consumption, while 50% were smokers. Obesity, defined as a BMI of 30 or higher, was present in 37.3% of the participants. The prevalence of a history of diabetes, high cholesterol and liver conditions was 18.9, 57.5, and 4.7%, respectively. Upon stratification by GGT/HDL quartiles, it was found that demographic factors including gender, age, ethnicity, education level, marital status, smoking habits, BMI, and the prevalence rates of diabetes, hypercholesterolemia, and liver disease all demonstrated statistical significance (P < 0.05). However, the PIR and alcohol consumption did not show statistically significant differences.
The mean GGT/HDL ratio was calculated at 19.79 (± 26.69), stratified into ordinal variables based on quartile distribution. Notable differences were identified across the quartiles regarding demographic characteristics, including gender, age, race, educational attainment, and marital status, along with lifestyle and health-related variables such as smoking status, BMI, diabetes, hyperlipidemia, and a history of liver disease (P < 0.05). No significant statistical variations were detected in income levels and alcohol consumption habits. The mean DSST score was 52.42 (± 16.72), with a significant variation observed as the quartiles of the GGT/HDL ratio increased (P = 0.005). The range of values for each continuous variable is provided in Table 1 of the supplementary materials.
Association of GGT/HDL with cognitive performance
Table 2 illustrates the results of our analysis using multivariate weighted linear regression to examine the association between the GGT/HDL ratio and cognitive function. In the initial crude model, elevated levels of the GGT/HDL ratio were significantly and negatively associated with DSST scores, irrespective of being treated as a continuous or categorical variable (P < 0.05), post hoc bonferroni comparisons revealed that Q4 was statistically significantly different from Q1. This correlation remained significant after adjusting for gender, age, and race in model 1 (P < 0.05), post hoc bonferroni comparisons revealed that Q2, Q3, and Q4 were all statistically significantly different from Q1. Upon full adjustment for potential confounders, the GGT/HDL ratio, when treated as a continuous variable, demonstrated a robust inverse relationship with DSST scores (β= -0.06, 95% CI: -0.08 to -0.04; P < 0.001). As a categorical variable, the highest quartile (Q4), compared to the lowest (Q1), exhibited a pronounced negative association with DSST scores (β=-3.72, 95% CI: -6.25 to -1.20, P = 0.006).
Dose-response relationships
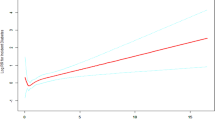
After controlling for covariates, we employed a restricted cubic splines model to characterize the dose-response relationship between the GGT/HDL ratio and DSST scores. Figure 1 illustrates our findings, revealing a significant inverse correlation between the GGT/HDL ratio and the cognitive function measured by DSST, with the relationship being linear (P for overall effect < 0.001, P for non-linearity = 0.169).
DM as a significant mediator between GGT/HDL and cognitive function scores
Table 3; Fig. 2 present our analysis of DM as a significant mediator in the association between the GGT/HDL ratio and DSST scores. The findings reveal that, with confounding factors adjusted for, an elevated GGT/HDL ratio is linked to a higher risk of developing DM (β = 0.002, P = 0.047), and higher risk levels of DM are associated with lower DSST (β=-5.661, P < 0.001). DM was found to mediate the association between the GGT/HDL ratio and cognitive function as measured by DSST, accounting for 13.8% of the total effect (P = 0.034).
All models were adjusted for age, gender, race, educational level, BMI, marriage status, drinking status, smoking status, and high cholesterol.
Path A represents the effect of “exposure” (X) on “mediator (M)”;
Path B represents the effect of “mediator (M)” on cognitive test scores “outcome” (Y);
Path C represents the total effect of “exposure” (X) on “outcome” (Y), without the adjustment for “mediator (M)”;
Path C’ represents the direct effect of “exposure” (X) on “outcome” (Y), after adjustment for “mediator (M).”;
Indirect effect represents an effect of “exposure (X)” on “(outcome) Y” mediated by mediator (M).
All models were adjusted for age, gender, race, educational level, BMI, marriage status, drinking status, smoking status, and high cholesterol.
Subgroup analysis
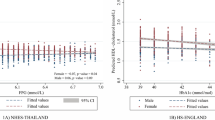
Figure 3 presents our subgroup analyses of participants, stratified by gender, age, race, education level, marital status, BMI, smoking habits, alcohol consumption habits, and the history of high cholesterol and liver disease. Post-adjustment for covariates, we observed a significant association between the GGT/HDL ratio and DSST scores within subgroups defined by gender, age, marital status, presence of high cholesterol, and history of liver disease. Additionally, among non-Hispanic whites, individuals with education levels exceeding high school, those with a BMI below 30, and in those who reported smoking and drinking habits, a substantial correlation between the GGT/HDL ratio and DSST scores was noted (P < 0.05). The subgroup analyses for other models can be found in Table 2 of the supplementary materials.
Adjusted for age, gender, race, education level, married status, BMI, smoking status, alcohol status, high cholesterol, liver conditions.
Sensitivity analysis
Finally, we conducted sensitivity analyses based on different diabetes diagnostic criteria and cognitive scales. The results revealed that when GGT/HDL was treated as a continuous variable, it showed a significant negative correlation with the DSST in both Model 1 and Model 3 (P < 0.05). When GGT/HDL was treated as a categorical variable, each Q4 in the models was negatively correlated with the DSST compared to Q1 (P < 0.05). In Model 1, every cognitive assessment scale for Q4 was negatively correlated with Q1, while Models 2 and 3 showed no significant association between GGT/HDL and cognitive scales, except for the DSST (for details, see Supplementary Table 3).
Based on these findings, we further conducted a weighted mediation analysis between different models and the DSST. We similarly observed that in Models 1 and 3, diabetes played a mediating role between GGT/HDL and DSST scores, accounting for 27.5% (P = 0.006) and 27.2% (P = 0.008) of the total effect, respectively (for details, see Supplementary Table 4).
Discussion
This study constitutes a cross-sectional examination of data from the NHANES spanning the years 2011 to 2014, with the objective of investigating the relationship between the GGT/HDL ratio and cognitive function in elderly individuals. After adjusting for covariates, a significant negative correlation was identified between the GGT/HDL ratio and DSST scores (P for non-linearity = 0.169). DM demonstrated independent associations with the GGT/HDL ratio and cognitive function and served as a mediator. This indicates that by optimizing the GGT/HDL ratio to mitigate the risk of DM, there may be a further reduction in the potential for cognitive dysfunction in older adults.
Earlier research has established a positive correlation between increased GGT/HDL ratios and the risk of NAFLD23,26. Jung et al.27in a longitudinal study found that the GGT/HDL ratio is a significant predictor of cardiovascular disease (CVD) risk in women, with a closer correlation in urban than in rural populations. Longitudinal research24 involving 15,453 Japanese individuals indicated that an elevated GGT/HDL ratio can predict the onset of T2DM. Zhao et al.28 further explored this finding and observed a curvilinear relationship between the ratio and T2DM risk, with increments below a ratio of 6.53 correlating to a 1.56-fold increased risk (HR = 2.57, 95%CI 1.20 to 5.49), after which the relationship plateaus. Gong et al.25 determined in a cross-sectional investigation that the GGT/HDL ratio is a robust predictor of MetS risk in T2DM patients (OR = 2.49, 95% CI 1.51 to 4.10). A recent retrospective cohort study29 recognized GGT/HDL as a prognostic indicator for MetS remission in adults who have undergone sleeve gastrectomy. In conclusion, existing research has identified a correlation between elevated GGT/HDL ratios and an increased risk of NAFLD, T2DM, CVD, and MetS. To our knowledge, no studies have yet explored the relationship between the GGT/HDL ratio and cognitive function in the elderly. Our findings indicate that a higher GGT/HDL ratio is associated with an increased risk of cognitive decline in older individuals, with the heightened risk of DM serving as a mediating factor.
The underlying mechanisms linking an increased GGT/HDL ratio to poorer cognitive function remain unclear. One potential explanation could involve the liver-brain axis, where communication between the liver and the brain occurs primarily through metabolic changes, signaling via the nervous and circulatory systems and epigenetic regulation30,31. Elevated GGT reflects liver dysfunction, which may lead to several effects: (1) excessive accumulation of toxic metabolites such as ammonia, lactate, manganese, and Aβ32; (2) increased levels of pro-inflammatory cytokines like interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α), which disrupt the blood-brain barrier7,25allowing ammonia and other exogenous toxins to enter the central nervous system, thereby inducing neuroinflammation; (3) potential structural changes in the brain, such as brain edema, neuronal morphological alterations, reduced gray matter volume, and decreased cerebral blood flow and perfusion, which can exacerbate brain atrophy30,33,34,35; (4) liver dysfunction may also lead to imbalances in neurotransmitter transmission, affecting substances like serotonin, GABA, norepinephrine, and dopamine30,36; and (5) abnormal liver function can result in glucose metabolism disturbances, leading to insulin resistance, which may further impair cognitive function30.
HDL, known as a “good” lipoprotein, has been found to have pro-inflammatory and pro-oxidative effects when reduced and is associated with increased risks of CVD and IR. While most studies suggest a positive correlation between reduced HDL and cognitive decline37,38,39some studies propose no relationship between serum HDL and cognitive disorders22,40potentially influenced by baseline age and follow-up duration16,41. Moreover, HDL plays a role in modulating glucose uptake in skeletal muscle and the secretion capacity of pancreatic β-cells. Consequently, reduced levels of HDL are correlated with an increased incidence of DM, as the decrement in HDL is associated with a higher prevalence of the condition42.Importantly, current research indicates that HDL functionality, rather than serum HDL levels, more accurately reflects disease biological variance17. Mendelian randomization studies and large-scale genome-wide association studies (GWAS) for AD have identified significant associations between lipoprotein metabolism and HDL particle gene sets with AD risk, involving genes that encode proteins for HDL biogenesis and HDL protein components, such as APOE, APOC1, APOJ, etc43,44. Variants in the ABCA1 gene are associated with the development of T2DM through their impact on lowering HDL levels, resulting in lipotoxicity to pancreatic β-cells24. In this study, we considered the GGT/HDL ratio as a whole and examined its correlation with cognitive function assessed by the DSST. Therefore, the HDL value alone cannot be used as a singular indicator to determine its impact on cognitive function.
Lastly, an elevated GGT/HDL ratio can effectively predict the development of NAFLD, T2DM, CVD, and MetS, which are recognized modifiable risk factors for cognitive disorders and can indirectly increase the risk of cognitive impairment.
GGT and HDL are standard laboratory markers that are readily available, cost-effective, and modifiable risk factors for cognitive impairment. Our research pioneers an investigation into the association between the GGT/HDL ratio and cognitive function in elderly individuals, revealing that an increased GGT/HDL ratio is associated with a heightened risk of cognitive decline, with DM serving as a mediator. Subgroup analyses, categorized by gender, age, marital status, hyperlipidemia history, and liver disease history, all demonstrated a significant association between the GGT/HDL ratio and DSST scores. The same significant association was observed in non-Hispanic white, individuals with education levels beyond high school, those with a BMI under 30, and among smokers and drinkers. These findings indicate that, in clinical settings, the GGT/HDL ratio can more sensitively identify populations at risk for cognitive disorders. This insight encourages clinicians to offer targeted advice to patients, potentially enabling early intervention to prevent cognitive decline or to slow the progression of related conditions.
The merits of this study are multifaceted: (1) We utilized a comprehensive, weighted approach to analyze a representative sample from a large-scale American database, ensuring the reliability of our findings; (2) We performed a dose-response analysis to explore the linear relationship between the GGT/HDL ratio and cognitive assessment; (3) We have examined the intermediary role of DM in the relationship between the GGT/HDL ratio and cognitive function, demonstrating that optimization of the GGT/HDL ratio could lower the risk of DM, consequently reducing the potential for cognitive impairment. To achieve this goal, clinicians can encourage individuals with a high GGT/HDL ratio to improve their lifestyle by promoting healthy eating, physical exercise, and reducing alcohol intake. These measures can help prevent and delay the onset of diabetes and reduce the risk of cognitive impairment45,46. However, our study is not without limitations: (1) As a cross-sectional study, it is beyond our scope to establish a causal link between the GGT/HDL ratio and cognitive impairment—additional prospective studies are necessary for further validation; (2) While our analysis relied exclusively on the DSST cognitive test outcomes from the NHANES database, this may not comprehensively represent an individual’s full spectrum of cognitive functions; (3) In our study, DM was defined based on a prior physician diagnosis and excluded those with blood sugar levels that were borderline, without specific differentiation between Type 1 and Type 2 DM, although some supplementation was made in the sensitivity analysis, this may still result in some missed diagnoses. Thus, future research could benefit from stratified analyses based on the subtypes of DM and a more stringent definition; (4) Although our study accounted for 10 confounding factors as covariates, it did not encompass others, such as hypertension, depressive symptoms, and medication usage, which could be considered in subsequent studies.
Methods
Data sources and study population
The data for this study were sourced from the United States National Health and Nutrition Examination Survey (NHANES) database47sponsored by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS) and approved by the Institutional Review Board. All participants provided informed consent. NHANES performs a biennial national health and nutrition survey using a complex, stratified, multistage probability sampling design that considers geographical and demographic variations. Disease prevalence is assessed through weighted calculations. These sampling weights account for the probabilities of selection, mitigating selection biases inherent in the sampling design and ensuring that the sample is representative of the non-institutionalized U.S. population. This enables the study’s results to be extrapolated to a wider demographic. Furthermore, by employing weights, the importance of individuals within the sample can be adjusted, enhancing the precision of the analytical outcomes.
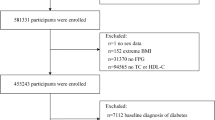
Our research incorporated data from two survey cycles, 2011–2012 and 2013–2014, initially including 19,931 subjects. The exclusion criteria were: (1) participants with incomplete cognitive function scale data (n = 16,917); (2) individuals with missing HDL data (n = 165); (3) individuals with missing GGT data (n = 12); (4) individuals with missing mediator and covariates data (n = 87). Ultimately, the study analyzed data from 2,750 individuals. The detailed selection process is depicted in (Fig. 4).
Study variables
The exposure factors consisted of fasting venous levels of GGT in units per liter and HDL in millimoles per liter. The DxC800 analyzer was utilized with an enzyme rate method to ascertain the activity of GGT in serum or plasma (BIOPRO_G, BIOPRO_H). During the 2011–2012 cycle, HDL was analyzed on the Roche Modular P chemistry analyzer. In the 2013–2014 cycle, HDL measurements were conducted on both the Roche Modular P and Roche Cobas 6000 chemistry analyzers, employing a direct assay method to determine the levels of HDL in serum or plasma (HDL_G, HDL_H).
The exposure variable under investigation is the ratio of GGT to HDL. The GGT/HDL ratio was calculated by GGT(U/L)/ HDL (mmol/L).
The Digit Symbol Substitution Test (DSST) serves as a sensitive measure for assessing various dimensions of cognitive function, including memory, attention, executive abilities, and spatial orientation48. This test is a component of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III). The DSST challenges participants to correctly match symbols with numbers within a 2-minute timeframe across 133 boxes49. The total score, reflecting the number of accurate matches, indicates cognitive performance, with higher scores signifying superior cognitive ability.
Mediating variable and covariates
The mediating variable is DM, defined as being told by a professional healthcare provider that you have diabetes.
The covariates encompass a spectrum of demographic and health indicators, including gender, age, race (Mexican American, Other Race, Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Asian), level of education (Less than high school, High school or equivalent, Above high school), marital status (Married or living with a partner, Widowed or divorced or separated or never married), Body Mass Index (BMI) categories (< 25, ≥ 25、<30,≥30), smoking status (Defined as individuals who have smoked 100 or more cigarettes in their lifetime), alcohol consumption (Defined as individuals who have consumed 12 or more standard alcoholic drinks per year), high cholesterol(Defined as being told by a professional healthcare provider that you have high cholesterol), liver conditions(Defined as being told by a professional healthcare provider that you have liver conditions).
Statistical analysis
All analyses accounted for sample weights, clustering, and stratification to generate nationally representative estimates, recognizing the complex sampling design of NHANES. According to NHANES analysis guidelines, the weights for this study were selected as ‘WTMEC2YR.’ The study covers the period from 2011 to 2014, involving two cycles, and the final weights were derived by halving the ‘WTMEC2YR’ weights from these two cycles. Baseline characteristics are compared using weighted chi-square tests for categorical variables and ANOVA for continuous variables. Continuous data are presented as the mean (standard deviation) (mean (SD)), while categorical data are expressed as percentages (%).
We used the Shapiro-Wilk test to assess the distribution of GGT/HDL, which was found to be skewed. Therefore, we conducted analyses of the GGT/HDL ratio as both a continuous and categorical variable, categorized by quartiles with the first quartile (Q1) serving as the reference group. Weighted linear regression was employed to estimate the beta coefficients (β) and 95% confidence intervals (CI), assessing the association between the GGT/HDL ratio and cognitive performance. The crude model was unadjusted for any covariates, while Model 1 included adjustments for gender, age, and race. Model 2 built upon Model 1 by further accounting for education level, marital status, BMI, smoking and alcohol consumption status, high cholesterol, and liver conditions. Following the adjustment for all covariates, we utilized restricted cubic splines (RCS) to model the dose-response relationship between the GGT/HDL ratio and cognitive performance. Mediation analysis explored the intermediary role of DM in the association between the GGT/HDL ratio and cognitive function, with adjustments for all covariates. We then performed subgroup analyses and conducted sensitivity analyses based on different definitions of diabetes. Model 1 defined diabetes as being diagnosed by a healthcare provider with a HbA1c level greater than or equal to 6.5%; Model 2 defined it as being diagnosed by a healthcare provider with a fasting blood glucose level greater than or equal to7 mmol/L; and Model 3 defined it as being diagnosed by a healthcare provider with a 2-hour postprandial blood glucose level (OGTT) greater than or equal to 11.1 mmol/L. These were then compared with other cognitive function measures (Consortium to Establish a Registry for Alzheimer’s Disease - Word List (CERAD-WL); Consortium to Establish a Registry for Alzheimer’s Disease – Delay Recall (CERAD-DR); Animal Fluency Test (AFT), and DSST).
Analyses were performed using statistical software R version 4.3.0, with a P < 0.05 indicating statistical significance. A Bonferroni-corrected threshold of P < 0.0083 was applied (0.05/6 comparisons). The mediation analyses were performed using the “mediation” R package and 10,000 bootstrap samples to examine whether the association between GGT/HDL exposure and cognitive function events was influenced by diabetes. We used Weighted linear regressions were performed using the “survey” package and the “survey”, “ggplot2”, and “ggsci” packages to RCS. Forest plots were visualized using the “forestplot” and “grid” packages.
Institutional review board statement
The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol. All participants signed written informed consent.
Conclusion
In conclusion, within a nationally representative sample of the American elderly, an increased GGT/HDL ratio is associated with a heightened risk of cognitive decline, with DM exhibiting a mediating effect. The results of our study may underpin future prospective studies aimed at elucidating the precise causal dynamics.
Data availability
Data availabilityThe National Health and Nutrition Examination Survey dataset is publicly available at the National Center for Health Statistics of the Center for Disease Control and Prevention (NHANES - National Health and Nutrition Examination Survey Homepage (cdc.gov)).
References
Estimation of the global prevalence of dementia. In 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public. Health 7, e105–e125 (2022).
Weng, X. et al. Association between mixed exposure of phthalates and cognitive function among the U.S. Elderly from NHANES 2011–2014: three statistical models. Sci. Total Environ. 828, 154362 (2022).
Alzheimer’s disease facts and figures. Alzheimers Dement https://doi.org/10.1002/alz.13016 (2023).
Morató, X. et al. A randomized, open-label clinical trial in mild cognitive impairment with EGb 761 examining blood markers of inflammation and oxidative stress. Sci. Rep. 13, 5406 (2023).
Gaikwad, S., Senapati, S., Haque, M. A. & Kayed, R. Senescence, brain inflammation, and oligomeric Tau drive cognitive decline in alzheimer’s disease: evidence from clinical and preclinical studies. Alzheimers Dement. 20, 709–727 (2024).
Błaszczyk, J. W. Pathogenesis of dementia. Int. J. Mol. Sci. 24, 543 (2022).
Asimakidou, E., Saipuljumri, E. N., Lo, C. H. & Zeng, J. Role of metabolic dysfunction and inflammation along the liver–brain axis in animal models with obesity-induced neurodegeneration. Neural Regeneration Res. 20, 1069 (2025).
Alzheimer’s disease facts and figures. Alzheimers Dement 20, 3708–3821 (2024).
Xie, W., Zheng, F., Yan, L. & Zhong, B. Cognitive decline before and after incident coronary events. J. Am. Coll. Cardiol. 73, 3041–3050 (2019).
Jeong, S. et al. Association of non-alcoholic fatty liver disease with incident dementia later in life among elder adults. Clin. Mol. Hepatol. 28, 510–521 (2022).
Pignalosa, F. C. et al. Diabetes and cognitive impairment: A role for glucotoxicity and dopaminergic dysfunction. Int. J. Mol. Sci. 22, 12366 (2021).
Wu, B. et al. Liver as a new target organ in alzheimer’s disease: insight from cholesterol metabolism and its role in amyloid-beta clearance. Neural Regeneration Res. 20, 695 (2025).
Wu, Y. et al. Hepatic soluble epoxide hydrolase activity regulates cerebral Aβ metabolism and the pathogenesis of alzheimer’s disease in mice. Neuron 111, 2847–2862e10 (2023).
Mitrić, A. & Castellano, I. Targeting gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic. Biol. Med. 208, 672–683 (2023).
Yavuz, B. B. et al. Serum elevated gamma glutamyltransferase levels May be a marker for oxidative stress in alzheimer’s disease. Int. Psychogeriatr. 20, 815–823 (2008).
Poliakova, T. & Wellington, C. L. Roles of peripheral lipoproteins and cholesteryl ester transfer protein in the vascular contributions to cognitive impairment and dementia. Mol. Neurodegeneration. 18, 86 (2023).
Button, E. B. et al. HDL from an alzheimer’s disease perspective. Curr. Opin. Lipidol. 30, 224–234 (2019).
Nakamura, Y. et al. Longitudinal association of hypertension and dyslipidemia with cognitive function in community-dwelling older adults: the SONIC study. Hypertens. Res. 46, 1829–1839 (2023).
Yu, Y. et al. Correlation between serum lipid profiles and cognitive impairment in old age: a cross-sectional study. Gen. Psychiatr. 36, e101009 (2023).
Sergi, D. et al. Lipids at the Nexus between cerebrovascular disease and vascular dementia: the impact of HDL-Cholesterol and ceramides. Int. J. Mol. Sci. 24, 4403 (2023).
Hottman, D. A., Chernick, D., Cheng, S., Wang, Z. & Li, L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 72PA, 22–36 (2014).
Peters, R. et al. Evaluation of high cholesterol and risk of dementia and cognitive decline in older adults using individual patient Meta-Analysis. Dement. Geriatr. Cogn. Disord. 50, 318–325 (2021).
Feng, G., Feng, L. & Zhao, Y. Association between ratio of γ-glutamyl transpeptidase to high-density lipoprotein cholesterol and prevalence of nonalcoholic fatty liver disease and metabolic syndrome: a cross-sectional study. Ann. Transl Med. 8, 634 (2020).
Xie, W., Liu, B., Tang, Y., Yang, T. & Song, Z. Gamma-glutamyl transferase to high-density lipoprotein cholesterol ratio: A valuable predictor of type 2 diabetes mellitus incidence. Front. Endocrinol. (Lausanne). 13, 1026791 (2022).
Gong, S., Gan, S., Zhang, Y., Zhou, H. & Zhou, Q. Gamma-glutamyl transferase to high-density lipoprotein cholesterol ratio is a more powerful marker than TyG index for predicting metabolic syndrome in patients with type 2 diabetes mellitus. Front. Endocrinol. (Lausanne). 14, 1248614 (2023).
Li, Q., Han, Y., Hu, H. & Zhuge, Y. Gamma-glutamyl transferase to high-density lipoprotein cholesterol ratio has a non-linear association with non-alcoholic fatty liver disease: A secondary prospective cohort study in non-obese Chinese adults. Front. Med. (Lausanne). 9, 995749 (2022).
Jung, D. H., Park, B., Ryu, H. E. & Lee, Y. J. Sex-specific associations of γ-glutamyltransferase to HDL-cholesterol ratio and the incident risk of cardiovascular disease: three Korean longitudinal cohorts from different regions. Front. Endocrinol. (Lausanne). 14, 1231502 (2023).
Zhao, Y., Xin, X. & Luo, X. P. The relationship between the ratio of gamma-glutamyltransferase to high-density lipoprotein cholesterol and the risk of diabetes mellitus using publicly available data: a secondary analysis based on a longitudinal study in Japan. Lipids Health Dis. 22, 7 (2023).
Lizarbe-Lezama, M. L. et al. Association between gamma Glutamyl transpeptidase to HDL-Cholesterol (GGT/HDL-C) ratio and metabolic syndrome resolution after sleeve gastrectomy. Diab Vasc Dis. Res. 21, 14791641241252553 (2024).
Cheon, S. Y. & Song, J. The association between hepatic encephalopathy and diabetic encephalopathy: the Brain-Liver Axis. Int. J. Mol. Sci. 22, 463 (2021).
Smith, M. L., Wade, J. B., Wolstenholme, J. & Bajaj, J. S. Gut microbiome-brain-cirrhosis axis. Hepatology 80, 465 (2024).
Yan, M. et al. Gut liver brain axis in diseases: the implications for therapeutic interventions. Signal. Transduct. Target. Ther. 8, 443 (2023).
Yilmaz, P. et al. Subclinical liver traits are associated with structural and hemodynamic brain imaging markers. https://doi.org/10.1111/liv.15549
Ghaznawi, R. et al. Reduced parenchymal cerebral blood flow is associated with greater progression of brain atrophy: the SMART-MR study. J. Cereb. Blood Flow. Metab. 41, 1229–1239 (2021).
Kisler, K., Nelson, A. R., Montagne, A. & Zlokovic, B. V. Cerebral blood flow regulation and neurovascular dysfunction in alzheimer’s disease. Nat. Rev. Neurosci. 18, 419–434 (2017).
De Cól, J. P. et al. Underlying mechanisms behind the Brain–Gut–Liver Axis and Metabolic-Associated fatty liver disease (MAFLD): an update. Int. J. Mol. Sci. 25, 3694 (2024).
Lee, J., Lee, S., Min, J. Y. & Min, K. B. Association between serum lipid parameters and cognitive performance in older adults. J. Clin. Med. 10, 5405 (2021).
Chernick, D., Zhong, R. & Li, L. The role of HDL and HDL mimetic peptides as potential therapeutics for alzheimer’s disease. Biomolecules 10, 1276 (2020).
Zuliani, G. et al. Relationship between low levels of High-Density lipoprotein cholesterol and dementia in the elderly. The InChianti study. J. Gerontol. Biol. Sci. Med. Sci. 65A, 559–564 (2010).
Marcum, Z. A. et al. Serum cholesterol and incident alzheimer’s disease: findings from the adult changes in thought study. J. Am. Geriatr. Soc. 66, 2344–2352 (2018).
Li, G. et al. Serum cholesterol and risk of alzheimer disease: a community-based cohort study. Neurology 65, 1045–1050 (2005).
Yuan, S. & Larsson, S. C. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia 63, 2359–2371 (2020).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing alzheimer’s disease risk. Nat. Genet. 51, 404 (2019).
Koch, M. et al. High density lipoprotein and its apolipoprotein-defined subspecies and risk of dementia. J. Lipid Res. 61, 445–454 (2020).
Wang, Z. et al. Dietary polyphenols to combat nonalcoholic fatty liver disease via the Gut-Brain-Liver axis: A review of possible mechanisms. J. Agric. Food Chem. 69, 3585–3600 (2021).
Wakabayashi, I. Associations between glycemic status and the ratio of γ-glutamyl transferase to HDL-C in middle-aged women. Lab. Med. lmae084 https://doi.org/10.1093/labmed/lmae084 (2024).
Questionnaires, N. H. A. N. E. S. Datasets, and Related Documentation. https://wwwn.cdc.gov/Nchs/Nhanes/
Rosano, C. et al. Digit symbol substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing. 45, 688–695 (2016).
Casagrande, S. S., Lee, C., Stoeckel, L. E., Menke, A. & Cowie, C. C. Cognitive function among older adults with diabetes and prediabetes, NHANES 2011–2014. Diabetes Res. Clin. Pract. 178, 108939 (2021).
Acknowledgements
We would like to express our sincere gratitude to all the participants and the entire NHANES staff involved in this study.
Funding
This work was supported by grants from the Key Research and Development Project of Zhejiang Provincial (2021C03106).
Author information
Authors and Affiliations
Contributions
Y.W. conceptualized the overall idea and wrote the main body of the manuscript; Y.W., Y.P., and L.J. analyzed the key results from the data and prepared the main tables and figures; T.M. and W.X. performed the sensitivity analyses and presented the corresponding results in tables; W.D. and J.X. revised the manuscript; and E.Y. oversaw the final revisions and project management. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Mao, Y., Xu, T. et al. A cross sectional study of the diabetes mediated GGT to HDL ratio and cognitive function in older adults. Sci Rep 15, 20928 (2025). https://doi.org/10.1038/s41598-025-06439-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06439-2