Abstract
This retrospective observational study evaluated the effectiveness of endoscopic healing (EH) and the durability of infliximab (IFX) in combination with azathioprine (AZA) versus IFX monotherapy in pediatric patients with Crohn’s disease (CD). EH was assessed after 1 year of treatment, whereas IFX durability and associated risk factors were evaluated over an extended follow-up period. Data from 108 pediatric patients were analyzed by comparing the EH rates, IFX trough levels (TLs), antibody-to-IFX (ATIs), and IFX durability between the AZA combination therapy (combination therapy group) and IFX monotherapy (monotherapy group) groups. Of the 108 patients who received IFX therapy, 85 (78.7%) received AZA combination therapy, and 23 (21.3%) received IFX monotherapy. The combination therapy group demonstrated superior EH rates (78.6 vs. 33.3%, p < 0.001), higher IFX TLs (4.6 µg/mL vs. 3.9 µg/mL, p = 0.016), lower ATI positivity (25.0% vs. 52.2%, p = 0.025), and prolonged IFX durability than the monotherapy group. Multivariable Cox proportional hazard regression analysis showed that ATI positivity (hazard ratio [HR] 5.33, 95% confidence interval [CI] 1.61–17.60, p = 0.006) and combination therapy with IFX and AZA (HR 0.13, 95% CI 0.03–0.51, p = 0.004) were associated with IFX durability.
Similar content being viewed by others
Introduction
Crohn’s disease (CD), a type of inflammatory bowel disease (IBD), is characterized by inflammation in the gastrointestinal tract1. With an increasing prevalence of CD, numerous studies have been conducted on its treatment2,3. Notably, the emergence of biologics is widely regarded as a pivotal development in the effective treatment of CD.
The landmark SONIC trial for CD demonstrated the superior effectiveness of infliximab (IFX) and azathioprine (AZA), a combination therapy, compared with IFX monotherapy for corticosteroid-free clinical remission (CR) in naïve patients with moderate-to-severe disease4. Furthermore, combination therapy is more effective for achieving CR than monotherapy and reduces the loss of response (LOR) rate in pediatric patients with CD5,6. In addition, combination therapy prevents the formation of anti-drug antibodies (ADAs), which are antibodies against biologics that reduce the drug effectiveness and maintain higher median IFX trough levels (TLs), improving the remission rate in CD7,8.
Beyond targeting CR in treating pediatric CD, treatment of the target of endoscopic healing (EH) has been associated with improvements in long-term outcomes, as mucosal inflammation is associated with long-term disease-related complications, even in CR9,10,11. However, a notable gap is present in the data on EH effectiveness of combination therapy compared with that of monotherapy12particularly in pediatric patients. Therefore, because of limited availability of data on this issue, the aim of this study was to evaluate the effectiveness of EH in combination therapy compared with that in monotherapy in pediatric patients with CD.
Results
Baseline characteristics
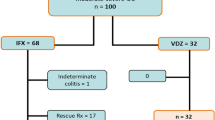
A total of 108 patients were included in this study. Of these patients, 21.3% (23/108) and 78.7% (85/108) were assigned to the monotherapy and combination therapy groups, respectively. Analysis of the monotherapy group revealed that 60.9% (n = 19) of the patients discontinued treatment during the induction period owing to drug-related side effects such as nausea, vomiting, elevated liver function test results, and leukopenia, while 17.4% (n 4) were identified as homozygous or compound heterozygous mutations in thioprine methyltransferase or nudix hydrolase 15, indicating poor metabolizers.
The mean age of the patients at diagnosis was 14.2 years, and 80.6% (87/108) were male. Four patients (3.7%) had a first-degree family history of IBD. No significant differences were observed in the baseline characteristics of the patients between the monotherapy and combination therapy groups. The disease phenotype, disease activity, Simple Endoscopic Score for CD (SES-CD) score, and other baseline characteristics are shown in (Table 1). In this study, IFX was administered to patients with a Pediatric Crohn’s Disease Activity Index (PCDAI) > 30 and/or worsening biochemical markers such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), or fecal calprotectin. The median disease duration of IFX initiation period was 2.27 months (1.77 months in the monotherapy group vs. 2.7 in the combination therapy group, p = 0.209). The clinical and biochemical data at the time of IFX initiation, including disease activity and IFX dose, are presented in (Supplementary Table 1). The median dose of AZA in combination groups was 36.2 ± 12.91 mg (0.61 ± 0.2 mg/kg), and the 6-thioguanine nucleotide (TGN) level was 245.25 ± 88.89 pmol/8 × 108 RBC after 1 year of treatment.
Comparison of treatment outcomes between monotherapy and combination therapy groups
No significant differences in the proportions of patients with CR and transmural healing (TH) were observed between the monotherapy and combination therapy groups at one year (CR: 95.8% vs. 96.4%, p > 0.99; TH: 13.6% vs. 27.4%, p = 0.291). Laboratory results and SES-CD were also comparable between the two groups (SES-CD: 1.0 vs. 0.0, p = 0.083). However, the proportion of patients with biochemical remission (BR) and EH at one year was significantly lower in the monotherapy group than in the combination therapy group (BR: 66.7% vs. 92.9%, p = 0.003; EH: 33.3% vs. 78.6%, p < 0.001).
The median IFX TLs were significantly lower in the monotherapy group than in the combination therapy group (3.9 µg/mL vs. 4.6 µg/mL, p = 0.016; Fig. 1). Furthermore, the proportion of patients with minimal maintenance of IFX threshold TLs for EH (5 µg/mL) was lower in the monotherapy group than in the combination therapy group (30.5% vs. 44.1%, p = 0.023)13. Moreover, antibodies to IFX (ATIs) positivity was higher in the monotherapy group than in the combination therapy group (52.2 vs. 25.0%, P = 0.025). Detailed treatment outcomes of the two groups are presented in (Table 2).
Comparison of IFX durability between monotherapy and combination therapy groups
The number of patients who underwent dose intensification was 15 (65.2%) in the monotherapy group and 47 (55.3%) in the combination therapy group (p = 0.519). Furthermore, no patient stopped IFX treatment before 1 year of treatment. However, one patient in the combination therapy group discontinued IFX treatment after 1 year of treatment because of disease worsening with an infusion reaction caused by ADA formation.
Univariate and multivariate Cox proportional hazard regression analyses revealed that ATI positivity (hazard ratio [HR] 5.33, 95% confidence interval [CI] 1.61–17.60, p = 0.006) and combination therapy (HR 0.13, 95% CI: 0.03–0.51, p = 0.004) were associated with IFX durability (Table 3).
Kaplan–Meier survival curves were used to evaluate IFX durability between the monotherapy and combination therapy groups. Notably, IFX durability was significantly higher in the combination therapy group than in the monotherapy group (p = 0.0026, log-rank test; Fig. 2). The 1-, 2-, and 5-year IFX durability rates were 67.1%, 39.4%, and 20.3%, respectively, in the monotherapy group and 73.7%, 49.1%, and 26.2%, respectively, in the combination therapy group.
Discussion
In this study, we demonstrated that IFX and AZA combination therapy resulted in superior EH and greater IFX durability than IFX monotherapy. The combination therapy group exhibited higher IFX TLs and lower ATI formation rates than the monotherapy group, indicating that pharmacokinetics played a crucial role in achieving better clinical and endoscopic outcomes.
Previous studies have documented the effectiveness of combination therapy in achieving CR. However, studies comparing combination therapy with monotherapy for EH are limited. Most studies have primarily focused on CR as the main endpoint, while EH, which is linked to long-term prognosis, has only recently gained attention4,7,8,14.
Despite the emphasis on CR, the STRIDE-II consensus update in 2021 underscores the growing significance of achieving EH as a therapeutic target because of its association with long-term outcomes in IBD11,15 However, research specifically addressing EH in pediatric populations remains limited12,16. This highlights a significant gap in the literature, particularly regarding treatment strategies targeting EH in pediatric patients. Consequently, limited guidance is available on the optimal treatment strategies for EH in these patients. Our study helps bridge this gap by demonstrating that early combination therapy improves CR and provides a significant advantage in achieving EH compared with IFX monotherapy. This indicates that initiating combination therapy early in the treatment course could be a promising strategy for achieving better outcomes, extending beyond CR to a more sustained EH.
In our cohort, 78.7% of patients received concomitant AZA, and 69.4% started IFX within three months of CD diagnosis, indicating a relatively short disease duration. The early introduction of biologics in pediatric patients with CD has been associated with improved outcomes by leveraging the therapeutic window12,17,18. For these reasons, the EH rates in this study (68.5%) may be relatively high compared with those in previous studies. Nevertheless, this did not affect the results, as the disease duration at IFX initiation was not significantly different between the two groups (p = 0.209).
TH is associated with favorable long-term outcomes, including long-term CR, fewer therapeutic modifications, and lower rates of hospitalization for CD and CD-related surgery19,20. In this study, the TH rate after one year of combination therapy was slightly higher than that after monotherapy (27.4% vs. 13.6%, p = 0.291), but the difference was not statistically significant. However, a previous study indicated that not all patients who achieved TH attained EH. Achieving TH is more challenging than achieving EH and often requires a longer treatment duration. Therefore, the one-year observation in our study may have been insufficient to evaluate the TH outcomes accurately.
The pharmacokinetic mechanisms underlying the effectiveness of combination therapy are likely related to increased IFX TLs and reduced ATI formation, both of which are believed to contribute to sustained therapeutic effects4,7,21,22. Although the exact mechanism by which thiopurines improve IFX pharmacokinetics remains unclear, it may involve a metabolic shift towards 6-TGN levels23,24. Although we could not measure 6-TGN levels in this study, combination therapy significantly increased the proportion of patients maintaining IFX threshold TLs (5 µg/mL) for EH (p = 0.016)25. Furthermore, ATI positivity was significantly lower in the combination therapy group (25.0 vs. 52.2%, p = 0.025), indicating that AZA reduced the immunogenicity. Polakovicova et al. reported that IFX TLs depend on AZA dose26. However, given the various side effects of AZA, studies have suggested that achieving a 6-TGN level of ≥ 125 pmol/8 × 108 RBC may be sufficient to optimize IFX therapeutic levels using combination therapy with AZA and reduce ATI development. Notably, ATI positivity and combination therapy were significantly associated with IFX durability.
Our findings also revealed that combination therapy enhanced the durability of IFX more effectively than monotherapy. This result is consistent with those of other studies that demonstrated that combination therapy significantly lowers the discontinuation rate of IFX4,6. The increased durability observed in our study is likely attributed to the pharmacokinetic advantages of AZA, which aid in maintaining higher IFX concentrations and preventing the formation of ATI, thereby extending the drug’s effectiveness.
However, evidence indicates that the advantages of combination therapy may diminish when therapeutic drug monitoring (TDM) is applied during treatment27. Specifically, IFX monotherapy with proactive dose adjustments yielded outcomes comparable to combination therapy28. Notably, patients in the combination therapy group required lower maintenance doses of IFX while achieving higher TLs and experiencing fewer dose escalations, which offers greater economic benefits29. Furthermore, no significant differences were observed in the steroid-free CR between the monotherapy and combination therapy groups during induction therapy. However, following maintenance therapy, combination therapy demonstrated a more substantial CR and was effective in delaying the reduction of LOR over a long period30. Collectively, the current evidence supports the view that combination therapy may provide greater long-term benefits in pediatric patients with CD.
Although combination therapy offers substantial benefits in terms of EH and IFX durability, the safety concerns associated with AZA, such as potential hepatotoxicity, bone marrow suppression, and increased risk of infections and malignancies, must be carefully managed31,32. However, with appropriate monitoring, thiopurine-S-methyltransferase (TPMT) and nudix hydrolase 15 (NUDT15) gene testing before initiating treatment and regular monitoring of 6-TGN and 6-methylmercaptopurine (6-MMP) metabolites can effectively manage these risks. Although concerns about thiopurine-related side effects persist, studies have demonstrated the benefits of combination therapy, including its ability to achieve both clinical and biochemical remission and promote EH, often outweighing these risks. This makes combination therapy a promising approach, particularly when carefully monitored to minimize the adverse effects.
This study had several limitations. As this was a single-center retrospective study, the follow-up schedules were less structured than those in prospective studies. However, all patients underwent regular examinations such as ileocolonoscopy and biopsy, adhering to the same principles. Furthermore, testing of AZA metabolites, including 6-thioguanine or 6-methilmercaptopurine, was introduced at our medical center midway through the study period, which meant that the 6-TGN concentration could not be measured in all patients. Nevertheless, this did not affect the finding that AZA as a combination therapy was effective in achieving EH. Further research is required to determine whether the EH rate varies with the AZA metabolite concentration. Finally, the number of patients differed between the two groups. Because most patients at our medical center receive combination therapy, enrolling them in monotherapy is challenging. Most patients who received monotherapy in this study were those who initially received AZA but discontinued the drug during the induction period because of side effects such as nausea, vomiting, and leukopenia or did not receive AZA because genetic mutations were identified in thioprine methyltransferase or nudix hydrolase 15.
In conclusion, combination therapy with IFX and AZA resulted in superior EH rates and greater IFX durability than monotherapy in pediatric patients with CD. Although monitoring thiopurine-related side effects is essential, the overall benefits of combination therapy in enhancing long-term outcomes make it a valuable treatment option for pediatric patients with CD.
Materials and methods
Ethics statement
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB File No. 2023-10-010) and adhered to the ethical guidelines outlined in the Declaration of Helsinki. The requirement for patients and guardians informed consent was waived as the data used in this study was waived as all data was de-identified.
Patients and data collection
This retrospective observational study was conducted at the Department of Pediatrics of the Samsung Medical Center between November 2012 and February 2022. Children and adolescents diagnosed with CD and treated with IFX and/or AZA at < 19 years of age were included. Patients with missing baseline clinicopathological data were excluded. CD was diagnosed on the basis of the revised Porto criteria of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition33.
Baseline clinicopathological and laboratory data at diagnosis, including sex, age, disease phenotype, growth indicators, and family history of IBD, were obtained from electronic medical records (EMR). Clinical data and laboratory results at diagnosis and one year after IFX initiation, including IFX treatment duration, dose intensification, pediatric Crohn’s disease activity index (PCDAI) score, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum albumin, simple endoscopic score for CD (SES-CD), TLs of IFX, and the presence of ATIs, were retrospectively collected from the EMR. ATIs were quantified using enzyme-linked immunosorbent assay (ELISA) kits (Matriks Biotek Laboratories, Ankara, Turkey) at an optical density of 450 nm, and IFX TLs were assessed using an IDK monitor infliximab drug level ELISA prior to administration. Ileocolonoscopy and magnetic resonance enterography (MRE) were performed at diagnosis and one year after biologic treatment.
Endpoints and definitions
The primary endpoint of this study was EH after one year of IFX treatment, and the secondary endpoint was IFX durability during the study period. Based on whether the patients received IFX monotherapy or combination therapy with IFX and AZA, they were divided into monotherapy and combination therapy groups.
Treatment outcomes, such as CR, BR), EH, and TH, were evaluated using laboratory tests, ileocolonoscopy, and MRE after 1 year of treatment. CR was defined as a PCDAI < 10 and BR as CRP < 0.5 mg/dL and/or FC < 200 mg/kg, respectively. EH was defined as SES-CD ≤ 2, which corresponds to complete mucosal healing to normal34. TH was defined as a wall thickness of < 3 mm in the absence of ulcers, edema, enhancement, and complications in all ileocolonic segments, as evaluated using MRE. IFX durability was defined as the continuous use of IFX, excluding situations requiring inevitable discontinuation of IFX, such as infusion reactions, surgery, or severe adverse events. We extended the follow-up period to more than 1 year to analyze IFX durability and risk factors for discontinuation.
At our medical center, we primarily implement a top-down strategy to treat pediatric patients with CD. IFX was administered instead of corticosteroids or other immunomodulators when the disease worsened with or without AZA. Both groups were administered 5 mg/kg IFX at 8-week intervals during the maintenance period. If no clinical and/or biological remission occurred during the treatment and a target of 5 µg/mL was not reached based on therapeutic drug monitoring (TDM), dose intensification was administered, which is a reactive TDM strategy. In addition, in the combination group, AZA was administered as a steady-dose escalation method. AZA is routinely initiated at a low dose (0.5 mg/kg/dose daily), and the dose is increased by 1–2 mg/kg/dose daily when TPMT and NUDT15 are confirmed as wild-type.
Statistical analyses
For statistical comparisons between the combination and monotherapy groups, Student’s t-test and Wilcoxon rank-sum test were used for continuous variables, whereas the chi-square or Fischer’s exact test was used for categorical variables.
Univariate and multivariate Cox proportional hazards regression analyses were used to investigate factors associated with IFX durability. Factors with p < 0.1 in the univariate analyses were included in the multivariate analyses. The results were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Kaplan–Meier analysis and the log-rank test were used to calculate IFX durability and overall statistical differences, respectively. Statistical significance was set at p < 0.05. All statistical analyses were performed using the Rex (Version 3.6.0, RexSoft Inc., Seoul, Korea).
Data availability
Data supporting the findings of this study are not publicly available due to privacy and ethical restrictions and can be obtained from the corresponding author upon request.
References
Torres, J., Mehandru, S. & Colombel, J. F. &Peyrin-Biroulet, L. Crohn’s disease. Lancet 389, 1741–1755. https://doi.org/10.1016/S0140-6736(16)31711-1 (2017).
Hong, S. J. et al. Characteristics and incidence trends for pediatric inflammatory bowel disease in Daegu-Kyungpook Province in korea: a Multi-Center study. J. Korean Med. Sci. 33, e132. https://doi.org/10.3346/jkms.2018.33.e132 (2018).
Rosen, M. J. & Dhawan, A. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 169, 1053–1060. https://doi.org/10.1001/jamapediatrics.2015.1982 (2015).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for crohn’s disease. N Engl. J. Med. 362, 1383–1395. https://doi.org/10.1056/NEJMoa0904492 (2010).
Kierkus, J. et al. Monotherapy with Infliximab versus combination therapy in the maintenance of clinical remission in children with moderate to severe Crohn disease. J. Pediatr. Gastroenterol. Nutr. 60, 580–585. https://doi.org/10.1097/MPG.0000000000000684 (2015).
Grossi, V. et al. Concomitant use of immunomodulators affects the durability of Infliximab therapy in children with crohn’s disease. Clin. Gastroenterol. Hepatol. 13, 1748–1756. https://doi.org/10.1016/j.cgh.2015.04.010 (2015).
Kansen, H. M. et al. Less Anti-infliximab antibody formation in paediatric Crohn patients on concomitant immunomodulators. J. Pediatr. Gastroenterol. Nutr. 65, 425–429. https://doi.org/10.1097/MPG.0000000000001551 (2017).
Francis, G. Withdrawal of immunosuppression in crohn’s disease treated with scheduled Infliximab maintenance. Gastroenterology 135, 2156–2157. https://doi.org/10.1053/j.gastro.2008.08.061 (2008).
Neurath, M. F. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 61, 1619–1635. https://doi.org/10.1136/gutjnl-2012-302830 (2012).
Ungaro, R. C. et al. Deep remission at 1 year prevents progression of early crohn’s disease. Gastroenterology 159, 139–147. https://doi.org/10.1053/j.gastro.2020.03.039 (2020).
Turner, D. et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for Treat-to-Target strategies in IBD. Gastroenterology 160, 1570–1583. https://doi.org/10.1053/j.gastro.2020.12.031 (2021).
Kang, B. et al. Mucosal healing in paediatric patients with Moderate-to-Severe luminal crohn’s disease under combined immunosuppression: escalation versus early treatment. J. Crohns Colitis. 10, 1279–1286. https://doi.org/10.1093/ecco-jcc/jjw086 (2016).
van Hoeve, K. et al. Higher Infliximab trough levels are associated with better outcome in paediatric patients with inflammatory bowel disease. J. Crohns Colitis. 12, 1316–1325. https://doi.org/10.1093/ecco-jcc/jjy111 (2018).
D’Haens, G. et al. Early combined immunosuppression or conventional management in patients with newly diagnosed crohn’s disease: an open randomised trial. Lancet 371, 660–667. https://doi.org/10.1016/S0140-6736(08)60304-9 (2008).
D’Haens, G., Geboes, K., Ponette, E. & Penninckx, F. Healing of severe recurrent ileitis with azathioprine therapy in patients with crohn’s disease. Gastroenterology 112, 1475–1481. https://doi.org/10.1016/s0016-5085(97)70027-1 (1997).
Giugliano, F. P. et al. Does azathioprine induce endoscopic and histologic healing in pediatric inflammatory bowel disease? A prospective, observational study. Dig. Liver Dis. 50, 240–246. https://doi.org/10.1016/j.dld.2017.10.017 (2018).
Kang, B. Early biologic treatment in pediatric crohn’s disease: catching the therapeutic window of opportunity in early disease by Treat-to-Target. Pediatr. Gastroenterol. Hepatol. Nutr. 21, 1–11. https://doi.org/10.5223/pghn.2018.21.1.1 (2018).
Walters, T. D. et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an Immunomodulator in children with crohn’s disease. Gastroenterology 146, 383–391. https://doi.org/10.1053/j.gastro.2013.10.027 (2014).
Sands, B. E. et al. Mucosal and transmural healing and Long-term outcomes in crohn’s disease. Inflamm. Bowel Dis. 31, 857–877. https://doi.org/10.1093/ibd/izae159 (2025).
Choi, S. Y. et al. Transmural healing evaluated by magnetic resonance enterography in paediatric patients with crohn’s disease receiving maintenance treatment with biologics. Aliment. Pharmacol. Ther. 56, 1146–1156. https://doi.org/10.1111/apt.17161 (2022).
Brandse, J. F. et al. Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 14, 251–258 e251-252 https://doi.org/10.1016/j.cgh.2015.10.029 (2016).
Zitomersky, N. L. et al. Antibodies to Infliximab are associated with lower Infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm. Bowel Dis. 21, 307–314. https://doi.org/10.1097/MIB.0000000000000284 (2015).
Mogensen, D. V. et al. A role for thiopurine metabolites in the synergism between thiopurines and Infliximab in inflammatory bowel disease. J. Crohns Colitis. 12, 298–305. https://doi.org/10.1093/ecco-jcc/jjx149 (2018).
Yarur, A. J. et al. Combination therapy with immunomodulators improves the pharmacokinetics of infliximab but not vedolizumab or ustekinumab. Clin. Gastroenterol. Hepatol. 21, 2908–2917 e2910 https://doi.org/10.1016/j.cgh.2022.10.016 (2023).
Papamichael, K. et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 17, 1655–1668 e1653 https://doi.org/10.1016/j.cgh.2019.03.037 (2019).
Polakovicova, V. et al. Positive Pharmacokinetic effect of azathioprine co-medication on Infliximab trough levels is dose-dependent. Dig. Liver Dis. 51, 1112–1116. https://doi.org/10.1016/j.dld.2019.05.001 (2019).
Colman, R. J. et al. Infliximab monotherapy vs combination therapy for pediatric crohn’s disease exhibit similar pharmacokinetics. Inflamm. Bowel Dis. 30, 1678–1685. https://doi.org/10.1093/ibd/izad307 (2024).
Lega, S. et al. Proactively optimized Infliximab monotherapy is as effective as combination therapy in IBD. Inflamm. Bowel Dis. 25, 134–141. https://doi.org/10.1093/ibd/izy203 (2019).
Drobne, D. et al. Optimised Infliximab monotherapy is as effective as optimised combination therapy, but is associated with higher drug consumption in inflammatory bowel disease. Aliment. Pharmacol. Ther. 49, 880–889. https://doi.org/10.1111/apt.15179 (2019).
Hoelz, H. et al. Pediatric IBD patients treated with Infliximab and proactive drug monitoring benefit from early concomitant Immunomodulatory therapy: A retrospective analysis of a 10-Year Real-Life cohort. Inflamm. Bowel Dis. 30, 2004–2018. https://doi.org/10.1093/ibd/izad277 (2024).
Debnath, P. et al. Thiopurine-induced myelosuppression with severe Sepsis in a patient with crohn’s disease: A case report. Indian J. Crit. Care Med. 25, 228–230. https://doi.org/10.5005/jp-journals-10071-23738 (2021).
Connell, W. R., Kamm, M. A., Ritchie, J. K. & Lennard-Jones, J. E. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 34, 1081–1085. https://doi.org/10.1136/gut.34.8.1081 (1993).
Levine, A. et al. ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 58, 795–806. https://doi.org/10.1097/MPG.0000000000000239 (2014).
Daperno, M. et al. Development and validation of a new, simplified endoscopic activity score for crohn’s disease: the SES-CD. Gastrointest. Endosc. 60, 505–512. https://doi.org/10.1016/s0016-5107(04)01878-4 (2004).
Funding
This work was supported by the Medical Research Fund of Kangbuk Samsung Hospital.
Author information
Authors and Affiliations
Contributions
Yoon Zi Kim: Data curation, Investigation; Validation; Writing—original draft; Writing—review & editing. Eun Sil Kim: Conceptualization; Data curation; Formal analysis; Investigation; Visualization; Validation; Writing—original draft; Writing—review & editing. Yiyoung Kwon: Data curationSeon Young Kim: Data curationHansol Kim: Data curationYon Ho Choi: Investigation; Supervision; Validation.Mi Jin Kim: Investigation; Supervision; Validation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, Y.Z., Kim, E.S., Kwon, Y. et al. Comparison of endoscopic healing and durability between combination therapy with infliximab and azathioprine versus infliximab monotherapy in pediatric Crohn’s disease. Sci Rep 15, 23025 (2025). https://doi.org/10.1038/s41598-025-06445-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06445-4