Abstract
Based on the current guidelines, there is no consensus on inpatient treatment including cerebral vasospasm prophylaxis and follow-up imaging for perimesencephalic nonaneurysmal subarachnoid hemorrhage (NASAH). To evaluate the daily practice of neurosurgeons within the Vascular Section of the European Association of Neurosurgical Societies (EANS) an online survey was performed from February 2023 to June 2023. Thirty-two questionnaires were answered from eighteen different countries. Most answers were provided from employees of University Hospitals (n = 27; 84.4%). Up to five NASAH cases per year were reported by 10 (31.3%), another 12 (37.4%) treat more than 20 cases. The majority of contributors (65.6%) estimate the complication rates in NASAH to be less than 2%. Inpatient monitoring was significantly influenced by the initial presentation and the distribution of blood observed in the CT scan, with significantly more patients being admitted to the intensive care unit in case 3 (p = 0.011) compared to case 1. Further, the therapeutic approaches differ in the blood pressure monitoring (p = 0.08). However, this finding did not achieve statistical significance. In case 1 and case 2 neither cerebral vasospasm prophylaxis nor transcranial doppler sonography is performed in 11 centers (34.4%) which decreases statistically significantly in case 3 (n = 2; 6.3%; p = 0.0014). This study confirms that, the amount of blood in the first native CT scan influences the treatment decision. However, clear intercontinental differences cannot be evaluated due to the small number of participants.
Similar content being viewed by others
Introduction
Nonaneurysmal subarachnoid hemorrhage (NASAH) is defined by hemorrhage in the subarachnoid space without a detectable aneurysm or previous trauma1.
Depending on the specific bleeding pattern, NASAH can be categorized into perimesencephalic (PM) and non perimesencephalic (NPM) SAH2. According to the 2013 guidelines of the European Stroke Organization, diagnostic approaches vary based on the initial bleeding pattern. Digital subtraction angiography (DSA) remains the gold standard for the exclusion of aneurysms, however, it is only recommended in patients with NPM SAH3.
In contrast, due to the lower rates of rebleeding, cerebral vasospasm (CV), and delayed cerebral ischemia, these guidelines do not recommend DSA in patients suffering from PM SAH after conducting a benefit-risk analysis3.
Recently, a nationwide survey conducted in Germany revealed a variety of treatment approaches in these patients, being influenced by the volume of blood identified in the initial cranial computed tomography (cCT) scan4.
This study aims to evaluate whether this variability in treatment approaches is also prevalent across Europe.
Materials and methods
This survey was distributed among members of the Vascular Section of the European Association of Neurosurgical Societies (EANS) and conducted via an online platform from February 2023 to June 2023.
The primary questions focused on the employment status of the respondents and details regarding patients with NASAH.
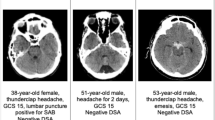
Additionally, three clinical case vignettes were presented, illustrating varying amounts of PM SAH in the initial native cCT images. These cases are designed to facilitate the evaluation of treatment strategies and are depicted in Fig. 1.
The distributed questionnaire is available in the supplementary file.
Aim of the study
This survey primarily focused on the caseload, the estimated complication rate, and the proposed treatment regimen of patients being admitted with NASAH to neurosurgical departments throughout hospitals of the EANS members.
The main aim was to analyze whether NASAH patients are treated differently in dependence of their clinical presentation and the amount of PM SAH detected in the initial cCT scan.
Statistical analysis
For categorical data, absolute numbers and percentages are given. Person-Chi2 test without Yates correction was used to map correlation. Contingency coefficient is applied. Reported p-values are two-sided and a significance threshold of p < 0.05 was applied to all tests. Data analysis were performed with IBM SPSS v. 28 (IBM Corp, Armonk, USA).
Ethical approval and consent to participate
This multicentric survey was carried out in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and later amendments. The Human Investigation Committee of the Ludwig-Maximilians-University Munich, Germany approved the survey (Reference Nr: 21–1297). Individual consent to participate was not necessary, since the survey was conducted without real information about the individual patients.
Results
Response rates
Thirty-two questionnaires were returned from eighteen different countries. Most were completed by employees from departments located in Italy (n = 6; 18.8%), Greece (n = 4; 12.5%), or the United Kingdom (n = 3; 9.4%). Questionnaires from countries with only one response were grouped under the heading “Others”. Details are shown in Fig. 2.
Employment status
Four (12.5%) of the respondents were employed as neurosurgical residents. Twice as many (n = 8; 25.0%) were consultants or head of the departments. The remainder (n = 12; 37.5%) were employed as senior consultants.
Most of the responding centers were university hospitals (n = 27; 84.3%). The remaining five were maximum care hospitals (n = 3; 9.4%), or private clinics (n = 2; 6.3%).
Estimated caseload
More than one third of the centers (n = 12; 37.5%) treat 10–20 NASAH patients per year, while a caseload of less than five cases annually is rare (n = 5; 15.6%). Detailed information is presented in Fig. 3.
Complication rate
Three centers (9.3%) report no complications in these patients. In addition, most contributors (n = 18; 56.3%) estimate the complication rate to be less than 2%. While complication rates exceeding 10% are stated by n = 3 (9.3%). No notable correlation was identified in the estimated complication rate and the hospital’s care status.
Inpatient monitoring
In the first case, 3 centers (9.3%) admit their patient to the intensive care unit (ICU), 12 (37.5%) to the intermediate care unit (IMCU), and 17 (53.1%) to the regular ward (RW). However, the management changes with increasing amount of PM SAH detected on the cCT scan. Comparing the answers for inpatient monitoring given for case 1 and case 3, statistical significance was reached comparing monitoring on the RW and ICU (p = 0.017; p = 0.011). Inpatient monitoring is shown in Fig. 4.
Blood pressure monitoring
One center did not use any blood pressure monitoring in all cases (3.1%), and another did not use it in case 3 (3.1%). The method used for blood pressure measurement was variable, with elevated numbers of invasive monitoring being used in case 3. Nonetheless, these results did not reach statistical significance (p = 0.08). Details are shown in Fig. 5.
Treatment regimens
Comparing all treatment regimens, the number of patients in the group “neither CV prophylaxis nor transcranial doppler sonography (TCD)” was statistically significantly reduced in case 3 (p = 0.0014). Details Fig. 6.
Follow-up imaging
Statistically significantly more centers perform a second DSA in case 3 compared to case 1 and case 2 (p = 0.027; p = 0.014). This DSA is most often scheduled during the hospital stay or within the first three months after the initial bleeding. Baseline data is summarized in Table 1.
Discussion
The current study presents the findings of our survey, encompassing diagnostic and therapeutic strategies in patients with NASAH - initially confirmed by one negative DSA - within neurosurgical departments of the EANS. Our results indicate statistically significant variations in the treatment protocols for inpatient monitoring, specifically regarding CV prophylaxis and TCD, as well as the execution of a follow-up DSA - based on the amount of PM SAH detected in the first native cCT.
NASAH is frequently classified as a “benign” form of bleeding, attributed to its lower complication rates5,6. Therefore, inpatient treatment including CV prophylaxis and repetitive TCD are rarely evaluated3. The lower complication rate is supported by many studies, exemplary a work conducted by Alrohimi and colleagues in 2024 compared aneurysmal SAH with NASAH, revealing a statistically significant disparity in the incidence of symptomatic vasospasm (40% vs. 2.6%; p = 0.01)6. This finding aligns with the estimated complication rate (> 5%), which were reported by 84.4% of our respondents. The estimated complication rates did not differ regarding the employment status of the respondents. These results are consistent with the results of our nationwide survey in Germany, with a complication rate minor 5% being reported from 76.3% respondents4.
Currently, DSA is not recommended for these patients based on a benefit-risk analysis3. Nevertheless, numerous neurosurgical departments continue to perform at least one initial DSA, and more and more data is being published regarding necessity and timing of follow-up DSA4,7,8. This reflects a cautious stance among clinicians, despite a recent study by Vogentseder and colleagues excluding any bleeding source in 51 patients with PM SAH9. Our study was able to confirm this cautious approach of neurosurgeons within the Vascular Section of the EANS, as evidenced by a statistically significant increase in the performance of a secondary DSA in case 3 compared to cases 1 and 2.
In relation to inpatient care, a statistically significant increase in the number of patients being admitted to the ICU in case 3 was observed when compared to the other cases (p = 0.011). Although this clinical pathway is not recommended, the already published survey conducted in Germany and this survey including experts of the Vascular Section of the EANS, revealed that clinicians adapt their daily practice to the individual needs and presentations of patients4. This finding underscores the variability in treatment protocols across different institutions and emphasizes the necessity for standardized guidelines tailored to the specific characteristics of NASAH.
In conclusion, while NASAH typically presents a less severe clinical profile compared to aneurysmal SAH, its diagnosis and management require a careful consideration of bleeding patterns and individual patient factors, including the risk of secondary aneurysm finding. Based on our hospital guidelines, we recommend an initial DSA to rule out vascular pathologies. In case of cerebral vasospasm, a high Fisher grade and/or a high World Federation of Neurosurgical Societies (WFNS) grade, a second DSA within the hospital stay should be performed. Furthermore, ongoing research and consensus building among healthcare providers are essential to establish effective treatment protocols and optimize patient outcomes.
Strengths and limitations
This survey was conducted within the vascular section of the EANS. Although centers from eighteen different countries participated, the response rate does not permit any significant conclusions regarding the geographical distribution of NASAH and its associated treatments. Furthermore, the number of NASAH patients treated annually and the complication rates are only estimated numbers which are not objectively assessed. As this survey exclusively focuses on DSA as a diagnostic tool, no inferences can be made concerning additional diagnostic modalities such as CT or MRI with contrast agents.
Data availability
Availability of materials and data. Data is available upon reasonable request. Request should be addressed to C.W.
References
Gupta, S. K. et al. Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: is it a benign entity? Surg. Neurol. 71, 566–571 (2009).
Rinkel, G. J. E. et al. Outcome in patients with subarachnoid haemorrhage and negative angiography according to pattern of haemorrhage on computed tomography. Lancet 338, 964–968 (1991).
Steiner, T. et al. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc. Dis. 35, 93–112 (2013).
Wolfert, C. et al. Management of perimesencephalic nonaneurysmal subarachnoid hemorrhage: a National survey. Sci. Rep. 13, 12805 (2023).
Akbik, F. et al. Diffuse Angiogram-Negative subarachnoid hemorrhage is associated with an intermediate clinical course. Neurocrit Care. 36, 1002–1010 (2022).
Alrohimi, A. et al. Aneurysmal versus benign. Perimesencephalic Subarachnoid Hemorrhage SVIN. 4, e001166 (2024).
Amantakul, A., Vuthiwong, W. & Khiawsa, N. The diagnostic yield of repeat computed tomography angiography in cases of spontaneous subarachnoid haemorrhage after negative initial digital Subtraction angiography. Pol. J. Radiol. 89, 179–186 (2024).
Mohan, M., Islim, A., Dulhanty, L., Parry-Jones, A. & Patel, H. CT angiogram negative perimesencephalic subarachnoid hemorrhage: is a subsequent DSA necessary? A systematic review. J. NeuroIntervent Surg. 11, 1216–1221 (2019).
Vogetseder, M. et al. Follow-Up imaging in Angiography-Negative spontaneous subarachnoid hemorrhage. World Neurosurg. 191, e496–e504 (2024).
Acknowledgements
We would like to express our gratitude to the EANS and the members of the EANS for their participation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study conception and design: C.W., B.S., A.R., E.S.Data acquisition, analysis and interpretation: C.W., B.S.Manuscript drafting as well as approval of the manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolfert, C., Sommer, B., Krauss, P. et al. Results of an European survey on the management of perimesencephalic nonaneurysmal subarachnoid hemorrhage. Sci Rep 15, 24401 (2025). https://doi.org/10.1038/s41598-025-06443-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06443-6