Abstract
Diabetic foot ulcers (DFUs) represent a significant challenge in diabetic care, with variable prognoses influenced by factors such as ulcer location, depth, and the presence of infection or ischemia. The Site, Depth, and Infection/Ischemia (SDI) classification system is a potential tool for predicting DFU outcomes, but its prognostic value requires further investigation. This retrospective cohort study, conducted at Qingdao Haici Hospital between January 2021 and December 2022, included 261 diabetic patients with DFUs located at or distal to the ankle joint. The SDI classification system was applied to categorize ulcers based on site, depth, and infection/ischemia. Patient outcomes, including healing time, minor and major amputations, and mortality, were assessed, with follow-up conducted through telephone or outpatient visits. Statistical analysis was performed using SPSS version 27, with p-values < 0.05 considered statistically significant. The study included 240 patients after exclusions. Significant differences in healing time, amputation rates, and mortality were observed across different SDI classifications. Patients with hindfoot ulcers (S3) had the longest healing time (9.3 months) and the highest mortality (23.1%). Deeper ulcers (D3) also exhibited longer healing times (4.3 months) and higher mortality (18.5%). Patients with both infection and ischemia (I3) had the longest healing time (5.6 months), highest amputation rates, and increased mortality. Statistical analyses revealed significant differences in healing time (p < 0.001), amputation rates (p = 0.000), and mortality (p = 0.017) across classification groups. The SDI classification system effectively predicts outcomes in DFU patients, with higher SDI grades associated with longer healing times, higher amputation rates, and increased mortality. This study highlights the clinical utility of the SDI system and underscores the need for its further implementation in clinical practice for better patient management and prognostic assessment.
Similar content being viewed by others
Introduction
Diabetic foot (DF) is a major and growing public health issue, significantly contributing to both morbidity and mortality among individuals with diabetes mellitus1. Diabetic foot ulcers (DFUs), a common and severe manifestation of this condition, are one of the primary causes of lower limb amputations and hospitalizations in diabetic patients, thereby imposing a substantial societal and economic burden globally2. The incidence of DFUs has been rising in parallel with the increasing prevalence of diabetes, emphasizing the urgent need for effective prevention, early detection, and treatment strategies3,4. DFUs are complex lesions that typically present with a prolonged course, marked by dynamic changes in both the clinical appearance and underlying pathology5. These ulcers often result from a combination of factors, including peripheral neuropathy, poor circulation, impaired immune response, and bacterial infections, all of which complicate healing and predispose to further complications, such as ischemia and infection6. Therefore, accurately assessing the severity and progression of DFUs is crucial to guide treatment decisions and predict clinical outcomes7. Early intervention can significantly reduce the risk of major complications, such as limb amputation, but only when the appropriate severity grading system is used to stratify risk effectively1,8.
Although numerous grading systems for DFUs exist, they often vary in complexity, specificity, and clinical applicability. Systems such as the Wagner classification and the University of Texas system are widely utilized; however, these methods can be cumbersome and lack consistency in prognostic prediction. There is a pressing need for a grading system that is both simple to apply in daily clinical practice and capable of providing reliable prognostic information. Ideally, such a system would be easy to communicate to both healthcare providers and patients, fostering better patient understanding and more effective management strategies9,10. The Site, Depth, and Infection/Ischemia (SDI) classification system addresses this need by offering a novel, three-dimensional approach to evaluating DFUs. The SDI system categorizes ulcers based on three critical factors: the site of the ulcer, the depth of tissue involvement, and the presence or absence of infection or ischemia. Each of these factors is divided into 3 to 4 distinct levels, providing a comprehensive and nuanced description of the ulcer’s severity. This stratification allows clinicians to more accurately assess the condition of the ulcer and tailor interventions accordingly11,12.
The primary objective of this study is to evaluate the predictive power of the SDI classification system in determining the prognosis of patients with DFUs. By applying the SDI framework to a cohort of DFU patients and analyzing their clinical outcomes, this study aims to assess the system’s ability to predict healing time, the need for surgical intervention, and the risk of major complications.
Methods
Study design
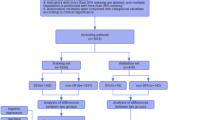
This retrospective cohort study was conducted from January 2021 to December 2022, with the primary objective of evaluating the prognostic value of the SDI classification system in DFUs. To be included in the study, patients were required to meet the following criteria: (1) a confirmed diagnosis of diabetes mellitus, (2) the presence of DFUs located at or distal to the ankle joint, (3) documented evidence of concurrent lower limb neuropathy and/or peripheral artery disease, and (4) an age range between 40 and 80 years, inclusive. Patients were excluded if they met any of the following conditions: (1) malignant tumors with a life expectancy of less than one year, (2) co-occurring autoimmune diseases, (3) sole foot trauma or ulcers not attributable to diabetes, or (4) psychiatric disorders that impaired the ability to comply with treatment protocols. The diagnostic criteria for evaluating DFUs followed established clinical guidelines, including those from the International Working Group on the Diabetic Foot (IWGDF)13. Assessments were conducted by clinicians with 8 + years of experience (podiatrists, diabetologists, and surgeons) during routine clinical evaluations. These were aligned with institutional protocols and international standards for diabetic foot care. The SDI classification system, including site, depth, and infection/ischemia assessments, was based on direct clinical examination and patient history review. A total of 261 patients were enrolled in the study. The study methodology, objectives, and protocols were designed in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines14. Informed consent was obtained from all subjects. The study was approved by the ethics committee of Rizhao People’s Hospital and Qingdao Hiser Hospital Affiliated of Qingdao University (approval number: 20241128057; Approval date: November 28, 2024), conducted in accordance with relevant guidelines and the Declaration of Helsinki. Data was handled confidentially, with all personal identifiers removed to ensure participant privacy.
Site, depth, and infection/ischemia (SDI) classification for diabetic foot ulcers
The assessment of DFUs in this study focused on three critical aspects: ulcer location, depth, and the presence of infection or ischemia. Each ulcer was graded using a standardized notation system denoted as SnDnIn, where “S” represents the ulcer’s location, “D” refers to the ulcer’s depth, and “I” denotes the presence of infection or ischemia (Table 1).
1. Site Assessment: For ulcers involving multiple anatomical regions of the foot, classification was based on the most proximal affected site, reflecting the area with the highest potential clinical impact. The grading system was defined as follows:
(1) S1: Ulcers confined exclusively to the forefoot, including the toes and metatarsal heads.
(2) S2: Ulcers extending beyond the forefoot to involve the midfoot, but not reaching the hindfoot.
(3) S3: Ulcers involving the hindfoot, with or without concurrent involvement of the forefoot or midfoot.
-
1.
2. Depth Assessment: The depth of the ulcer was classified into several stages, with further categorization based on the extent of tissue involvement, including superficial ulcers confined to the epidermis, partial-thickness ulcers involving deeper layers of skin, and full-thickness ulcers extending to underlying structures such as tendons, bones, or joints.
-
2.
3. Infection and Ischemia Assessment:
(1) Infection severity was evaluated following the guidelines established by the International Working Group on the Diabetic Foot (IWGDF), in line with the IDSA/IWGDF infection classification15. This included clinical signs of infection such as erythema, warmth, purulent discharge, and systemic indicators like fever or increased white blood cell count.
(2) The evaluation of ischemia was primarily based on patient-reported symptoms, such as intermittent claudication or rest pain, and clinical examinations. A key component of this assessment was the palpation of dorsal and posterior tibial artery pulses, which provide critical insight into the patient’s vascular status and the likelihood of ischemic involvement in the ulceration.
Ulcer grading examples
As illustrated below, the grading system was applied to two representative DFUs for clarity:
The patient was classified as S2D3I1, indicating a midfoot ulcer, full-thickness extending to the joint or bone, infected but not ischemic (Fig. 1).
The patient was classified as S1D3I3, indicating a forefoot ulcer, full-thickness extending to the joint or bone, with both infection and ischemia (Fig. 2).
Patient grouping and follow-up
Upon initial classification of DFUs based on severity, patients were categorized into distinct groups according to three critical dimensions: site, depth, and the presence of infection or ischemia. Statistical analysis for each patient group was performed, focusing on several key prognostic parameters, including ulcer healing time (defined as complete epithelialization of the wound with no drainage or need for dressings during at least two consecutive clinical assessments), the incidence of minor and major amputations, and mortality rates. For patients discharged without experiencing any of these outcomes, monthly follow-ups were scheduled, either through telephone interviews or outpatient visits. If any signs of deterioration, complications, or urgent concerns were reported during telephone follow-ups, patients were promptly advised to return for in-person clinical evaluation. All patients included in the final analysis were followed for a minimum of 12 months or until the occurrence of a predefined outcome (healing, amputation, or death), ensuring adequate time for outcome assessment. Patients who were lost to follow-up or who had not reached any of the specified outcomes at the time of the last follow-up were excluded from the study cohort. Additionally, the analysis of healing time excluded patients who had deceased during the study period. By the conclusion of the study, 7 patients were excluded due to loss to follow-up, and 14 patients were excluded because they had not reached any of the predefined outcomes. As a result, the final analysis included a total of 240 patients.
Statistical analysis
Data analysis in this study was performed using SPSS version 27 software (IBM Corp., Armonk, NY, USA). For continuous variables that did not follow a normal distribution, the median was used to represent the central tendency, and comparisons between groups were conducted using the Kruskal-Wallis test. For variables with a normal distribution, the mean was employed to represent the central tendency, and between-group comparisons were made using one-way analysis of variance (ANOVA). Categorical data were presented as proportions or percentages, and the chi-square test was applied for comparisons between different groups. A p-value of less than 0.05 was considered statistically significant.
Results
Frequency distribution of SDI classification in diabetic foot ulcer patients
The classification of the 240 DFU patients according to the SDI grading system is summarized in Table 1. The frequency distribution across the three key dimensions—site, depth, and infection/ischemia—is presented as follows. In terms of site, the majority of patients (151 out of 240) had ulcers located in the forefoot (S1), while a smaller proportion of patients had ulcers in the midfoot (S2, 63 patients) and hindfoot (S3, 26 patients). Regarding depth, 131 patients had ulcers that involved deeper layers of the skin, such as the deep fascia and muscle (D2), while 81 patients presented with full-thickness ulcers extending to the joint or bone (D3). A smaller proportion of patients had superficial ulcers involving only the epidermis (D1, 28 patients). For infection/ischemia, the largest group of patients (95 patients) had ulcers with infection alone (I1). A significant number of patients also exhibited ischemia (I2, 43 patients), while a substantial proportion had both infection and ischemia (I3, 71 patients). Only 31 patients had no infection or ischemia (I0) (Table 2).
Prognosis of DFU patients by different location classifications
The prognosis of DFU patients was assessed based on ulcer location, revealing significant differences in healing time, amputation rates, and mortality. Patients with forefoot ulcers (S1) had the shortest healing time (2.1 months), with 28.5% requiring minor amputations, 2.0% requiring major amputations, and 6.0% experiencing mortality. In contrast, patients with midfoot ulcers (S2) had a longer healing time (3.2 months), with 20.1% requiring minor amputations, 6.3% requiring major amputations, and 11.1% experiencing mortality. Patients with hindfoot ulcers (S3) had the longest healing time (9.3 months), with 15.3% requiring minor amputations, 38.5% requiring major amputations, and 23.1% experiencing mortality. Statistical analysis revealed significant differences in healing time (p < 0.001) and major amputation rates (p = 0.000) between the groups. Mortality rates were also significantly higher in the S3 group (23.1%) compared to the S1 and S2 groups (6.0% and 11.1%, respectively) (p = 0.017). However, no significant difference was observed in minor amputation rates (p = 0.235) (Table 3).
Prognosis of DFU patients by different depth classifications
The prognosis of DFU patients was stratified by ulcer depth, with notable differences in healing time, amputation rates, and mortality. Patients with superficial ulcers (D1) had the shortest healing time (1.2 months), with 21.4% requiring minor amputations, 3.6% requiring major amputations, and 7.1% experiencing mortality. In contrast, patients with deeper ulcers (D2) had a longer healing time of 2.9 months, with 22.1% requiring minor amputations, 3.8% requiring major amputations, and 3.8% experiencing mortality. Patients with the most severe ulcers (D3) had the longest healing time of 4.3 months, with 30.9% requiring minor amputations, 13.6% requiring major amputations, and 18.5% experiencing mortality. Statistical analysis indicated significant differences in healing time (p < 0.001) and mortality (p = 0.001) across the depth groups. However, there were no significant differences in minor amputation rates (p = 0.325) between the groups (Table 4).
Prognosis of DFU patients based on different infection/ischemia classifications
The prognosis of DFU patients was further categorized based on the presence of infection or ischemia, revealing substantial differences in healing time, amputation rates, and mortality. Patients without infection or ischemia (I0) had the shortest healing time (0.9 months), with 16.1% requiring minor amputations and no patients requiring major amputations or experiencing mortality. In contrast, patients with infection alone (I1) had a healing time of 1.9 months, with 17.9% requiring minor amputations, 2.1% requiring major amputations, and 6.3% experiencing mortality. Patients with ischemia (I2) had a longer healing time of 3.5 months, with 25.6% requiring minor amputations, 14.0% requiring major amputations, and 16.3% experiencing mortality. The group with both infection and ischemia (I3) exhibited the longest healing time (5.6 months), with 38.0% requiring minor amputations, 12.7% requiring major amputations, and 12.7% experiencing mortality. Statistical analysis revealed significant differences in healing time (p = 0.000), minor amputations (p = 0.016), and major amputations (p = 0.006) between the groups, with the most severe outcomes observed in the I3 group. Mortality was notably higher in the I3 group, but the difference was not statistically significant (p = 0.052) (Table 5). Notably, no cases of major amputation occurred in the I0 group, indicating that all patients who underwent major surgical interventions had documented infection, ischemia, or both. This supports the internal validity of the SDI classification in accurately reflecting ulcer severity.
Discussion
This study addresses this gap by proposing a comprehensive definition of DF as any tissue damage or structural alterations occurring at or beyond the ankle joint, attributable to lower limb neuropathy and/or peripheral vascular disease in diabetic patients. This study presents one of the first clinical evaluations of the SDI classification system for DFUs, offering a novel three-dimensional framework for stratifying ulcer severity. Unlike traditional systems that assess depth or infection in isolation, the SDI system integrates anatomical location, ulcer depth, and the presence of infection and/or ischemia into a unified prognostic model. Our findings demonstrate that each SDI dimension independently correlates with clinical outcomes such as healing time, amputation risk, and mortality. These results underscore the clinical value of SDI as a comprehensive and practical tool to guide individualized treatment planning, facilitate risk stratification, and improve prognostic accuracy in DFU management.
One of the key findings of this study is the significant variability in the prognosis of DFU patients, depending on the location, depth, and presence of infection/ischemia. The SDI (Site, Depth, Infection/Ischemia) classification system was employed to categorize the severity of DFUs, and it was found to be effective in predicting various clinical outcomes, including healing time, amputation rates, and mortality. The SDI system’s ability to stratify DFUs based on site, depth, and infection/ischemia provides valuable insights into the expected disease course, enabling clinicians to make more informed decisions regarding treatment strategies and amputation risks. The system proved particularly useful in distinguishing between the severity of ulcers located in different regions of the foot. Patients with hindfoot ulcers (S3) experienced the worst outcomes, with significantly longer healing times (9.3 months), a higher rate of major amputations (38.5%), and increased mortality (23.1%) compared to patients with forefoot or midfoot ulcers (S1 and S2). These findings align with existing literature, which consistently shows that ulcer location is a key predictor of healing times and amputation risk16,17.
The results of this study also reinforced the important role of infection and ischemia in the prognosis of DFUs. Patients with both infection and ischemia (I3) exhibited the longest healing times (5.6 months), the highest rates of major amputations (12.7%), and the highest mortality rates (12.7%) compared to other groups. This underscores the severe impact that infection and ischemia can have on DFU outcomes, as these factors contribute to delayed wound healing, increased tissue damage, and a greater likelihood of complications such as gangrene and sepsis. The SDI system’s ability to differentiate between infection and ischemia allows clinicians to tailor treatment interventions accordingly, facilitating early interventions that target the underlying causes of poor healing, such as infection control and vascular revascularization. The findings from this study corroborate the well-established understanding that infection and ischemia are significant predictors of adverse DFU outcomes, including the risk of amputation and prolonged hospitalization18,19. Moreover, the combination of infection and ischemia is widely recognized as one of the most challenging clinical scenarios in DFU management, often requiring aggressive and multi-disciplinary treatment approaches20.
The SDI classification system was selected due to its three-dimensional approach, which comprehensively evaluates DFUs by incorporating the ulcer’s site, depth, and the presence or absence of infection and ischemia. This multidimensional classification offers a more detailed assessment compared to traditional methods, allowing for more accurate prediction of clinical outcomes, including healing time, the need for surgical intervention, and the risk of amputation and mortality. Moreover, the SDI system has shown potential in addressing the limitations of previous classification systems, particularly in differentiating the roles of infection and ischemia in the ulcer’s progression. Several other classification systems, such as the Wagner and Texas Classification Systems, have been widely used in clinical practice21,22. The Wagner classification categorizes ulcers based on depth and the presence of infection, while the Texas system adds ischemia as a separate component, providing a more detailed assessment of vascular status23,24. However, these systems often focus on limited aspects of the ulcer, such as either location or depth, and may not fully integrate the interactive roles of infection and ischemia. In contrast, the SDI system integrates site, depth, and vascular and infectious factors into a unified framework, offering a more holistic approach to ulcer classification25,26. This makes it ideal for use in both outpatient and inpatient settings, where quick and reliable assessments are necessary. The SDI system’s straightforward approach allows healthcare providers to classify DFUs efficiently and accurately, facilitating timely interventions and promoting better patient outcomes. The use of the SDI system is particularly beneficial in situations where rapid decision-making is required, such as in emergency settings or when planning for surgical interventions like debridement or amputation.
The limitations of this study should be acknowledged. First, the retrospective design inherently relies on the accuracy and completeness of existing medical records, introducing potential bias. Second, the assessment of ischemia was primarily based on clinical indicators, with objective vascular tests (e.g., ABI, Doppler) inconsistently recorded and angiographic data largely unavailable, which may have affected ischemia grading. Third, the SDI system does not incorporate other prognostically relevant variables such as glycemic control, systemic inflammatory markers, or adherence to offloading protocols. Additionally, the study was conducted at a limited number of centers with a moderate sample size, potentially limiting generalizability to broader populations and healthcare settings. Finally, factors such as patient compliance, caregiver support, and local access to advanced wound care or revascularization services were not formally assessed. Future prospective, multicenter studies incorporating standardized vascular assessments and broader sociodemographic variables are needed to validate and refine the SDI system across diverse clinical contexts.
Conclusions
The findings of this study indicate that as the SDI grading of diabetic foot ulcer patients increases across site, depth, and infection/ischemia dimensions, there is a clear extension in healing time, along with higher risks of amputation and mortality. These results demonstrate that the SDI grading system is effective in predicting patient outcomes, highlighting its clinical utility and the need for further implementation in clinical practice.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E. & Hicks, C. W. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 46 (1), 209–221 (2023).
Yang, L., Rong, G. C. & Wu, Q. N. Diabetic foot ulcer: challenges and future. World J. Diabetes. 13 (12), 1014–1034 (2022).
Monteiro-Soares, M. et al. Diabetic foot ulcer classifications: A critical review. Diabetes Metab. Res. Rev. 36 (Suppl 1), e3272 (2020).
Monteiro-Soares, M. et al. Classification of foot ulcers in people with diabetes: A systematic review. Diabetes Metab. Res. Rev. 40 (3), e3645 (2024).
Thewjitcharoen, Y. et al. Changing the patterns of hospitalized diabetic foot ulcer (DFU) over a 5-year period in a multi-disciplinary setting in Thailand. BMC Endocr. Disorders. 20 (1), 89 (2020).
Akkus, G. & Sert, M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J. Diabetes. 13 (12), 1106–1121 (2022).
Niță, O. et al. Evaluating classification systems of diabetic foot ulcer severity: A 12-Year retrospective study on factors impacting survival. Healthcare (Basel) 11(14). (2023).
Aldana, P. C., Cartron, A. M. & Khachemoune, A. Reappraising diabetic foot ulcers: A focus on mechanisms of ulceration and clinical evaluation. Int. J. Low Extrem Wounds. 21 (3), 294–302 (2022).
Brocklehurst, J. D. The validity and reliability of the SINBAD classification system for diabetic foot ulcers. Adv. Skin. Wound Care. 36 (11), 1–5 (2023).
Husain, M. & Agrawal, Y. O. Antimicrobial remedies and emerging strategies for the treatment of diabetic foot ulcers. Curr. Diabetes Rev. 19 (5), e280222201513 (2023).
Sen, P., Demirdal, T. & Emir, B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab. Res. Rev. 35 (7), e3165 (2019).
Khashim, Z., Samuel, S., Duraisamy, N. & Krishnan, K. Potential biomolecules and current treatment technologies for diabetic foot ulcer: an overview. Curr. Diabetes Rev. 15 (1), 2–14 (2019).
van Netten, J. J. et al. The international working group on the diabetic foot: stories and numbers behind three decades of Evidence-Based guidelines for the management of Diabetes-Related foot disease. Diabetes Ther. 15 (1), 19–31 (2024).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61 (4), 344–349 (2008).
Monteiro-Soares, M. et al. Guidelines on the classification of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 40 (3), e3648 (2024).
Armstrong, D. G., Lavery, L. A. & Harkless, L. B. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 21 (5), 855–859 (1998).
Mathioudakis, N. et al. The society for vascular surgery wound, ischemia, and foot infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J. Vasc Surg. 65 (6), 1698–1705e1691 (2017).
Ince, P., Kendrick, D., Game, F. & Jeffcoate, W. The association between baseline characteristics and the outcome of foot lesions in a UK population with diabetes. Diabet. Med. 24 (9), 977–981 (2007).
Gershater, M. A. et al. Complexity of factors related to outcome of neuropathic and neuroischaemic/ischaemic diabetic foot ulcers: a cohort study. Diabetologia 52 (3), 398–407 (2009).
Baig, M. S. et al. An overview of diabetic foot ulcers and associated problems with special emphasis on treatments with antimicrobials. Life (Basel) 12(7). (2022).
Nanda, R., Nath, A., Patel, S. & Mohapatra, E. Machine learning algorithm to evaluate risk factors of diabetic foot ulcers and its severity. Med. Biol. Eng. Comput. 60 (8), 2349–2357 (2022).
Hernandez-Guedes, A., Arteaga-Marrero, N., Villa, E., Callico, G. M. & Ruiz-Alzola, J. Feature ranking by variational dropout for classification using thermograms from diabetic foot ulcers. Sensors (Basel) 23(2). (2023).
Viadé, J. et al. A novel assessment, diagnostic and treatment system for diabetic foot. J. Foot Ankle Res. 16 (1), 84 (2023).
Lee, E. J., Jeong, I. S., Kim, I. J., Cho, Y. H. & Kim, Y. J. Risk assessment and classification for foot ulceration among patients with type 2 diabetes in South Korea. Int. J. Nurs. Pract. 28 (3), e13012 (2022).
Chen, Y. L. et al. Establishment and reliability evaluation of prognostic models in diabetic foot. Altern. Ther. Health Med. 29 (8), 534–539 (2023).
Sharma, H. et al. The efficacy of inflammatory markers in diagnosing infected diabetic foot ulcers and diabetic foot osteomyelitis: systematic review and meta-analysis. PLoS One. 17 (4), e0267412 (2022).
Acknowledgements
We thank every patient and staff who participated in the study.
Funding
The research was funded by the 2021 Shandong Provincial Medical and Health Science and Technology Development Plan (No. 202104130109) for the study on the role of LincRNA-p21 in regulating the occurrence and development of abdominal aortic aneurysm via the Hippo-YAP signaling pathway. It was also supported by the 2021 Qingdao Medical and Health Scientific Research Plan (No. 2021-WJZD046) for the clinical tongue diagnosis analysis of Gangrene-Limb Arteriosclerosis Obliterans in Traditional Chinese Medicine.
Author information
Authors and Affiliations
Contributions
The conceptualization of the study was led by Chun-Hua Zhang, Chun-Yan Jiao, Li Li, Chen-Hui Sui, Ting Ji, Fan Wang, Jin-Jun Wang, and Xiao-Na Liu. Data curation was performed by Chun-Hua Zhang, Chun-Yan Jiao, Li Li, and Chen-Hui Sui. Formal analysis was carried out by Chun-Hua Zhang, Chun-Yan Jiao, Ting Ji, and Fan Wang. The methodology was developed by Chun-Hua Zhang, Chun-Yan Jiao, Li Li, Chen-Hui Sui, Ting Ji, Fan Wang, Jin-Jun Wang, and Xiao-Na Liu. Resources and software management were handled by Chun-Hua Zhang, Chun-Yan Jiao, Li Li, Chen-Hui Sui, Ting Ji, and Fan Wang. The original draft was written by Chun-Hua Zhang, while the review and editing of the manuscript were conducted by Jin-Jun Wang and Xiao-Na Liu.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Rizhao People’s Hospital and Qingdao Hiser Hospital Affiliated of Qingdao University (approval number: 20241128057; Approval date: November 28, 2024). All procedures involving human participants were conducted in accordance with ethical standards set by the institutional and national research committees, as well as the 1964 Helsinki Declaration and its subsequent amendments. Informed consent was obtained from all participants or their legal guardians.
Consent for publication
Informed consent was obtained from all participants. Patients provided consent for the publication of their data or images.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, CH., Jiao, CY., Li, L. et al. Prognostic value of the site, depth, and infection/ischemia classification system in diabetic foot ulcers: a retrospective cohort study. Sci Rep 15, 27003 (2025). https://doi.org/10.1038/s41598-025-06509-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06509-5