Abstract
This study aims to investigate if there is a difference in the safety and efficacy of catheter ablation of atrial fibrillation (AF) between patients with bioprosthetic mitral valve replacement (MVR) and those with mechanical MVR. A total of 23,794 patients who underwent the first-time catheter ablation of AF were screened. Patients with a history of surgical AF ablation, left atrial appendage closure, or resection were excluded from the study. There were 21 patients in the bioprosthetic MVR group and 85 patients in the mechanical MVR group were included in the study. After a median follow-up of 17.9 (14.4, 21.4) months, AF recurrence rates were similar between the two groups (52.4% vs. 50.6%, log-rank p = 0.527). Persistent AF was independently associated with AF recurrence (HR 1.83, [95% CI 1.00–3.33], p < 0.05). Mitral isthmus block rates were 78.6% in the bioprosthetic MVR group and 69.6% in the mechanical MVR group (p = 0.508). No instances of catheter entrapment were reported in this study. Two pseudoaneurysm and one acute cerebral infarction occurred in the mechanical MVR group. The safety and effectiveness of AF catheter ablation were comparable between patients with mechanical MVR and those with bioprosthetic MVR.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting 2–4% of adults1. Mitral valve diseases are strongly associated with AF development, with 30–40% of patients with rheumatic mitral valve disease experiencing AF2,3,4. Long-term follow-up reveals that 41–47% of patients with mitral valve disease developed AF5. Mitral valve diseases may contribute to the occurrence of AF by causing volume overload and left atrial enlargement6. The high prevalence of persistent AF continues even after mitral valve surgery corrected hemodynamic abnormalities, as seen in previous study7.
Prosthetic valve replacement is a primary surgical intervention for mitral valve disease, with options including mechanical and bioprosthetic mitral valves. Mechanical valves offer long-term durability. However, lifelong warfarin anticoagulation is required in the patients with mechanical mitral valve replacement (MVR). In contrast, bioprosthetic MVR do not require long-term anticoagulation. However, it is prone to have structural valve deterioration and have a higher risk of reoperation in the patients with bioprosthetic MVR8. Catheter ablation has been established as an effective treatment for AF, with evidence supporting its safety and effectiveness in patients with mechanical MVR9. However, there was a paucity of data on the impact of catheter ablation of AF in patients with bioprosthetic MVR. This study aims to investigate whether there is a difference in the safety and efficacy of catheter ablation of AF in patients with bioprosthetic MVR versus those with mechanical MVR.
Method
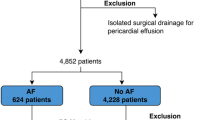
A total of 23,794 patients who underwent the first-time catheter ablation of AF in Beijing Anzhen Hospital from January 2015 to June 2023 were screened. Patients aged 18 years or older with a diagnosis of AF and a history of MVR were included. Patients were excluded if they had a history of catheter ablation, surgical AF ablation, left atrial appendage closure or resection. Of the 23,794 patients, 351 had undergone MVR. Among these, 130 had surgical AF ablation during valve replacement, 112 patients had previous catheter ablation or left atrial appendage closure or resection, and 3 declined follow-up. Finally, 106 patients were enrolled in the study. Among them 21 patients were enrolled in the bioprosthetic MVR group and 85 patients in the mechanical MVR group. Inform consents were obtained from all patients prior to the ablation procedure. This study was approved and supervised by the Ethics Committee of Beijing Anzhen Hospital and all methods were carried out in accordance with relevant guidelines and regulations.
Catheter ablation of AF
All anti-arrhythmic drugs except amiodarone were discontinued for a minimum of 5 half-lives before catheter ablation. The procedure was performed under fasting, conscious sedation and uninterrupted anticoagulation. During the procedure, heparin was injected intravenously to maintain the activated clotting time between 300 and 400 s. AF ablation strategy was described previously10. The left atrial geometry was reconstructed in the CARTO 3 system, with either a 3.5 mm tip ablation catheter (Navi-Star Thermocool SmartTouch® Catheter or Navi-Star Thermocool SmartTouch SF® Catheter, Biosense-Webster, USA) utilizing point by point or PentRay Nav eco tip catheter (Biosenes Webster, USA) with fast anatomy mapping. In patients with paroxysmal AF, circumferential pulmonary vein ablation (CPVA) was performed, with the ablation endpoint being complete pulmonary veins isolation (PVI). If sinus rhythm was not restored after PVI, the physician may perform additional ablation based on their personal experience. After CPVA in patients with persistent AF, left atrial roofline, mitral isthmus (MI), and cavotricuspid isthmus (CTI) were routinely targeted.Coronary sinus, superior vena cava, fractionated potentials, and the vein of Marshall (VOM) were targeted at the physician’s discretion. In cases where AF was not terminated, a 200 J direct current cardioversion was performed to restore sinus rhythm. Additional ablation was conducted as necessary to ensure PVI and linear block.
Data collection and follow-up
Antiarrhythmic drugs were administered orally for 3 months following the procedure. Patients in the bioprosthetic MVR group received new oral anticoagulants, while those in the mechanical MVR group received warfarin with a target international normalized ratio (INR) range of 2.0–3.0. 24 h-Holter was performed monthly during the first 3 months, followed by assessments at 6 months after the procedure and every 6 months thereafter. Scheduled follow-up was implemented by telephone interview or outpatient follow-up to collect recurrence events at 3, 6, months and every 6 months thereafter. Follow-up data were collected by professionally trained personnel. If the patient had palpitations or other symptoms suggestive of arrhythmia, ECG examination was performed in the local hospital at any time.
AF recurrence was defined as any recurrence of atrial arrhythmias lasting 30 s or more. Recurrence occurring within the first 3 months post-procedure was defined as early recurrence, while recurrence detected beyond 3 months was defined as late recurrence.
Statistical analysis
Statistical analyses were performed by SPSS 26.0 software. Continuous variables with a normal distribution were presented as mean ± standard deviation and compared using the independent-samples t-test. For continuous variables with non-normal distribution, medians and interquartile ranges were reported, and comparisons were made using the non-parametric Mann–Whitney U-test. Categorical variables were presented as numbers and percentages and compared by the χ2 or Fisher’s exact test. Kaplan–Meier analysis with log-rank test was used to evaluate AF recurrence-free survival between groups. Cox univariate and multivariate regression analyses were performed to identify independent predictors of AF recurrence after the catheter ablation. Variables with p value ≤ 0.05 in the univariate analysis were included in the multivariate analysis. A p value < 0.05 was considered statistically significant.
Results
Baseline characteristics
As shown in Table 1, patients in the bioprosthetic MVR group were older (62.8 ± 9.3 years vs. 57.6 ± 9.5 years, p < 0.05) than patients in the mechanical MVR group, and had a lower proportion of females (47.6% vs. 74.1%, p < 0.05), and higher HAS-BLED scores (1.0 [IQR 1.0–2.0] vs. 1.0 [IQR 0–1.0], p < 0.05). The prevalence of rheumatic heart disease was lower in the bioprosthetic MVR group compared to the mechanical valve group (42.9% vs. 72.9%, p < 0.05), while the proportions of hypertension (61.9% vs. 34.1%, p < 0.05) and coronary heart disease (33.3% vs. 5.9%, p < 0.05) were higher. Patients in the bioprosthetic MVR group had a smaller mitral ring area (2.2 ± 0.4 mm2 vs. 2.7 ± 0.6 mm2, p < 0.05) and a lower left ventricular ejection fraction (55.8 ± 9.5% vs. 60.3 ± 7.4%, p < 0.05). No significant differences were observed between the two groups in blood pressure, BMI, CHA2DS2-VASc score, other comorbidities, left atrial diameter, left interventricular septum, left ventricular end-diastolic diameter, and left ventricular end-systolic diameter.
Electrophysiology study and ablation
The ablation strategies and outcomes for the two groups were summarized in Table 2. There were 13 cases in the bioprosthetic MVR group and 38 cases in the mechanical valve group used PentRay catheter for fast anatomy mapping without catheter entrapment. All patients included in the study underwent CPVA. Bilateral PVI was successfully achieved in all patients. Among the 14 patients in the bioprosthetic MVR group who underwent MI ablation, 5 received ethanol infusion in the VOM. In the mechanical MVR group, 10 out of 56 patients who underwent MI ablation received ethanol infusion in the VOM. There was no significant difference in MI block rate (78.6% vs. 69.6%, p = 0.508) between the bioprosthetic and mechanical MVR group. During the procedure of ablation, 8 patients in the bioprosthetic MVR group and 19 in the mechanical MVR group patients experienced atrial flutter (p = 0.104). Complications in the mechanical MVR group included 2 cases of pseudoaneurysm and 1 case of acute cerebral infarction. For the pseudoaneurysms, local compression was applied, and follow-up vascular ultrasound confirmed closure of the arterial breaches. One patient experienced acute cerebral infarction on the first postoperative day, with an INR of 2.77. No procedure-related complications were observed in the bioprosthetic MVR group, and no catheter entrapment occurred in both the groups.
Follow-up
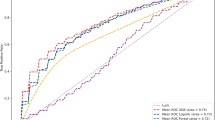
After a median follow-up of 17.9 (14.4, 21.4) months, no significant difference in AF recurrence rate was observed between the bioprosthetic MVR group and the mechanical MVR group (52.4% vs. 50.6%, log-rank p = 0.527, Fig. 1). In univariate Cox analyses, factors significantly associated with recurrences included age, persistent AF, and hypertension (Table 3). In multivariate Cox analysis, persistent AF was identified as an independent risk factor for AF recurrence after catheter ablation (HR 1.83, [95% CI 1.00–3.33], p < 0.05).
Eight patients in the mechanical MVR group experienced bleeding events with clinical symptoms, mainly presenting as hematuria and nasal hemorrhage. All the patients in the mechanical MVR group took warfarin, with INR values ranging from 2.37 to 4.00 at the time of bleeding. No significant clinical bleeding events were observed in the bioprosthetic MVR group (p = 0.352).
Sensitivity analyses
Due to the presence of certain baseline differences, we employed propensity score matching to balance the groups. Utilizing a 1:1 ratio and the nearest neighbor matching method, the variables including age, sex, coronary artery diseases, history of hypertension, left ventricular ejection fraction, and mitral orifice area were matched. Twenty-one patients who underwent bioprosthetic MVR and 21 patients who underwent mechanical MVR were matched. Survival analysis revealed that there was no statistically significant difference in AF recurrence rate between the bioprosthetic MVR group and the mechanical MVR group (52.4% vs. 47.6%, log-rank p = 0.705).
Discussion
This study found that the survival rates of patients without AF recurrence after catheter ablation were comparable between those with bioprosthetic MVR and those with mechanical MVR. Persistent AF was an independent predictor of recurrence following catheter ablation.
Mitral valve disease is a major pathophysiological cause of AF. Up to 50% of the patients undergoing mitral valve surgery had atrial fibrillation11. Conversely, AF is also a common complication following mitral valve surgery, with an incidence as high as 25%12. Concomitant valvular surgery during the maze procedure is usually recommended for patients with both AF and mitral valve disease. However, the proportion of surgical AF ablation in complex mitral valve surgery remains only around 64.6%11. Catheter ablation may be considered for patients without surgical ablation during MVR surgery or for those with new-onset AF after mitral valve surgery. The recurrence rate of catheter ablation was 44.8% in the patients with persistent AF who had undergone rheumatic valve surgery13. Almorad et al.14 reported a recurrence-free survival rate of atrial arrhythmias of 31.7% after the first catheter ablation of AF following mitral valve surgery. Several studies have investigated the efficacy and safety of catheter ablation of AF in patients with mechanical MVR or mitral valvuloplasty15,16,17. Lang et al.17 observed that sinus rhythm was maintained in 73% of patients with AF and mechanical MVR 1 year after catheter ablation. However, little published data reported the safety and efficacy of catheter ablation of AF in patients with bioprosthetic MVR. Zhao et al.18 reported that the long-term outcomes of AF following cardiac valve replacement were comparable between mechanical and bioprosthetic valves. However, in their study, patients underwent either MVR or aortic valve replacement, and it was not specified whether those with bioprosthetic valve replacement underwent MVR or aortic valve replacement. As a result, it is unclear whether valve material influences AF recurrence in patients who have undergone MVR. In our study, AF recurrence following catheter ablation did not differ between mechanical and bioprosthetic MVR. Catheter ablation of AF in patients with bioprosthetic MVR appears to be both safe and effective.
The STAR AF II study reported that linear ablation, when performed in addition to PVI in patients with non-valvular persistent AF, did not change the outcomes of AF recurrence19. However, it remains unclear whether additional linear ablation is necessary for patients with rheumatic heart disease. In addition, atrial flutter was also frequently observed in patients following mitral valve surgery20,21. In this study, 67.0% of patients had rheumatic heart disease, and 48.8% of those with paroxysmal AF had atrial flutter either before or during the ablation procedure. Therefore, PVI plus linear ablation was performed in these patients. During the MI line ablation, physicians should approach the mechanical mitral ring with caution to avoid mitral valve entrapment. Sometimes, it is difficult to reach the anatomical boundary of the ablation line to achieve MI block. In patients with bioprosthetic MVR, the anatomical boundary of the ablation line is more easily accessible, as valve entrapment is not a concern. Therefore, we hypothesized that the MI linear block rate might be higher in patients with bioprosthetic MVR compared to those with mechanical valve replacement. However, in this study, no statistical difference was observed in the bidirectional MI linear block rate between the two groups. Possible explanations include: (1) The pouch structure in the isthmus of the mitral valve after valve replacement may be a key factor influencing the achievement of MI linear block. Long et al.22 and Deng et al.23 both reported that the isthmus of the mitral valve in patients with mechanical MVR often develops pouch-like structures, which could significantly increase the difficulty of achieving conduction block across the MI line. (2) Statistical Class II errors might be caused by the small sample size of this study.
During the follow-up period of our study, pseudoaneurysm and acute cerebral infarction were observed after catheter ablation in patients after mechanical MVR. Previous studies reported femoral pseudoaneurysm, transient ischemic attack, mitral valve prosthesis disk embolization, and catheter entrapment within the prosthetic valve in patients with MVR undergoing catheter ablation of AF17,24,25. Given the small simple size of the study, it may be that the three procedure-related complication cases in the mechanical MVR group were coincidental. Nonetheless, in our study, the rate of procedure-related complications was higher in patients with mechanical MVR. Compared to the mechanical MVR group, the incidence of bleeding events following AF catheter ablation was lower in the bioprosthetic MVR group. Non-vitamin K antagonist oral anticoagulations were used for anticoagulation in the bioprosthetic MVR group, while warfarin was recommended for patients with mechanical MVR. Due to multiple interactions with food and drugs, INR values in warfarin users were prone to fluctuation. The INR values in the mechanical MVR group ranged from 2.37 to 4.0 when bleeding events occurred. Previous studies have shown that non-vitamin K antagonist oral anticoagulations were associated with lower rates of intracranial hemorrhage, major hemorrhage, fatal bleeding events, and cardiovascular death compared to warfarin, while being noninferior to warfarin in preventing stroke or systemic embolism26,27.
Study limitations
The small sample size from a single center was the primary limitation of this study. However, the study population was screened from 23,794 patients with clearly reported selection criteria. Additionally, the incidence of valvular disease combined with AF in China was higher than that in developed countries. To the best of our knowledge, this study was still the first and the largest study to explore catheter ablation of AF in patients with bioprosthetic MVR. Although the limited sample size led to certain baseline characteristic differences, these factors were not significantly associated with AF recurrence in the Cox regression analysis. Due to the retrospective study, the study did not clearly distinguish the order between receiving valve surgery and diagnosis of AF. It may be related to the differences in the mechanism underlying postoperative AF recurrence in patients who underwent MVR. The VOM ethanol infusion is primarily used for patients with persistent AF. In this study, the prevalence of persistent AF was higher in the bioprosthetic MVR group compared to the mechanical MVR group in this study. Thus, the data showed a higher proportion of the VOM ethanol infusion in the bioprosthetic MVR group (without statistical significance). The role of the VOM ethanol infusion in valvular AF needs to be further explored in future studies.
Conclusions
In conclusion, this study demonstrated the rate of AF recurrence after catheter ablation was not significantly different between the patients with bioprosthetic MVR and mechanical MVR. The safety and effectiveness of catheter ablation of AF were comparable between the patients with mechanical MVR and bioprosthetic MVR. But lower incidence of procedure-related complication was reported in the patients with bioprosthetic MVR.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to the patient privacy information involved but are available from the corresponding author on reasonable request.
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 139, e56–e528 (2019).
Diker, E. et al. Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease. Am. J. Cardiol. 77, 96–98 (1996).
Rowe, J. C., Bland, E. F., Sprague, H. B. & White, P. D. The course of mitral stenosis without surgery: Ten- and twenty-year perspectives. Ann. Intern. Med. 52, 741–749 (1960).
Olesen, K. H. The natural history of 271 patients with mitral stenosis under medical treatment. Br. Heart J. 24, 349–357 (1962).
Grigioni, F. et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: Determinants and long-term outcome. J. Am. Coll. Cardiol. 40, 84–92 (2002).
Chugh, S. S. et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 129, 837–847 (2014).
Jessurun, E. R. et al. Mitral valve surgery and atrial fibrillation: Is atrial fibrillation surgery also needed?. Eur. J. Cardiothorac. Surg. 17, 530–537 (2000).
Yu, J. & Wang, W. Bioprosthetic versus mechanical mitral valve replacement for rheumatic heart disease in patients aged 50–70 years. Front Cardiovasc. Med. 9, 904958 (2022).
Hussein, A. A. et al. Radiofrequency ablation of atrial fibrillation in patients with mechanical mitral valve prostheses safety, feasibility, electrophysiologic findings, and outcomes. J. Am. Coll. Cardiol. 58, 596–602 (2011).
Dong, J.-Z. et al. Prospective randomized comparison between a fixed ‘2C3L’ approach versus stepwise approach for catheter ablation of persistent atrial fibrillation. Europace 17, 1798–1806 (2015).
Mehaffey, J. H. et al. Variability and utilization of concomitant atrial fibrillation ablation during mitral valve surgery. Ann. Thorac. Surg. 111, 29–34 (2021).
Kernis, S. J. et al. Atrial fibrillation after surgical correction of mitral regurgitation in sinus rhythm: Incidence, outcome, and determinants. Circulation 110, 2320–2325 (2004).
Liu, X. et al. Efficacy of catheter ablation and surgical CryoMaze procedure in patients with long-lasting persistent atrial fibrillation and rheumatic heart disease: A randomized trial. Eur. Heart J. 31, 2633–2641 (2010).
Almorad, A. et al. Long-term clinical outcome of atrial fibrillation ablation in patients with history of mitral valve surgery. Front. Cardiovasc. Med. 9, 928974 (2022).
Kim, J. O. et al. Clinical characteristics and rhythm outcome of catheter ablation of hemodynamically corrected valvular atrial fibrillation. J. Cardiol. 73, 488–496 (2019).
Deneke, T., Mügge, A. & Shin, D.-I. Catheter ablation of valvular atrial fibrillation: things have changed?. J. Cardiovasc. Electr. 21, 1199–1201 (2010).
Lang, C. C. et al. Transcatheter radiofrequency ablation of atrial fibrillation in patients with mitral valve prostheses and enlarged atria: Safety, feasibility, and efficacy. J. Am. Coll. Cardiol. 45, 868–872 (2005).
Zhao, L. et al. Long-term outcomes of catheter ablation of atrial fibrillation post-cardiac valve replacement. Int. J. Cardiol. 225, 82–86 (2016).
Verma, A. et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 372, 1812–1822 (2015).
Adachi, T. et al. Left septal atrial tachycardia after open-heart surgery: relevance to surgical approach, anatomical and electrophysiological characteristics associated with catheter ablation, and procedural outcomes. Circ. Arrhythm. Electrophysiol. 8, 59–67 (2015).
Markowitz, S. M. et al. Lesional tachycardias related to mitral valve surgery. J. Am. Coll. Cardiol. 39, 1973–1983 (2002).
Long, D. et al. Mitral isthmus ablation in patients with prosthetic mitral valves. Chin. Med. J.-Pek. 123, 2532–2536 (2010).
Deng, W.-N. et al. Pouched mitral isthmus is associated with incomplete linear block in atrial fibrillation patients with mechanical mitral valve replacement. J. Cardiovasc. Electrophysiol. 26, 501–508 (2015).
Wang, X. et al. Heart rhythm disorders and pacemakers: Pulmonary vein isolation combined with substrate modification for persistent atrial fibrillation treatment in patients with valvular heart diseases. Heart 95, 1773–1783 (2009).
Bridgewater, B. J., Levy, R. D. & Hooper, T. L. Mitral valve prosthesis disk embolization during transeptal atrioventricular junction ablation. J. Interv. Cardiol. 7, 535–537 (1994).
Patel, M. R. et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 365, 883–891 (2011).
Connolly, S. J. et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151 (2009).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82170310); ZHONGNANSHAN MEDICAL FOUNDATION OF GUANGDONG PROVINCE (ZNSA-2020017); and Beijing Xinlian Zhicheng Cardiovascular Health Public Welfare Foundation.
Funding
This work was supported by the National Natural Science Foundation of China (82170310); ZHONGNANSHAN MEDICAL FOUNDATION OF GUANGDONG PROVINCE (ZNSA-2020017); and Beijing Xinlian Zhicheng Cardiovascular Health Public Welfare Foundation.
Author information
Authors and Affiliations
Contributions
De-Yong Long, Jian-Zeng Dong, Xin Du, Cai-Hua Sang, Rong-Hui Yu, Chen-Xi Jiang, Nian Liu, Song-Nan Li, Wei Wang, Xue-Yuan Guo, Xin Zhao, Chang-Yi Li, Song Zuo, Meng-Meng Li, Chang-Qi Jia, Yue-Xin Jiang, Wen-He Lv, Ri-Bo Tang, and Chang-Sheng Ma completed material preparation. Ze-Yang Wu, Yu-Kun Li, Xue-Si Wang, Zhuo-Hang Du and Xiao-Ying Liu finished data collection and analysis. Xiao-Ying Liu wrote the main manuscript text and prepared all tables and figure. Ri-Bo Tang was responsible for the revision of the article and the supervision of the entire study. All authors commented on previous versions of the manuscript. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, XY., Long, DY., Dong, JZ. et al. Safety and effectiveness of catheter ablation of atrial fibrillation in patients with mitral valve replacement mechanical versus bioprosthetic valves. Sci Rep 15, 26408 (2025). https://doi.org/10.1038/s41598-025-06592-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06592-8