Abstract
Previous studies have established associations between the C-reactive protein to lymphocyte ratio (CLR), inflammatory load index (IBI), and neutrophil to albumin ratio (NAR) with various conditions. However, the evidence for such associations in individuals with albuminuria remains unclear. This study aimed to investigate the relationship between inflammatory biomarkers and the presence of albuminuria by utilizing data derived from the National Health and Nutrition Examination Survey (NHANES). Participants were classified into two groups according to their urinary albumin-to-creatinine ratio (UACR): those who presented with albuminuria and those who did not. The association between inflammatory biomarkers and the occurrence of albuminuria was assessed through multivariate regression models. To explore possible non-linear relationships, restricted cubic spline (RCS) analysis was applied. Furthermore, subgroup and sensitivity analyses were conducted to ensure the reliability and consistency of the findings. The study included 18,876 participants, of whom 2,321 were classified in the albuminuria group and 16,555 in the non-albuminuria group. The results from a fully adjusted logistic regression model indicated a significant positive relationship between the natural logarithm-transformed inflammatory markers—Ln-CLR, Ln-IBI, and Ln-NAR—and the risk of presence albuminuria. Specifically, each unit increase in Ln-CLR, Ln-IBI, and Ln-NAR was linked with a 12%, 15%, and 79% heightened risk of albuminuria, respectively. RCS analysis showed a linear relationship between Ln-CLR, Ln-IBI and Ln-NAR and albuminuria. Subgroup analyses demonstrated consistent findings across various populations, and numerous sensitivity tests confirmed the reliability of these outcomes. This study demonstrates a positive correlation between CLR, IBI, and NAR and the presence albuminuria in US adults. Given these results, early monitoring of these inflammatory markers in high-risk populations is essential for identifying the onset of albuminuria and mitigating kidney damage progression.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) represents a significant global health challenge. As of 2017, it affected approximately 697.5 million people worldwide, with a prevalence rate of about 9.1%, and resulted in 1.2 million deaths globally1. Forecasts predict that deaths due to CKD could escalate to 4.4 million by 20502. The Global Burden of Disease Study now lists CKD as the 11th leading cause of death globally3. CKD also imposes substantial economic burdens on healthcare systems and societies due to its progressive nature, highlighting the importance of early detection of individuals at risk to prevent or delay progression to end-stage renal disease (ESRD). Albuminuria, marked by increased urinary albumin excretion, is a crucial indicator of early kidney damage. The UACR is essential for evaluating albuminuria, with values above 30 mg/g (or 3.4 mg/mmol) signifying its presence4,5. Despite its importance, screening for albuminuria is underutilized in clinical practice. For example, a national cohort study in the United States revealed that approximately two-thirds of potential cases among hypertensive or diabetic patients remained undetected6. This lack of detection can delay necessary medical interventions and increase the risk of adverse outcomes. Therefore, enhancing early screening for albuminuria is crucial, enabling timely interventions and improved patient outcomes.
Chronic inflammation has come to the front as a major player in the pathogenesis of kidney injury, from inception to progression. Emerging evidence underlines the relationship between inflammation and renal injury, especially on the association of albuminuria and inflammation responses. Extensive studies have established strong correlations between albuminuria and various inflammatory markers like systemic immune-inflammation index (SII)7,8. Other studies have also associated high levels of SII with diabetic kidney disease in patients with type 2 diabetes (T2DM)9. Population-based cohort studies have shown that inflammation biomarkers, such as white blood cell count, TNF-α, and IL-6, are positively related to the outcomes of prevalent CKD10. Reviews have also pointed out the negative impact of inflammatory mediators like IL-6 on renal function11. In conclusion, inflammation has a complex and great relevance with the development and progression of kidney damage.
The introduction of novel indicators such as the CLR, IBI, and NAR has shown potential in reflecting the body’s inflammatory and immune states. Extensive research has established correlations between these markers and the prognosis of various tumor types, including gastric cancer and colorectal cancer12,13,14,15. Additionally, these markers have been linked to several non-oncological diseases, such as myocardial infarction, Parkinson’s disease, osteoarthritis, and rheumatoid arthritis16,17,18,19. However, the relationship between CLR, NAR, and IBI with albuminuria remains uncertain. To address this knowledge gap, our study utilized data from NHANES to explore the correlation between CLR, NAR, and IBI with albuminuria.
Method
Data source and participants
The NHANES is a comprehensive program that measures the nutritional and health status of American children and adults. Conducted regularly by the National Center for Health Statistics (NCHS) of the U.S. Center for Disease Control and Prevention (CDC), the NHANES has been approved by the NCHS Research Ethics Review Board. Written informed consent has been obtained from all survey participants. Data from this cross-sectional research were used in the study. All methods were conducted in compliance with the relevant guidelines and regulations.
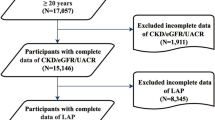
The present study focused on NHANES data from 2003 to 2010, the only survey cycle that included data on the inflammatory indices (CLR, IBI, NAR) as well as the UACR. Participants were excluded: (1) aged under 20 years, (2) incomplete data on the inflammatory indices and UACR, and (3) pregnancy. From a total of 41,156 participants in NHANES from 2003 to 2010, after excluding those under 20 years (n = 18,983), with incomplete data on UACR (n = 1,494), CLR (n = 1,080), IBI and NAR (n = 129), and those who were pregnant (n = 594), 18,876 participants remained for final analysis (Fig. 1).
Assessment of inflammatory indices
Blood samples were collected from NHANES participants in a standardized manner at mobile examination centers (MEC). CLR, IBI, and NAR were designated as exposure variables for this analysis. These indicators represent emerging biomarkers of systemic inflammation. The ratios were calculated as follows: CLR = C-reactive protein (CRP)/lymphocyte count; IBI = CRP × neutrophil count/lymphocyte count; NAR = neutrophil count/albumin.
Assessment of albuminuria
In order to determine the UACR, participants’ urine was collected in a systematic manner and examined by laboratories that have been accredited. This ratio, which is an important indicator in nephrology, was determined by dividing the milligram (mg) concentration of urine albumin by the gram (g) concentration of urinary creatinine. Albuminuria was classified as a UACR of 30 mg/g (or 3.4 mg/mmol) or above, according to previously established criteria, whereas values below this cutoff were deemed non-albuminuria. In this study, albuminuria served as the primary outcome variable.
Covariates
There may be other factors at play that distort the results of the exposure variable’s association with albuminuria. The present study’s multivariable-adjusted models synthesised these findings from prior research. Demographic factors encompassed gender, age, race, and degree of education. In the conducted analysis, a comprehensive approach was used to incorporate various factors that could potentially influence the results, including socioeconomic status. Specifically, the poverty income ratio (PIR) was included as a covariate. Additionally, several clinical biomarkers were examined for their role in the analysis. These biomarkers included albumin levels (g/dL), serum uric acid concentration (mg/dL), total cholesterol levels (TC, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), and the estimated glomerular filtration rate (eGFR, mL/min/1.73 m2). The eGFR can be calculated using the widely accepted CKD-EPI creatinine equation20. Alongside these biomarkers, several health behavior factors were also considered in the analysis, particularly the influence of alcohol consumption and smoking status. For the classification of alcohol consumption, participants were grouped into five distinct categories based on their self-reported levels of alcohol intake. These categories included individuals who reported never drinking alcohol (< 12 lifetime drinks), former drinkers (≥ 12 lifetime drinks and abstinence in the past year), and those classified as mild (average of ≤ 1 drink per day for women or ≤ 2 drinks per day for men over the past year), moderate (average of 1–3 drinks per day for women or 2–4 drinks per day for men over the past year), or heavy drinkers (average of ≥ 4 drinks per day for women or ≥ 5 drinks per day for men over the past year). Regarding smoking, participants were classified according to their lifetime cigarette consumption. A smoker was defined as an individual who had smoked 100 cigarettes or more in their lifetime, while those who had smoked fewer than 100 cigarettes were considered non-smokers. In addition to these health behaviors, illness status was another critical variable assessed in the analysis. The presence of chronic conditions such as diabetes and hypertension was used to classify participants’ illness status. Hypertension was defined as a medical diagnosis by a physician or healthcare professional, or participant’s systolic blood pressure (SBP) was measured at 140 mmHg or higher or diastolic blood pressure (DBP) was measured at 90 mmHg or higher. Additionally, participants who were currently using antihypertensive medications to manage their blood pressure were also classified as having hypertension21. For the diagnosis of diabetes, participants were required to either report a history of the disease, have a HbA1c level greater than 6.5%, or exhibit elevated blood glucose levels that met the diagnostic criteria. These criteria included fasting blood glucose levels of 126 mg/dL (or 7.0 mmol/L) or higher, or random blood glucose levels exceeding 200 mg/dL (or 11.1 mmol/L) when measured at any time of day. Furthermore, participants currently undergoing treatment with glucose-lowering medications or Insulin to control their blood glucose levels were also considered to have diabetes22.
Statistical analysis
In order to establish the baseline demographic characteristics, the continuous variables that followed a normal distribution were expressed as the mean ± standard deviation, whilst those with a skewed distribution were reported as the median accompanied by the interquartile range (IQR). Categorical variables were described using frequency and percentage. To assess differences between participants grouped by albuminuria, analysis of variance (ANOVA) was employed for normally distributed continuous variables, the Kruskal-Wallis test—a non-parametric comparison method—was used for skewed continuous variables, and Chi-square tests were conducted for categorical variables. A systematic evaluation of missing data in the final analytic cohort revealed that all variables exhibited missingness proportions below 10% (Table S1). Therefore, no data imputation was performed. In addition, to analyze the relationship between various indicators and albuminuria status, a multivariate logistic regression analysis was conducted, employing three distinct models to account for potential confounders. In the first model (Model 1), no adjustments were made for any covariates, and the analysis focused purely on the relationship between the independent variables and the albuminuria status. The second model (Model 2) incorporated several demographic variables, including gender, age, race, and educational level, to adjust for basic population-level characteristics. Finally, in the third model (Model 3), additional adjustments were made for a broader range of factors. These included socioeconomic factors such as the PIR, clinical factors such as BMI, TC, HDL-C, ALT, AST, serum uric acid, and eGFR. Lifestyle factors, including drinking and smoking status, as well as the presence of hypertension and diabetes, were also adjusted for in this model. The statistical analysis revealed that the inflammatory markers demonstrated a significant right-skewed in their distribution, indicating the need for data transformation to ensure accurate regression analysis (Fig. S1). To address this, the values of the inflammatory indices were transformed using the natural logarithms (Ln-CLR, Ln-IBI, and Ln-NAR). This transformation was necessary to stabilize variance and meet the assumptions required for reliable multivariate regression analysis. Subsequently, a further analysis of the non-linear associations between the inflammatory indices data and albuminuria prevalence was conducted using a RCS. When non-linear associations were identified, a threshold effects analysis model was employed to analyze the link and inflection points between the inflammatory index and albuminuria prevalence. Furthermore, this study incorporated a subgroup analysis. The population was segmented based on diverse factors, and interaction effects were analyzed to determine if the association between inflammatory markers and albuminuria varied significantly across different population strata. A suite of sensitivity analyses was conducted to ensure the robustness of our findings.
Statistical analysis was performed using R software (version 4.3.2), with significance determined at P < 0.05.
Results
Baseline characteristics of participants
This study included 18,876 subjects, comprising 49.86% females and 50.14% males, with a median age of 49 years (IQR 35–65). Compared to those without albuminuria, individuals with albuminuria demonstrated significantly higher prevalences of factors such as older age, BMI, smoking status, diabetes, hypertension and serum uric acid. They also had markedly lower lower levels of educational attainment, PIR, albumin levels, TC, HDL-C, ALT and eGFR. The disparities between these groups were significant (p < 0.05). No significant differences were found concerning gender and AST levels (all p > 0.05) (Table 1).
Association between inflammatory indices and albuminuria
Multiple logistic regression analyses consistently showed positive correlations between the three inflammatory biomarkers and the occurrence of albuminuria in both the unadjusted and adjusted models. Notably, Ln-NAR exhibited the highest odds ratio (OR) among the biomarkers across all models. In model 1, where no covariates were adjusted, the unadjusted ORs were as follows: Ln-CLR: OR = 1.33, 95% CI: 1.28–1.39; Ln-IBI: OR = 1.32, 95% CI: 1.27–1.37; Ln-NAR: OR = 2.27, 95% CI: 1.98–2.59. In model 2, after adjusting for gender, age, race, and education level, the adjusted ORs were: Ln-CLR: OR = 1.24, 95% CI: 1.19–1.30; Ln-IBI: OR = 1.26, 95% CI: 1.21–1.31; Ln-NAR: OR = 2.40, 95% CI: 2.11–2.73. In model 3, with all covariates adjusted, the fully adjusted ORs were: Ln-CLR: OR = 1.12, 95% CI: 1.06–1.18; Ln-IBI: OR = 1.15, 95% CI: 1.10–1.21; Ln-NAR: OR = 1.79, 95% CI: 1.53–2.10. These findings indicate a positive correlation between Ln-CLR, Ln-IBI, and Ln-NAR and the prevalence of albuminuria, with an increase of 1 unit in Ln-CLR, Ln-IBI, and Ln-NAR corresponding to an increase in the prevalence of albuminuria of 12%, 15%, and 79%, respectively. Subsequently, the continuous indicators were categorized into four quartiles. In model 3, participants in the highest quartile (Q4) of each biomarker had a significantly higher likelihood of exhibiting albuminuria, with all p-values < 0.05. Specifically, participants in Q4 for Ln-CLR, Ln-IBI, and Ln-NAR had a 27%, 53%, and 74% higher incidence of albuminuria, respectively, compared to those in Q1. These findings underscore the substantial associations observed between elevated quartiles of these biomarkers and an augmented risk of albuminuria (Table 2).
To further explore the non-linear dynamics between exposure variables and the incidence of albuminuria, RCS analysis was performed. The RCS analysis revealed distinct dose-response patterns between inflammatory biomarkers and albuminuria prevalence. Ln-CLR demonstrated a linear association with albuminuria (Fig. 2A, P-non-linear = 0.085). Similarly, Ln-IBI (Fig. 2B, P-non-linear = 0.128) and Ln-NAR (Fig. 2C, P-non-linear = 0.105) exhibited monotonic increasing trends without significant threshold effects. All biomarkers maintained strong overall associations with albuminuria risk (P-overall < 0.001 for each), suggesting continuous rather than threshold-dependent relationships.
Restricted cubic model of the association between inflammatory indices and albuminuria incidence after adjusting for gender, age, race, education level, PIR, BMI, smoking status, drinking status, diabetes, hypertension, albumin, serum uric acid, TC, HDL-C, ALT, AST and eGFR. (A). Ln-CLR and albuminuria; (B). Ln-IBI and albuminuria; (C). Ln-NAR and albuminuria.
Subgroup analyses
To evaluate the stability of associations among three inflammatory biomarkers and the occurrence of albuminuria across different demographic categories, multiple subgroup analyses were conducted. The preliminary examination revealed notable interactions between Ln-CLR and gender (P for interaction < 0.05), with stronger associations observed in males (OR = 1.17, 95% CI: 1.10–1.25) compared to females (OR = 1.08, 95% CI: 1.01–1.16) (Fig. 3A). Nonetheless, in alternative subgroups, these interactions did not reach significance (all P for interaction > 0.05). Likewise, the analyses identified a notably strong association between Ln-IBI and increased albuminuria prevalence, especially in males, with significant interaction effects (P for interaction < 0.05) (Fig. 3B). Additionally, the subgroup analysis for Ln-NAR revealed a significant interaction only with alcohol consumption status (P for interaction < 0.05) (Fig. 3C). The association between Ln-NAR and albuminuria risk was significantly stronger among individuals who consumed alcohol mildly compared to those with other drinking patterns. This finding highlights the specific influence of mild alcohol consumption on the relationship between Ln-NAR and albuminuria risk.
Sensitive analyses
In the subsequent sensitivity analysis, outliers were identified based on the interquartile range (IQR) and subsequently excluded from the dataset23,24. Following the removal of these outliers, logistic regression was re-conducted using the fully adjusted model. The results are as follows: Ln-CLR: OR = 1.10, 95% CI: 1.04–1.16; Ln-IBI: OR = 1.13, 95% CI: 1.08–1.19; Ln-NAR: OR = 1.82, 95% CI: 1.50–2.20. Participants in the Q4 of each inflammatory indicator were found to be significantly more likely to have albuminuria. Specifically, for Ln-CLR, the OR was 1.24 (95% CI: 1.08–1.50); for Ln-IBI, the OR was 1.48 (95% CI: 1.25–1.75); and for Ln-NAR, the OR was 1.72 (95% CI: 1.42–2.07) (Table 3 A). A secondary sensitivity analysis was subsequently performed, whereby the outcome variable was altered to macroalbuminuria and all relevant covariates were taken into account. The diagnosis of macroalbuminuria was made based on UACR ≥ 300 mg/g. After full adjustment for covariates, Ln-NAR demonstrated the strongest association, with each 1-unit increase corresponding to a 156% higher prevalence of macroalbuminuria. Participants in Q4 for Ln-NAR had a 115% higher incidence of albuminuria compared to those in Q1. For Ln-IBI, while continuous analysis showed significant associations, the Q4 subgroup demonstrated non-significant associations compared to the Q1 (P > 0.05). Notably, Ln-CLR exhibited attenuated effects in fully adjusted models, with neither continuous nor quartile-based analyses reaching statistical significance (all P > 0.05). These findings indicate that Ln-NAR maintains robust associations with macroalbuminuria even after comprehensive adjustment, whereas Ln-CLR and Ln-IBI show diminished effects when accounting for confounders (Table 3B).
These sensitivity analyses strengthen the robustness of our findings by addressing potential biases introduced by outliers and validating the consistency of the associations under different outcome definitions. This methodological approach enhances the reliability and generalizability of the conclusions derived from the study.
Discussion
This study analyzed 18,876 individuals from the NHANES database, categorized into albuminuria and non-albuminuria groups based on their UACR levels. After adjusting for demographic, socioeconomic, laboratory, health behavior, and disease status factors, elevated levels of inflammatory markers were associated with the prevalence of albuminuria. Specifically, participants in the highest quartiles of Ln-CLR, Ln-IBI, and Ln-NAR exhibited significantly increased risks of albuminuria, with ORs of 1.27, 1.53, and 1.74, respectively. RCS analysis showed a linear relationship between inflammatory markers and albuminuria, indicating consistent associations with higher risks of albuminuria. Subgroup analyses confirmed the consistency of these findings across different population groups. Moreover, the implementation of sensitivity analyses reinforced the stability and reliability of the observed associations between elevated inflammatory markers and the presence of albuminuria. Consequently, this study suggests that levels of CLR, IBI, and NAR may serve as potential biomarkers associated with albuminuria.
Numerous studies have linked the immune-inflammatory response with various diseases. As indicators of systemic inflammation, CLR, IBI, and NAR have been implicated in the etiology and progression of numerous chronic diseases. For instance, a comprehensive prospective cohort study involving 259,435 women demonstrated that elevated CLR levels were associated with an increased risk of breast cancer25. Moreover, a retrospective study involving 2,471 patients with colorectal cancer found that higher CLR levels were associated with poor tumor differentiation, advanced TNM stage and correlating with a poorer prognosis regardless of the cancer stage12. Cross-sectional studies have also revealed positive correlations between elevated IBI levels and cardiovascular disease, and between NAR levels and the prevalence of chronic obstructive pulmonary disease (COPD); furthermore, high NAR levels have been linked to increased all-cause mortality in COPD patients26,27.
Although direct studies connecting these three inflammatory markers to albuminuria are scarce, previous pathophysiological research supports the role of inflammation in renal damage. Detailed pathway map analysis of gene sets linked with eGFR in 157 European patients across nine CKD categories showed inflammation as a principal pathway in CKD progression11. Post-kidney injury, damaged cells and tissues release DAMPs, which engage with pattern recognition receptors such as TLRs to initiate the innate immune response28,29,30. This interaction induces the production of chemokines, and ROS, further exacerbating cellular necrosis and tissue damage11. Additionally, DAMPs trigger inflammasome and type I interferon responses, enhancing macrophage and leukocyte infiltration into the renal interstitium and amplifying inflammation31,32. Oxidative stress activates NF-κB, which promotes the production of various inflammatory mediators, intensifying the inflammatory response28,33. This activation perpetuates a positive feedback loop between inflammation and oxidative stress, contributing to chronic inflammation that ultimately leads to renal fibrosis and functional decline11,34,35. Moreover, the chronic release of fibrotic cytokines related to inflammation, such as IL-13, can trigger EMT, resulting in renal fibrosis and chronic renal failure28. EMT is defined as a process where epithelial cells lose polarity and cell-cell junctions and acquire mesenchymal features. TGF-β mediates its activity through the Smad pathway, which induces proliferation of fibroblasts and deposition of collagen, contributing to renal fibrosis36,37,38,39. In conclusion, the mechanism by which inflammation leads to kidney damage is complex, and further research is required to develop a comprehensive understanding of the mechanisms by which inflammation contributes to kidney damage.
Further stratification into subgroups showed extremely high incidence rates for albuminuria among males aged 50 years and above, with a PIR factor of >3.5, being a smoker, and having no previous conditions related to diabetes-on the same lines at all of the biomarkers analyzed. In addition, RCS analysis revealed a significant linear association between all examined biomarkers and the incidence of albuminuria. These findings indicate that Ln-CLR, Ln-IBI, and Ln-NAR could serve as critical biomarkers for future clinical guidelines aimed at screening and follow-up of high-risk populations.
Some key strengths underlying the current study include that the indicators are widely available and therefore can be more widely applied. We have taken extra care to adjust for as many possible confounding factors, thus enhancing the reliability of our results. Besides, sensitivity analyses conducted reinforce stability and dependability of the results. Nevertheless, some limitations do apply in this study. Being a cross-sectional study, it is limited to the establishment of a cause-and-effect relationship between high inflammatory markers and albuminuria, which future prospective studies have to clarify. Furthermore, the nature of inflammatory markers in this study has limited predictive value, thus suggesting that further studies might want to incorporate other biomarkers in order to increase the predictive value of their findings. Lastly, the generalizability of the findings of the study is somewhat limited in that all the participants were from the United States; thus, the applicability of such results in other countries or ethnicities requires further research to application.
Conclusion
In summary, our results highlight a strong association between elevated levels of inflammatory markers and the presence of albuminuria. These findings emphasize the critical role that maintaining a low level of inflammation plays in reducing the risk of albuminuria and promoting overall long-term health. Future clinical practices should focus on closely monitoring inflammatory markers in specific populations and implementing targeted interventions. Early intervention strategies for individuals with high inflammatory levels could optimize outcomes, improving the precision and efficacy of clinical decision-making and enhancing patient health outcomes.
Data availability
All the data can be accessed through the NHANES website (https://www.cdc.gov/nchs/nhanes/).
Abbreviations
- CLR:
-
C-reactive protein to lymphocyte ratio
- IBI:
-
Inflammatory load index
- NAR:
-
Neutrophil to albumin ratio
- UACR:
-
Urinary albumin-to-creatinine ratio
- NHANES:
-
National Health and Nutrition Examination Survey
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- HDL-C:
-
High-density lipoprotein cholesterol
- HbA1c:
-
Glycosylated hemoglobin A1c
- eGFR:
-
Estimated glomerular filtration rate
- BMI:
-
Body mass index
- PIR:
-
Poverty income ratio
- TC:
-
Total Cholesterol
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- OR:
-
Odds ratio
- RCS:
-
Restricted cubic spline
References
GBD Chronic Kidney Disease Collaboration. Global, regional, and National burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 395, 709–733. https://doi.org/10.1016/S0140-6736(20)30045-3 (2020).
GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: a forecasting analysis for the global burden of disease study 2021. Lancet 403, 2204–2256. https://doi.org/10.1016/S0140-6736(24)00685-8 (2024).
Ferrari, A. J. et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet 403, 2133–2161. https://doi.org/10.1016/S0140-6736(24)00757-8 (2024).
Ortiz, A. & Asociación Información Enfermedades Renales Genéticas (AIRG-E), European Kidney Patients’ Federation (EKPF), Federación Nacional de Asociaciones para la Lucha Contra las Enfermedades del Riñón (ALCER), Fundación Renal Íñigo Álvarez de Toledo (FRIAT), Red de Investigación Renal (REDINREN), Resultados en Salud 2040 (RICORS2040), Sociedad Española de Nefrología (SENEFRO) Council, Sociedad Española de Trasplante (SET) Council, Organización Nacional de Trasplantes (ONT). RICORS2040: the need for collaborative research in chronic kidney disease. Clin. Kidney J. 15, 372–387. https://doi.org/10.1093/ckj/sfab170 (2022).
Cho, Y-T. et al. Diagnosis of albuminuria by tryptic digestion and matrix-assisted laser desorption ionization/time-of-flight mass spectrometry. Clin. Chim. Acta. 420, 76–81. https://doi.org/10.1016/j.cca.2012.12.016 (2013).
Chu, C. D. et al. Estimated prevalence and testing for albuminuria in US adults at risk for chronic kidney disease. JAMA Netw. Open. 6, e2326230. https://doi.org/10.1001/jamanetworkopen.2023.26230 (2023).
Li, X., Cui, L. & Xu, H. Association between systemic inflammation response index and chronic kidney disease: a population-based study. Front. Endocrinol. (Lausanne). 15, 1329256. https://doi.org/10.3389/fendo.2024.1329256 (2024).
Qin, Z. et al. Systemic Immune-Inflammation index is associated with increased urinary albumin excretion: A Population-Based study. Front. Immunol. 13, 863640. https://doi.org/10.3389/fimmu.2022.863640 (2022).
Guo, W. et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011–2018. Front. Endocrinol. (Lausanne). 13, 1071465. https://doi.org/10.3389/fendo.2022.1071465 (2022).
Shankar, A. et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 80, 1231–1238. https://doi.org/10.1038/ki.2011.283 (2011).
Stenvinkel, P. et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int. Rep. 6, 1775–1787. https://doi.org/10.1016/j.ekir.2021.04.023 (2021).
Meng, Y. et al. Prognostic role and clinical significance of C-reactive protein-lymphocyte ratio in colorectal cancer. Bioengineered 12, 5138–5148. https://doi.org/10.1080/21655979.2021.1960768 (2021).
Song, R. et al. Prognostic value of Inflammation-Immunity-Nutrition score and inflammatory burden index for hepatocellular carcinoma patients after hepatectomy. J. Inflamm. Res. 15, 6463–6479. https://doi.org/10.2147/JIR.S386407 (2022).
Pelc, Z. et al. Prognostic value of inflammatory burden index in advanced gastric Cancer patients undergoing multimodal treatment. Cancers (Basel). 16, 828. https://doi.org/10.3390/cancers16040828 (2024).
Xie, H. et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J. Cachexia Sarcopenia Muscle. 14, 869–878. https://doi.org/10.1002/jcsm.13199 (2023).
Zhai, J. et al. Association between the inflammatory burden index and rheumatoid arthritis and its all-cause mortality: data from NHANES 1999–2018. Front. Med. (Lausanne). 11, 1421497. https://doi.org/10.3389/fmed.2024.1421497 (2024).
Jia, H., Yin, K., Zhao, J. & Che, F. Association of inflammation/nutrition-based indicators with parkinson’s disease and mortality. Front. Nutr. 11, 1439803. https://doi.org/10.3389/fnut.2024.1439803 (2024).
Xiong, Z. et al. Inflammatory burden index: associations between osteoarthritis and all-cause mortality among individuals with osteoarthritis. BMC Public. Health. 24, 2203. https://doi.org/10.1186/s12889-024-19632-1 (2024).
He, L. et al. The relationship between C-reactive protein to lymphocyte ratio and the prevalence of myocardial infarction in US adults: A cross-sectional study. Heliyon 9, e17776. https://doi.org/10.1016/j.heliyon.2023.e17776 (2023).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and Cystatin C. N Engl. J. Med. 367, 20–29. https://doi.org/10.1056/NEJMoa1114248 (2012).
Byrd, J. B. & Brook, R. D. Hypertension. Ann. Intern. Med. 170, ITC65–ITC80. https://doi.org/10.7326/AITC201905070 (2019).
Harreiter, J. & Roden, M. [Diabetes mellitus: definition, classification, diagnosis, screening and prevention (Update 2023)]. Wien Klin. Wochenschr. 135, 7–17. https://doi.org/10.1007/s00508-022-02122-y (2023).
Mramba, L. K. et al. Detecting potential outliers in longitudinal data with time-dependent covariates. Eur. J. Clin. Nutr. 78, 344–350. https://doi.org/10.1038/s41430-023-01393-6 (2024).
Anagnostou, E. et al. Identifying jitter outliers in single fiber electromyography: comparison of four methods. Muscle Nerve. 63, 217–224. https://doi.org/10.1002/mus.27093 (2021).
Zhang, Y. et al. Healthy lifestyles, systemic inflammation and breast cancer risk: a mediation analysis. BMC Cancer. 24, 208. https://doi.org/10.1186/s12885-024-11931-5 (2024).
Yu, F. & Peng, J. Association between inflammatory burden index and cardiovascular disease in adult americans: evidence from NHANES 2005–2010. Heliyon 10, e38273. https://doi.org/10.1016/j.heliyon.2024.e38273 (2024).
Cao, K., Miao, X. & Chen, X. Association of inflammation and nutrition-based indicators with chronic obstructive pulmonary disease and mortality. J. Health Popul. Nutr. 43, 209. https://doi.org/10.1186/s41043-024-00709-x (2024).
Yeh, T-H., Tu, K-C., Wang, H-Y. & Chen, J-Y. From acute to chronic: unraveling the pathophysiological mechanisms of the progression from acute kidney injury to acute kidney disease to chronic kidney disease. Int. J. Mol. Sci. 25, 1755. https://doi.org/10.3390/ijms25031755 (2024).
Sato, Y. & Yanagita, M. Immune cells and inflammation in AKI to CKD progression. Am. J. Physiol. Ren. Physiol. 315, F1501–F1512. https://doi.org/10.1152/ajprenal.00195.2018 (2018).
Qu, L. & Jiao, B. The interplay between immune and metabolic pathways in kidney disease. Cells 12, 1584. https://doi.org/10.3390/cells12121584 (2023).
Kimura, T., Isaka, Y. & Yoshimori, T. Autophagy and kidney inflammation. Autophagy 13, 997–1003. https://doi.org/10.1080/15548627.2017.1309485 (2017).
Kadatane, S. P. et al. The role of inflammation in CKD. Cells 12, 1581. https://doi.org/10.3390/cells12121581 (2023).
Song, N., Thaiss, F. & Guo, L. NFκB and Kidney Injury. Front. Immunol. https://doi.org/10.3389/fimmu.2019.00815 (2019).
Huang, G., Zhang, Y., Zhang, Y. & Ma, Y. Chronic kidney disease and NLRP3 inflammasome: pathogenesis, development and targeted therapeutic strategies. Biochem. Biophys. Rep. 33, 101417. https://doi.org/10.1016/j.bbrep.2022.101417 (2023).
Zhang, H. & Wang, Z. Effect and regulation of the NLRP3 inflammasome during renal fibrosis. Front. Cell. Dev. Biol. 7, 379. https://doi.org/10.3389/fcell.2019.00379 (2019).
McWilliam, S. J. et al. The complex interplay between kidney injury and inflammation. Clin. Kidney J. 14, 780–788. https://doi.org/10.1093/ckj/sfaa164 (2021).
Wen, J-H. et al. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front. Immunol. 13, 946832. https://doi.org/10.3389/fimmu.2022.946832 (2022).
Huang, R., Fu, P. & Ma, L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal. Transduct. Target. Ther. 8, 129. https://doi.org/10.1038/s41392-023-01379-7 (2023).
Fu, Y. et al. Inflammation in kidney repair: mechanism and therapeutic potential. Pharmacol. Ther. 237, 108240. https://doi.org/10.1016/j.pharmthera.2022.108240 (2022).
Author information
Authors and Affiliations
Contributions
X.M. participated in the design of the study, conducted analysis and wrote the article. Y.Q., C.Q. and H.L. collaborated on visualizing tables and figures and formatting the paper. Y.S. are responsible for the Writing review & editing. The manuscript has been approved by all authors and is ready for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The NHANES project has been approved by the Ethics Review Committee of the National Center for Health Statistics of the U.S.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, X., Qian, Y., Qian, C. et al. Association between inflammation indicators and albuminuria in US adults: a cross-sectional study. Sci Rep 15, 21496 (2025). https://doi.org/10.1038/s41598-025-06540-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06540-6