Abstract
The treatment of sepsis is challenging due to unclear mechanisms. Propionate is increasingly seen as critical to sepsis pathophysiology by bridging gut microbiota and immunity, but the mechanisms remain unclear. Our study analysed differences in propionate metabolism in peripheral blood mononuclear cells from septic patients and healthy controls using single-cell RNA-seq (scRNA-seq) data. Differentially expressed genes (DEGs) analysis, pathway enrichment, transcription factor (TF) prediction, intercellular communication, and trajectory inference were used to explore the role of propionate metabolism in sepsis. We constructed a sepsis diagnostic model using LASSO and machine learning (XGBoost, CatBoost, NGBoost) with bulk RNA-seq data. scRNA-seq analysis revealed that propionate metabolism was highest in plasma cells (PCs), which can be classified into high and low metabolism groups, identifying 9,155 DEGs. High propionate metabolism was associated with metabolism such as short-chain fatty acids, while low metabolism was related to negative regulation of wound healing. The DoRothEA regulator algorithm showed TFs such as IRF4, ARID3A, FOXO4, and ATF2 were activated in high propionate metabolism subgroups, whereas NR5A1, BCL6, and CDX2 were activated in low subgroups. Cell-cell communication revealed that both groups interacted primarily with B cells and neutrophils, with the high propionate metabolism PCs showing more significant interactions. The receptor-ligand pairs primarily involved were VEGFA-FLT1 and VEGFB-FLT1, and the high propionate metabolism PCs and B cells might interact through BMP8B-BMPR2. Trajectory analysis indicated differentiation from B cells, first to low, then high propionate metabolism PCs. Finally, the LASSO algorithm identified 13 key genes, with the CatBoost model achieving perfect diagnostic performance (AUC = 1.000). These 13 key genes were validated through in vitro experiments. Collectively, these findings suggest that propionic acid metabolism may be a potential target for diagnosing and treating sepsis, offering new insights into its pathophysiology.
Similar content being viewed by others
Introduction
Sepsis is a serious, life-threatening illness caused by the body’s uncontrolled response to infection, impacting millions of people globally, with a mortality of ranging from approximately 17–33%. However, the treatment of sepsis is still limited to the control of infection and supportive therapy1. The understanding of the pathophysiology of sepsis is insufficient, and tools for prognosis need more exploitation.
Propionate is a member of short-chain fatty acids (SCFAs), which are mainly produced by gut microbes during the metabolism of a variety of substrates, including amino acids, carbohydrates, lactic acid, and 1, 2-propylene glycol. Bacteroidetes, Firmicutes, Proteobacteria, and Trichospirillaceae are considered to be major propionic acid-producing bacteria2. Propionate is considered to have a protective function during sepsis. Propionate level is lower in sepsis patients’ stools, and it correlates with a worse prognosis3. Indole-3-propionic acid (IPA) has been proven to enhance the phagocytosis of macrophages via aryl hydrocarbon receptor (AhR)3 and can alleviate sepsis-related liver injury by activating pregnane X receptor (PXR)4and meanwhile militates sepsis-related brain injury by inhibiting the activation of inflammasome NLRP3 and the secretion of IL-1β in microglia5. Propionate plays an important role in the inflammation process. By combining with the surface receptor (FFA2, FFA3, GPR109A) or inhibiting histone deacetylases (HDACs), it mediates the protective or pathogenic role in inflammation6. In application, IPA may treat intestinal flora disorder during sepsis, thus decreasing the level of serum inflammatory factors and the mortality of sepsis patients7.
Though several studies have indicated the key role of propionate in sepsis, there is a lack of insight targeting the mechanism of propionate in sepsis and an overall illustration of the propionate metabolism pathway, which has become a barrier to the application of propionate in the treatment and risk prediction of sepsis.
Our study is devoted to clarifying how propionate is involved in the development of sepsis. Single-cell sequencing (scRNA-seq), bulk-RNA sequencing (bulk RNA-seq), and machine learning were applied to probe into the site of propionate metabolism in peripheral blood and further illustrate the pathways, intracellular communication, and cell differentiation in which propionate metabolism is involved. Based on three bulk RNA-seq datasets, we screened out the hub genes for the determination of propionate metabolism level and constructed a prediction model. We also performed in-vitro validation.
Materials and methods
Data collection and Preparation
The sepsis scRNA-seq dataset EGAS00001006283 was obtained from the European Genome–phenome Archive (https://ega-archive.org/studies/EGAS00001006283)8, including 6 healthy controls, 7 patients after cardiac surgery and 26 sepsis patients. In addition, bulk RNA-seq data and related clinical information were screened and analyzed for GSE570659 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc= GSE57065), GSE9523310 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc= GSE95233) and GSE28750 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc= GSE28750), which included 82 patients and 25 healthy controls, 51 septic patients and 22 healthy controls and 10 septic patients and 20 healthy controls, respectively.
Single-cell RNA-sequencing analysis
Integration of count matrices and clinical information to generate objects through the Seurat package in R software. Firstly, we filtered out poor quality cells and genes (Cells expressing < 100 or > 4,000 genes, > 10% mitochondrial reads, > 2% hemoglobin reads, or a log10 (UMI per gene) < 0.6; Genes expressed in < 10 cells or with a total count < 3 were also removed). Then, the expression matrix was normalized and scaled using the Seurat package with default settings11. The Harmony package was implemented for assessing and eliminating batch effects in each sample. A principal component analysis (PCA) based on 2,000 highly variable genes was performed to reduce the dimensionality of the expression matrix. Unsupervised cell clusters were acquired by graph-based clustering approach (The top 20 principal components were selected, resolution = 0.5), and visualized by UMAP dimensionality reduction. The clusters were annotated to known biological cell types based on the expression of typical markers in the original literature. Differential expressed genes (DEGs) among each cell type were recognized by the FindAllMarkers function with the following criteria: logFC > 0.25; min.pct > 0.25; adjusted p-value < 0.05 (Benjamini-Hochberg).
Pathway activity analysis
For pathway activity analysis, normalized gene expression data from each cell cluster (scRNA-seq) were implemented in the R “GSVA” package (https://github.com/rcastelo/GSVA) to evaluate the enrichment of related gene sets12. The gene sets used for Gene Set Variation Analysis (GSVA) were downloaded from the Molecular Signatures Database (MSigDB) website (https://www.gsea-msigdb.org/gsea/msigdb). Plasma cells(PCs) in scRNA-seq datasets were divided into high- and low-propionate PCs according to the median GSVA score of a propionate metabolism-related transcriptional signature termed “GOBP_PROPIONATE_METABOLIC_PROCESS.v2023.2.Hs.gmt”.
Transcription factor enrichment and cell-cell communication analysis
The DoRothEA regulator, which we used to infer transcription factors (TFs) activity from the scRNA-seq dataset, was restricted to human TFs with evidence level A-C and minimum regulator size 513. We calculated a consensus activity score as implemented in the decoupleR R package14. We tested for differential activity between high- and low-propionate PCs groups using a linear mixed model with a random intercept for individuals, adjusting the p values for multiple testing using the Benjamini-Hochberg method. Cell-cell communication analysis of scRNA-seq data using the CellChat R software package15. CellChat is a tool for quantitatively inferring and analyzing intercellular communication networks using scRNA-Seq data. Significance in the interaction between distinct cell subpopulations is attributed to ligand-receptor pairs identified by CellChat, with a p value below 0.05.
Pathway enrichment analysis
Genes were functionally annotated by assessing the enrichment of Gene Ontology (GO) terms (biological process, cellular component terms, molecular functions). The R ‘clusterProfiler’ package was used to visualize the related pathways with an adjusted p value less than 0.0516.
Trajectory inference analysis
Slingshot (R package v1.8) analysis was performed using TSNE as the dimensionally reduced input17. Slingshot utilizes a minimum spanning tree method to recognize lineages of cells starting at the same origin and then diverging to unique endpoints, where it then calculates pseudotime for the cells on the lineage using principal curves.
Feature selection and model construction via machine learning-based integration
To identify hub genes associated with propionate metabolism in plasma, the least absolute shrinkage and selection operator (LASSO) regression technique based on DEGs between the high and low propionate PCs groups in the scRNA-seq dataset was used. Ten-fold cross-validation was used to select the minimal penalty term. Next, based on the hub genes selected by the LASSO algorithm, a total of 3 machine learning models are integrated, including Xtreme Gradient Boosting (XGBoost), Categorical Boosting (CatBoost), and Natural Gradient Boosting (NGBoost). All models underwent subsequent testing in the test cohort. To evaluate the performance of each model, the area under the curve (AUC) values were calculated for the testing and validation cohorts. The model with the highest average AUC value in the testing cohort was considered the best model.
The cecal ligation and puncture (CLP) model
The animal study protocol was reviewed and approved by the Animal Policy and Welfare Committee of Ruijin Hospital. The studies conducted were documented following the ARRIVE guidelines (Animals in Research: Reporting of In Vivo Experiments) (Table S1)18. All methods were performed in accordance with the relevant guidelines and regulations. Male C57BL/6 mice (8 weeks old, 20–25 g) were purchased from Phenotek Biotechnology (Shanghai) Co., Ltd. All mice were housed under specific pathogen-free conditions with free access to sterilized purified water and food. Prior to the start of the experiments, mice were kept at room temperature (25℃) for at least one week with a 12-hour light/dark cycle. The CLP model was developed to simulate the progression of sepsis. The mice were anesthetized with 1% pentobarbital sodium, and their abdominal cavities were surgically opened to expose the cecum. Then, the cecum is ligated using surgical sutures at 1/2 from the bottom of the ileal valve19. Subsequently, a 22-gauge needle was used to puncture the remaining cecum. The cecum was then returned to the abdominal cavity, and the abdomen was sutured closed. Resuscitation involved injecting 5 mL/100 g of prewarmed saline solution subcutaneously into all the mice. The Sham group underwent anesthesia and abdominal opening but did not undergo cecal ligation and puncture. Three mice were assigned to each group using the random number table method. Six mice underwent the removal of their right eyeballs for blood collection 8 h post-sepsis induction, followed by euthanasia via cervical dislocation.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted and purified from fresh blood of different groups of mice using VeZol reagent, following the manufacturer’s protocol. cDNA was synthesized from 1 µg of total RNA Isolation Kit (Vazyme #RC202). qRT-PCR was performed using the ChamQ Universal SYBR qPCR Master Mix (Vazyme #Q711). Relative gene expression levels were quantified using the 2−ΔΔCt method, with beta-actin as the internal control. The primer sequences are shown in Table S2.
Statistical analysis
All statistical analysis was performed using R version 4.2.2 or GraphPad Prism version 9. Data are expressed as mean ± standard error of the mean (SEM). Comparison of data between the two groups was performed using the unpaired t-test. Data visualization utilized the R package ggplot2. p < 0.05 was considered statistically significant.
Results
Single-cell RNA sequencing analysis
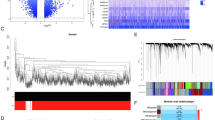
We first analyzed differences in propionate metabolism in peripheral blood from sepsis patients (n = 26) and healthy controls (n = 6) using the scRNA-seq dataset (EGAS00001006283). We found that propionate metabolism was most active in PCs (Fig. 1A, B, C). We then categorized PCs into two groups with high and low propionate metabolism and screened for differential genes according to p < 0.05, abs(logFC) > 0.25, with a total of 9155 DEGs.
Subgrouping and differences of propionate metabolism PCs. (A) t-SNE plots for showing major immune cell subsets in sepsis patients and the healthy control group. (B) Propionate levels of different immune cells in sepsis patients. (C) PA metabolism levels in different immune cells. (D) Pathway enrichment analysis of high and low propionate metabolism PCs using DEGs. (E) TFs enrichment and scoring of high and low propionate metabolism PCs. plasma cells, PCs; differentially expressed genes, DEGs.
We performed pathway enrichment analysis using DEGs and found that PCs with high propionate metabolism were mainly enriched in cellular biological processes such as SCFAs, succinyl-CoA, antimicrobial peptide-mediated humoral immune response, anti-microbial humoral immune response, antimicrobial humoral response, myotubular cell development, fatty acids, immunoglobulin production, monocarboxylic acids, and production of molecular mediators of the immune response; PCs with low propionate metabolism mainly enriched in negative regulation of wound healing, regulation of nuclear division, mast cell activation, differentiation of epithelial cells involved in kidney development, negative regulation of nuclear division, chromosome segregation, regulation of nuclear division in cranial neuromorphogenesis mitosis, differentiation of keratinocytes, and localization of proteins in the chromatin regions of chromosome centers (Fig. 1D).
The DoRothEA regulator algorithm showed that TFs such as IRF4, ARID3A, FOXO4, and ATF2 had increased activity in PCs with high propionate metabolism, whereas TFs such as NR5A1, BCL6, and CDX2 played important roles in PCs with low propionate metabolism (Fig. 1E).
Cell-cell communication analysis and trajectory analysis
CellChat for delineating intricate networks of cell-to-cell communication. We found that both groups mainly interacted with B cells and neutrophils, more significantly with highly propionate-metabolizing PCs (Fig. 2A, B). The receptor ligands were mainly VEGFA-FLT1 and VEGFB-FLT1, and the intercellular interactions between PCs and B cells with high propionate metabolism might be through BMP8B-BMPR2 (Fig. 2C).
Cell communication and trajectory analysis of propionate metabolism in PCs in sepsis patients. (A) Pathways involved in cell communication between propionate metabolism PCs and other immune cells. (B) Strength of communication between different types of cells. (C) Heatmap of receptor ligands during cell communication. (D) The trajectory analysis of propionate metabolism PCs. plasma cells, PCs.
The trajectory analysis showed that both of these cells differentiated from B cells, which then differentiated into low propionate metabolizing PCs and finally into high propionate metabolizing PCs (Fig. 2D).
Bulk RNA-seq analysis
We selected two sepsis bulk datasets, where GSE57065 (sepsis (n = 82) – healthy (n = 25)) was used as the training set, GSE95233 (sepsis (n = 51) – healthy (n = 22)) was used as the testing set and GSE28750 (sepsis (n = 10) – healthy (n = 20)) was used as the validation set. Firstly, we performed the merging, and after eliminating the batch effect, the training set and testing set were well distributed (Fig. 3A, B). Using two groups of high and low propionate metabolism PC differential genes inside the bulk data as inputs, the LASSO algorithm was used for dimensionality reduction and finally, 13 hub genes were identified to construct the prediction model (Fig. 3C-E). Immune infiltration analysis revealed that LMAN2, NT5DC2, PHPT1SEC31A, and ST6GAL1 were mainly positively correlated with PC infiltration, and CFLAR was negatively correlated with PC infiltration (Fig. 3F).
Establishing the machine learning prediction model. (A) and (B) The distribution of the training set and the testing set before and after batch effect elimination. (C) LASSO coefficient path diagram for risk factors. (D) Cross-validation curves. (E) 13 hub genes identified after LASSO regression analysis. (F) Immune infiltration analysis of propionate metabolism PCs. The AUC of the CatBoost model in the testing (G) and validation set (H). (I) The top 6 hub genes selected according to the CatBoost model. P < 0.05; **, P < 0.01; ***, P < 0.001. P < 0.05 was considered statistically significant. area under the curve, AUC.
Three machine learning algorithms, XGBoost, CatBoost, and NGBoost, were trained to predict the risk of sepsis development using the training set, and it was found that the CatBoost model achieved the best AUC value (1.000) in the testing set, and was considered the best model (Fig. 3G). Furthermore, the CatBoost model also demonstrated excellent performance with a high AUC value in an independent validation set, further confirming its robustness and generalizability (Fig. 3H). Based on the CatBoost model, the top 13 central genes were selected: EDEM3, TMEM167A, FNDC3B, CKAP4, REEP5, CFLAR, LMAN2, PHPT1, NT5DC2, SEC31A, UBA1, TPP2, ST6GAL1 (Fig. 3I).
In vitro verification
To verify the accuracy of bioinformatics analysis, qRT-PCR analysis was performed on peripheral blood of CLP and Sham mice. The expression of the 13 hub genes was measured. qRT-PCR results showed that, compared with the Sham group, the mRNA expression levels of CKAP4, FNDA3B, THEM167A, NT5DC2, SEC31A, EDEM3, LAMN2, TPP2 in the CLP group was significantly higher (p < 0.05), while mRNA expression level of ST6GAL1, PHPT1 in CLP group was significantly lower (p < 0.05). CFLAR, UBA1 and REEP5 didn’t show difference between groups (Fig. 4). These results further confirmed the reliability of our bioinformatics predictions and highlighted the potential of the identified hub genes as diagnostic biomarkers for sepsis.
Validation of hub gene expression by qRT-PCR in CLP and sham mice. Expression levels of 13 hub genes in Sham and CLP mice validated by unpaired t-test. Data are shown as mean ± SEM(NS indicates not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001,****, P < 0.0001). cecal ligation and puncture, CLP; standard error of the mean, SEM.
Discussion
We conducted relatively thorough research into the role of propionate metabolism in the process of sepsis. We first performed scRNA-seq analysis and found out PCs had the highest metabolic score. We divided PCs into two groups according to their propionate metabolism level and subsequently did pathway enrichment, transcription factor enrichment, and intercellular communication analysis. Quasi-temporal analysis was performed to depict the differentiation trajectory. Based on two bulk RNA sequencing datasets, we used the LASSO algorithm to screen out key genes and then constructed a model for predicting sepsis by propionate metabolism using machine learning methods. In vitro experiments were carried out to validate these hub genes.
By comparing the scRNA-seq data of sepsis patients and the health control group, we found that propionate metabolism is the most active in PCs of peripheral blood with sepsis. Previous studies reported that SCFAs can regulate B cell metabolism, supporting B cells’ activation, differentiation, and antibody production by providing more energy20. Our findings are consistent with the conclusion and meanwhile extended it to sepsis patients. From the enteric cavity to the periphery, SCFAs have a strong bio-gradient, which means different organs absorb different SCFAs selectively, while the impact of such distribution specificity on the body has not yet been clear21.
We divided PCs into two groups with high and low levels of propionate metabolism. We conducted differential gene enrichment to explain the difference in function between the two types of PCs. PCs with high propionate metabolism are predominantly enriched in cellular biological processes such as SCFAs metabolism, succinyl lauroyl acid metabolism, antimicrobial peptide-mediated humoral immune response, antimicrobial humoral immune reaction, antibacterial humoral response, myotube cell development, fatty acid metabolic process, immunoglobulin production, monocarboxylic acid metabolic process, and the production of immune response mediators. These processes are mostly associated with anti-infection and immune responses. In contrast, PCs with low propionate metabolism are mainly enriched in processes like negative regulation of wound healing, regulation of nuclear division, and mast cell activation. Macrocytes have been shown to impair the phagocytic function of resident macrophages and the migratory function of neutrophils and aggravate sepsis22,23. This can explain the protective effect of propionate in sepsis and provides clear guidance for molecular-level research.
DoRothEA regulator algorithm showed that certain TFs including IRF4, ARID3A, FOXO4, and ATF2 had enhanced activity in high propionate metabolism PCs. IRF4, a member of the interferon regulatory factor family, plays a key role in the development, differentiation, and decision of cell fate in multiple immune cells. Previous studies discovered that SCFAs enhanced the IRF4 expression in B cells, which is indispensable in differentiation and antibody production20. TLR9 maintains the stability of lipid metabolism and regulates the production of IL-10 via IRF424. To our knowledge, IL-10 has vital immunosuppression effects, which stimulate the differentiation of T cells, regulate intestinal inflammation, and promote intestinal homeostasis25,26,27. This reminds us that B cells potentially limit the excessive immune response during sepsis. Recent research indicates that INF-α secretion is enhanced in B cells of high ARID3A expression, and such an effect can be activated by the stimulation of TLR928. Combined with our findings, propionate metabolism in PCs may also be engaged in the network of TLR9, IRF4, and ARID3A, affecting the immune response and the metabolism of nutrient substances. However, we are still not clear whether high propionate metabolism PCs secrete more INF-α and IL-10, which needs further research to enlighten immune conditioning therapy in sepsis. FOXO proteins are a subgroup of the Forkhead box family (FOX) proteins, which play a critical role in the differentiation of B cells. A high expression of FOXO4 was found to regulate gut flora and alleviate inflammation by inhibiting the NF-κB pathway in enterocytes29.
The study of intracellular communication revealed that both groups of PCs interact with B cells and neutrophils, especially in the high propionate metabolism group. PCs talk to B cells and neutrophils mainly via cytokines, including B cell–activating factor (BAFF), interleukins, and TNFs30,31. Our research adds VEGFA-FLT1 and VEGFB-FLT1 to the process. Specifically, high propionate metabolism PCs communicate via BMP8B-BMPR2. VEGFs are highly specific pro-angiogenic growth factors that play essential physiological roles in angiogenesis, vascular maintenance, and formation. They are crucial for inducing endothelial cell survival, proliferation, migration, angiogenesis, and increasing vascular permeability32. Additionally, VEGFs have been found to exhibit immunosuppressive functions within the tumor microenvironment33. BMP8B was found effective in lipid metabolism, inducing the browning of white adipose tissue and activating VEGF, thereby promoting vascular and neural remodeling of adipose tissue34,35. So far, there is a lack of evidence that B cells or PCs are capable of secreting BMP8B or VEGF, and their signaling pathway and functions remain unclear.
Meta-analysis has proved that machine-learning models show better performance compared with traditional scoring36. Different from previous models, ours centers on patients’ propionate metabolism level, predicting the probability of sepsis by 13 genes from a single perspective, which is not addressed by previous studies. In this study, we used bulk RNA-seq data of differential genes of high- and low-propionate-metabolizing PCs as input. We applied the LASSO algorithm for dimensionality reduction to identify 13 hub genes. We then attempted modeling using three machine learning algorithms—XGBoost, CatBoost, and NGBoost—and ultimately found that CatBoost achieved the highest AUC value. Compared with the other two, CatBoost has a better capability of handling class features and missing data and preventing overfitting. XGBoost has a relatively faster computing speed and better model performance, while NGBoost has probability output and quantization of uncertainty37,38,39. Several studies confirm the accuracy and reliability of XGBoost in the prediction of the onset and mortality risk of sepsis40,41. Liu et al. exploited a model with external verification to precisely predict the need for intubation in ICU patients within 24 to 96 h of admission42. This strengthened the rationality of our choice of algorithm. Moreover, integrated algorithms may improve forecasting, which deserves some insight into subsequent analysis43.
Beyond propionate metabolism, we also examined other relevant metabolic pathways and immune-modulatory pathways as potential confounders in sepsis. SCFAs influence protein modifications, regulate protein activity, and impact cellular signal transduction in sepsis44. Pentanoate has been proved to have anti-inflammatory and immunoregulatory effects by promoting histone H4 acetylation at the IL-10 promoter through the provision of acetyl-CoA and inhibition of HDAC activity, thus inducing IL-10 production in lymphocytes45. Additionally, exogenous SCFAs supplementation has been shown to downregulate IL-6 levels46a pathway that interacts with vascular endothelium during cytokine storms and mediates sepsis-induced muscle atrophy via the gp130/JAK2/STAT3 pathway, potentially contributing to ICU-acquired weakness47,48. These findings suggest that propionate may exert anti-sepsis effects beyond its plasma concentrations, warranting further investigation.
To our knowledge, this is the first study on the role of propionate in the development of sepsis. By combining scRNA-seq analysis, bulk RNA-seq analysis, and machine learning, we clarify the site and the key molecules and pathways in propionate metabolism. This is highly significant for advancing our understanding of propionate metabolism and developing new approaches for the prediction and treatment of sepsis. Additionally, compared with traditional inflammatory biomarkers, propionate metabolism genes possess the following advantages. Firstly, propionate metabolism level reflects the abundance and activity of the relative microbiomes, which potentially elucidates the underlying mechanisms of the internal environment disturbance in sepsis patients. Moreover, the variability in the expression levels of propionate metabolism-related genes among sepsis patients may account for differences in their responsiveness to treatment. Lastly, inflammatory biomarkers are susceptible to short-term factors like stress, while gene expression profiles provide a more stable and long-term indication of inflammatory risk, thereby enhancing their predictive potential and clinical utility.
Our study has some limitations. Firstly, our research needs an in-depth insight into cell behaviors on molecular and pathway levels, and the significance and details of transcription factors, cell communications, and cell difference trajectory need to be clear. Secondly, our prediction model has yet to undergo external validation to confirm its effectiveness. Thirdly, our study is limited by the sample size of the database and was conducted only in a small-scale population. Future research should aim to conduct larger-scale experiments to derive more robust conclusions.
Conclusion
In this study, we present evidence that PCs are crucial in propionate metabolism in sepsis patients. TFs including IRF4, ARID3A, FOXO4, and ATF2 had enhanced activity in high propionate metabolism PCs, and PCs communicate with B cells and neutrophils via BMP8B-BMPR2, VEGFA-FLT1 and VEGFB-FLT1. 13 hub genes screened out from bulk RNA-seq datasets helped us construct a sepsis prediction model. Additionally, we conducted in vitro validation by qRT-PCR, which confirmed the differential expression of these 13 hub genes in sepsis. Our study may provide new insights into the pathophysiological mechanisms of sepsis and potential avenues for future therapeutic exploration.
Data availability
The sepsis scRNA-seq dataset EGAS00001006283 was obtained from the European Genome–phenome Archive (https://ega-archive.org/studies/EGAS00001006283);The bulk RNA-seq data and related clinical information were screened and analyzed for GSE57065(https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc= GSE57065), GSE95233(https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc= GSE95233) and GSE28750 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc= GSE28750); The gene sets used for Gene Set Variation Analysis (GSVA) were downloaded from the Molecular Signatures Database (MSigDB) website (https://www.gsea-msigdb.org/gsea/msigdb).
References
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247 (2021).
Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M. & Larraufie, P. Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49 (2021).
Huang, Z. B. et al. Gut microbiota-derived Indole 3-propionic acid partially activates Aryl hydrocarbon receptor to promote macrophage phagocytosis and attenuate septic injury. Front. Cell. Infect. Microbiol. 12, 1015386 (2022).
Wang, S. et al. Indole-3-propionic acid alleviates sepsis-associated acute liver injury by activating pregnane X receptor. Mol. Med. 29, 65 (2023).
Fang, H. et al. Sepsis-Induced gut dysbiosis mediates the susceptibility to Sepsis-Associated encephalopathy in mice. mSystems 7, e0139921 (2022).
Li, M. et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 831, 52–59 (2018).
Fang, H. et al. Indole-3-Propionic acid as a potential therapeutic agent for Sepsis-Induced gut microbiota disturbance. Microbiol. Spectr. 10, e0012522 (2022).
Kwok, A. J. et al. Neutrophils and emergency granulopoiesis drive immune suppression and an extreme response endotype during sepsis. Nat. Immunol. 24, 767–779 (2023).
Cazalis, M. A. et al. Early and dynamic changes in gene expression in septic shock patients: a genome-wide approach. Intensive Care Med. Exp. 2, 20 (2014).
Tabone, O. et al. Endogenous Retroviruses Transcriptional Modulation After Severe Infection, Trauma and Burn. Front Immunol. 9, 3091 (2018).
Hao, Y. et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 42, 293–304 (2024).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7 (2013).
Garcia-Alonso, L., Holland, C. H., Ibrahim, M. M., Turei, D. & Saez-Rodriguez, J. Benchmark and integration of resources for the Estimation of human transcription factor activities. Genome Res. 29, 1363–1375 (2019).
Badia-i-Mompel, P. et al. DecoupleR: ensemble of computational methods to infer biological activities from omics data. Bioinf. Adv. 2, vbac016 (2022).
Jin, S. et al. Inference and analysis of cell-cell communication using cellchat. Nat. Commun. 12, 1088 (2021).
Xu, S. et al. Using clusterprofiler to characterize multiomics data. Nat. Protoc. 19, 3292–3320 (2024).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genom. 19, 477 (2018).
Kilkenny, C., Browne, W. J., Cuthi, I., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 41, 27–31 (2012).
Drechsler, S. & Osuchowski, M. Cecal ligation and puncture. Methods Mol. Biol. 2321, 1–8 (2021).
Kim, M., Qie, Y., Park, J. & Kim, C. H. Gut microbial metabolites fuel host antibody responses. Cell. Host Microbe. 20, 202–214 (2016).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 7, 189–200 (2016).
Dahdah, A. et al. Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. J. Clin. Invest. 124, 4577–4589 (2014).
Carvalho, M., Benjamim, C., Santos, F., Ferreira, S. & Cunha, F. Effect of mast cells depletion on the failure of neutrophil migration during sepsis. Eur. J. Pharmacol. 525, 161–169 (2005).
Wang, P. et al. Tlr9 deficiency in B cells leads to obesity by promoting inflammation and gut dysbiosis. Nat. Commun. 15, 4232 (2024).
Saraiva, M. & O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10, 170–181 (2010).
Mantovani, A. & Marchesi, F. IL-10 and macrophages orchestrate gut homeostasis. Immunity 40, 637–639 (2014).
Sun, M. et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 9, 3555 (2018).
Ward, J. M. et al. Human effector B lymphocytes express ARID3a and secrete interferon alpha. J. Autoimmun. 75, 130–140 (2016).
Zhou, W. et al. FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology 137, 1403–1414 (2009).
Li, Y. et al. The regulatory roles of neutrophils in adaptive immunity. Cell. Communication Signal. 17, 147 (2019).
Parsa, R. et al. BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J. Exp. Med. 213, 1537–1553 (2016).
Nieves, B. J., D’Amore, P. A. & Bryan, B. A. The function of vascular endothelial growth factor. Biofactors 35, 332–337 (2009).
Ribatti, D. Immunosuppressive effects of vascular endothelial growth factor. Oncol. Lett. 24, 369 (2022).
Pellegrinelli, V. et al. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat. Commun. 9, 4974 (2018).
Whittle, A. J. et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012).
Fleuren, L. M. et al. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 46, 383–400 (2020).
Prokhorenkova, L., Gusev, G., Vorobev, A., Dorogush, A. V. & Gulin, A. CatBoost: unbiased boosting with categorical features. Preprint At. https://doi.org/10.48550/ARXIV.1706.09516 (2017).
Duan, T. et al. NGBoost: Natural Gradient Boosting for Probabilistic Prediction. Preprint at (2019). https://doi.org/10.48550/ARXIV.1910.03225
Chen, T., Guestrin, C. & XGBoost A Scalable Tree Boosting System. (2016). https://doi.org/10.48550/ARXIV.1603.02754
Zhang, Y., Xu, W., Yang, P. & Zhang, A. Machine learning for the prediction of sepsis-related death: a systematic review and meta-analysis. BMC Med. Inf. Decis. Mak. 23, 283 (2023).
Yang, Z., Cui, X. & Song, Z. Predicting sepsis onset in ICU using machine learning models: a systematic review and meta-analysis. BMC Infect. Dis. 23, 635 (2023).
Liu, J. et al. Development and external validation of an interpretable machine learning model for the prediction of intubation in the intensive care unit. Sci. Rep. 14, 27174 (2024).
Gupta, J., Majumder, A. K., Sengupta, D., Sultana, M. & Bhattacharya, S. Investigating computational models for diagnosis and prognosis of sepsis based on clinical parameters: opportunities, challenges, and future research directions. J. Intensive Med. 4, 468–477 (2024).
Zhang, L. et al. Protein modification by short-chain fatty acid metabolites in sepsis: a comprehensive review. Front. Immunol. 14, 1171834 (2023).
Luu, M. et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 10, 760 (2019).
Liao, H. et al. Short chain fatty acids protect the cognitive function of Sepsis associated encephalopathy mice via GPR43. Front. Neurol. 13, 909436 (2022).
Kang, S. & Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 53, 1116–1123 (2021).
Zanders, L. et al. Sepsis induces Interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J. Cachexia Sarcopenia Muscle. 13, 713–727 (2022).
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 82241044, 82172152, and 82102244) and Shanghai “Rising Stars of Medical Talents” Youth Development Program(SHWSRS(2024)_70).
Author information
Authors and Affiliations
Contributions
DC and JL designed the research. WS, DjC, HQ, and SZ collected the data. WS, and DjC analyzed the data. SZ, XP, LY and SH performed the literature search. WS, DjC, LY and HQ drafted the article. DC and JL jointly supervised the study. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, L., Shang, W., Chen, D. et al. Identifying propionate metabolism-related genes as biomarkers of sepsis development and therapeutic targets. Sci Rep 15, 24531 (2025). https://doi.org/10.1038/s41598-025-06463-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06463-2
Keywords
This article is cited by
-
Research advances on the role of programmed endothelial cell death in sepsis
Cell Death Discovery (2025)