Abstract
In this study, the Microbially Induced Carbonate Precipitation (MICP) technique was integrated with four distinct biopolymers to evaluate the wind erosion resistance and durability of desert sand soil in Kashi, Xinjiang. The samples were analyzed using Scanning Electron Microscopy (SEM), Energy Dispersive Spectroscopy (EDS), and X-ray Diffraction (XRD). SEM images revealed that the biopolymers effectively filled the interstitial spaces between calcium carbonate crystals and sand particles, thereby enhancing the cohesion of the soil matrix. XRD analysis indicated a significantly higher relative abundance of calcite mineral mass in samples incorporating biopolymers compared to those without biopolymers. EDS analysis demonstrated that samples containing biopolymers exhibited markedly elevated normalized concentrations of calcium ions, suggesting enhanced precipitation efficiency. Collectively, these findings confirm a substantial improvement in the bearing capacity, wind erosion resistance, and durability of the samples treated with biopolymers.
Similar content being viewed by others
Introduction
Kashi, Xinjiang (Fig. 1), is situated in the southern part of the Xinjiang Uygur Autonomous Region, with a strategic location advantage of “connecting five people and eight countries, and serving as a key link between Europe and Asia”. As a gateway to the west, Kashi serves as the starting point for the “China-Pakistan Economic Corridor” and plays a crucial role in commodity trade. In 2016, Kashi was designated as the main node city of both the “Belt and Road” initiative and the China-Central Asia-West Asia Economic Corridor, presenting new opportunities for foreign trade development in the region1. However, Kashi is bordered by the Taklimakan Desert to its east, leading to land desertification that contributes to frequent sandstorms2,3,4. These severe weather events5 result in large amounts of sand and dust being whipped up into cloudy air with significantly reduced horizontal visibility. This has serious implications for residents’ daily lives, economic activities, and even national economic objectives. It is imperative to mitigate or eliminate these harmful dust storms. One approach to addressing this issue is through soil consolidation to reduce wind erosion on desert surfaces. However, traditional methods such as physical, chemical or plant-based reinforcement may lead to environmental pollution or high costs that are not aligned with requirements for low-carbon sustainable development3,4. Physical approaches, such as constructing sand-retention barriers (e.g., fences and straw squares), represent conventional approaches in desertification control. These structures, however, are prone to burial by aeolian sediment transport due to their restricted vertical dimensions. The plant-based reinforcement method focuses on establishing xerophytic vegetation, yet exhibits limitations in arid zones: artificial plantations demonstrate low survival rates attributable to hydrological deficits, coupled with inherently slow biomass accumulation under xeric stress. Chemical stabilization involves the application of polymer-based compounds with soil-binding or hygroscopic characteristics to dune surfaces. While effective in the short term, this strategy raises concerns regarding cost-effectiveness and ecological impacts, as certain synthetic additives possess persistent toxicity or exhibit recalcitrance to biodegradation, thereby posing contamination risks6. Therefore, identifying an environmentally friendly and efficient soil consolidation method is essential for effective sand and dust control7.
Source of experimental sand. (The map was generated by ArcGIS10.7. https://pan.xunlei.com/s/VNyxo_kKlrC3AHr-s3wg2XVgA1?pwd=zu37 or https://pan.baidu.com/s/1lFJ4dmp5AbeRJiztYBoIbw?pwd=6789).
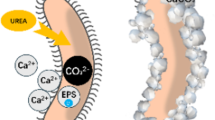
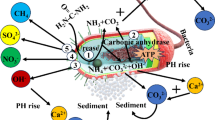
Microbially Induced Carbonate Precipitation (MICP) solidified sand is known for its resistance to wind erosion and dust as well as environmentally friendly characteristics8,9,10. MICP is a biologically driven technology that involves the deposition of calcium carbonate and can be categorized into biological control and biological induction mechanisms. In biologically controlled mineralization, organisms regulate the nucleation and growth of mineral crystals, ultimately influencing the morphology and characteristics of crystals11. In this study, colonies are typically cultured first, with urea hydrolysis being accelerated using urease produced by the colonies. For instance, Bacillus exhibits strong biological activity in high alkali and high salt environments, continuously releasing highly active urease during metabolism12,13,14. Under the catalysis of urease, urea is hydrolyzed to \({\text{NH}}_{2}\text{COOH}\) (carbamate) and \({\text{NH}}_{3}\) (Formula (1)). The former will further hydrolyze to \({\text{NH}}_{3}\) and \({\text{H}}_{2}{\text{CO}}_{3}\) (Formula (2)), which generally react in water to reach ionization equilibrium (Eq. (3), (4)). The \({\text{CO}}_{3}^{2-}\) generated in this process combines with \(\text{C}{a}^{2+}\) to produce \({\text{CaCO}}_{3}\)(Formula (5)–(7)), which is widely found on the surface and pores of soil particles. This process results in cementing originally loose soil particles to form a relatively compact particle structure15, leading to the formation of a hard crust on the surface of the soil, the production of calcium carbonate induced by the biostimulus is depicted in Fig. 2.
In the study of MICP’s resistance to wind erosion, the direct effect of MICP curing on bare soil significantly influences its resistance to wind erosion and dust raising. Gao Yufeng et al.16 demonstrated that the curing effect of MICP can be enhanced by appropriately increasing the volume, concentration, and treatment solution concentration. The Bang team conducted surface microbial treatment on well-graded sand and poorly graded sand under different ambient temperature and humidity conditions, revealing that soil with good MICP consolidation gradation exhibits better resistance to wind erosion and dust lifting in low humidity and high temperature environments17,18. Hadas Raveh-Amit et al. studied wind erosion control of loess in the Negev Desert (Israel), finding that spraying a 0.5 M solution into the soil can reduce dry crack area and length by two times19. Hao Meng’s research examined the potential of MICP to reduce wind erosion in desert soils, showing that this method significantly improves bearing capacity and wind erosion resistance by forming crusts, suggesting its promise for protecting desert soils from wind erosion20. Shafii et al. successfully applied three different enzymes to control soil erodibility, concluding that enzyme-treated soil samples exhibited higher erosion resistance than untreated samples21. Recent research results indicate that MICP has shown great potential for controlling wind erosion and suppressing dust22,23,24,25,26,27,28.
Sodium alginate (SA) biopolymer-assisted enzyme-induced carbonate precipitation (EICP) was utilized to investigate the stability of Nafud desert sand. The results indicated that the combination of EICP and SA significantly increased the erosion resistance rate by 100% compared with untreated samples29. Zhao et al. explored the use of biopolymers to enhance EICP by retaining carbonates around soil particles, aiming to improve soil water retention and reduce permeability. In their study, xanthan gum and guar gum biopolymers, as well as inert polycellulose hydrogels, were employed to enhance EICP. It was concluded that both guar gum and xanthan gum have a higher potential to retain water and reduce water evaporation, thereby enhancing carbonate precipitatio30. Biopolymers are natural polymers produced by plants and organisms in the environment, which are hydrophilic, binding, environmentally friendly, and widely used in food and medical fields31. In recent years, many researchers have found that biopolymers such as gellan gum, agar gel, guar gum, and xanthan gum can improve the engineering characteristics of soil32. For example, under dry conditions, the compressive strength of natural clay is 1050 kPa, however it can be increased to 4660 kPa with 1% gellan gum or 3190 kPa with agar gel addition33. Previous studies34,35,36,37 also demonstrated that wind-induced soil erosion can be controlled using biopolymers alone. The results showed that samples treated with biopolymer had higher wind dispersion resistance than those treated with water only. In order to further enhance the wind erosion resistance of desert soil for ideal conditions, the combination of bio-polymer gelatin, gellan gum, guar gum, xanthan gum, and MICP was used.
When MICP is employed as a standalone solution, although it can enhance bearing capacity strength. Nevertheless, the sample typically necessitates multiple cycles of MICP treatment to achieve a superior curing outcome. Simultaneously, Cui et al.38 disclosed that microbial-solidified sand exhibits pronounced brittle failure characteristics. Using biopolymer alone results in a gel structure with superior toughness; however, its compressive and shear strengths are relatively lower, and primarily in shallow reinforcement, it is challenging to penetrate deeply into the sand. When MICP is combined with biopolymers, the resulting biopolymer can provide additional nucleation sites for the recombination of calcium carbonate crystals. This leads to the formation of larger calcium carbonate crystals, facilitating the development of “biopolymer-calcium carbonate” aggregates and enhancing the strength of particle bonding. The incorporation of biopolymer reduces the porosity and equivalent pore size of the sample while improving overall compactness. Additionally, biopolymers may serve as carbon sources, thereby prolonging microbial activity, sustaining MICP over an extended period, and increasing the content of calcium carbonate crystals39,40.
In this paper, the wind erosion resistance of the sample was evaluated through a small wind tunnel test. The crust thickness of the sample was measured over a period of 60 days, and the bearing capacity of the micro-penetration instrument was also measured. The mechanical properties of MICP and different biopolymer treated sands were studied by conducting acid resistance tests and freeze–thaw cycle tests on the samples. Additionally, the acid corrosion resistance and freeze–thaw cycle of the samples were preliminarily discussed. Elemental analysis was carried out using energy dispersive spectroscopy (EDS), while scanning electron microscopy (SEM) and X-ray diffraction (XRD) were used to further analyze the microstructure and mechanism of different treatments.
Experimental materials and methods
Sand
The sand utilized in the investigation was sand retrieved from a depth of 10 cm (77°22 ′49 "E, 39°1′ 11" N) proximate to the Taklimakan Desert. The Size distribution diagram of sand particles is delineated in Fig. 3, while its physical and chemical attributes are expounded in Table 1. X-ray diffraction analysis, as depicted in Fig. 4, intimated that the minerals in the sand preponderantly encompass approximately 64.1% SiO2, 13.9% CaO, and 9.5% Al2O3. Furthermore, Fig. 5 showcases the species and proportion of bacteria extant in sandy soil.
Annotation
Cu: The Coefficient of uniformity (Cu) reflects the distribution of different particle groups of different sizes and is one of the indicators to judge whether the soil particle size is well graded. Its expression is as follows:
among them, on the cumulative curve of soil particle size,
\({d}_{10}\) is the particle size of 10% by sifting weight,
\({d}_{60}\) is the particle size of 60% by sifting weight.
Cc: Cc represents the Coefficient of curvature, and the formula for calculating the Coefficient of curvature is:
in this formula:
\({d}_{10}\) denotes the particle size corresponding to 10% by weight, also known as the effective particle size.
\({d}_{30}\) denotes the particle size corresponding to 30% by weight.
\({d}_{60}\) denotes the particle size corresponding to 60% by weight, also referred to as the control size or limiting size.
The curvature coefficient is an index used to evaluate the continuity of the slope of the cumulative curve of soil particle size distribution.
pH: The pH value, also known as the “potential of Hydrogen”, represents the negative logarithm of the hydrogen ion concentration. It is utilized to quantify the acidity or alkalinity of a substance.
Cementation solution
The ratio of cementation solution used in the test is: yeast extract 0.1 g/L, ammonia chloride 12.5 mM, sodium acetate 42.5 mM, urea 350 mM, calcium chloride 250 mM, pH8.441, the amount of spray is 30 ml each time7.
Stimulation solution
The ratio of stimulation solution used in the test is: yeast extract 0.1 g/L, ammonia chloride 12.5 mM, sodium acetate 42.5 mM, urea 350 mM, pH8.441.
Biopolymer
There are four types of biopolymer used in the experiment: xanthan gum, gellan gum, guar gum, and gelatin. The physical and chemical properties of these biopolymer are presented in Table 2, while their appearance is depicted in Fig. 6. According to previous research42, both Xanthan gum and guar gum exhibit optimal curing effects at a dosage of 1%. Therefore, the concentration of biopolymer used in this study is also set at 1%.
Test scheme
Sample processing
The method for treating the sample is detailed in Table 3.
Wind tunnel test
The wind tunnel comprises the tunnel body, the power component, and the measurement and control section, as depicted in Fig. 7 and Table 4. It is primarily classified based on the speed range of the experimental section, falling under the Eiffel type wind tunnel category. The technical specifications of the wind tunnel tester are as follows: Device dimensions: 1650 × 450 × 410 mm; Experimental section dimensions: 200 × 200 mm; Acrylic thickness of experimental section: 5–8 mm; Wind speed in the experimental section: 12 m/s, which also serves as the speed for this wind erosion test. This equipment is utilized for determining sample wind erosion and calculating the percentage of sample weight loss before and after conducting a wind tunnel test.
Measurement of bearing capacity
The micro-penetration instrument used in the bearing capacity test is sourced from Changzhou 80 Future Intelligent Technology Co., LTD. This series of micro-penetration instruments serves as a convenient and practical tool for geotechnical engineers and engineering investigation technicians during on-site foundation soil description and construction tank inspection. Such as Fig. 8, WXGR-2.0. The instrument measures the penetration resistance, which is then converted into bearing capacity according to the established conversion standard. The basic principle is to reflect the mechanical characteristics of different depths in the test sample according to the resistance force encountered by the probe in the process of continuous penetration, and each sample is measured three times to obtain the average value.
Measurement of crust thickness
The thickness of the crust in the sample was measured using a measuring scale. After conducting the test, the crust thickness at three different positions(Fig. 9) for each sample was measured with a caliper. The mean value (± standard deviation of three tests) was then calculated and reported as the result.
Acid resistance test
Preparation of acidic solution43: In this experiment, 0.5 mol/L acetic acid was used, and the chemical properties of acetic acid are listed in Table 5. After weighing the initial weight of each test block (m1), three samples were taken from each block and immersed in a 0.5 mol/L acetic acid solution for 24 h with thorough exposure by turning the surfaces. Subsequently, the samples were removed from the solution, washed carefully with water, furthermore, the sample was subjected to drying, and then re-weighed (m2) to measure the mass loss rate after immersion. This process was repeated five cycles to calculate the bearing capacity loss rate after acid etching. The mass loss rate calculation formula is presented as follows:
where: \({\text{m}}_{1}\) is the weight before pickling.
\({\text{m}}_{2}\) is the weight after pickling.
\(\text{m}\) is the mass loss rate.
Freeze–thaw cycle test
In the freeze–thaw cycle test, the samples were categorized and frozen in a constant temperature refrigerator at − 20 °C, and then thawed at room temperature. The number of freeze–thaw cycles ranged from 1 to 5 cycles. The mass loss rate of the samples was calculated, and the bearing capacity loss rate after the freeze–thaw cycle was measured.
Results and discussion
Bearing capacity analysis
As illustrated in Fig. 10, the bearing capacity of various samples gradually increased within 60 days, with the maximum bearing strength of stimulation solution and cementation solution added reaching 369.67 kPa, which was 348.23 kPa higher than that of undisturbed sand. This finding is consistent with previous studies20. To enhance the uniformity of MICP, four types of biopolymer were individually added. The experimental results revealed that the addition of biopolymer gelatin increased the bearing capacity by 390.23 kPa compared to undisturbed sand and by 42 kPa compared to only cementation solution and stimulation solution. Specifically, the bearing capacity reached up to 411.67 kPa for gelatin-added samples, 393.33 kPa for guar gum-added samples, 391.67 kPa for gellan gum-added samples, and 388.67 kPa for xanthan gum-added samples-in ascending order from smallest to largest effect according to part of reference44.
The rigid rod shaped helical structure of xanthan gum confers exceptional stability and facilitates the formation of hydrogen bonds and electrostatic interactions with sandy soil particles, resulting in enhanced soil particle compaction, reduces pores between them, enhances adhesion, and effectively improves stiffness and bearing capacity of sandy soil. Compared to xanthan gum, guar gum contains a large number of special hydroxyl groups that can combine with soil particles to form a hydrogel network. This network improves the bearing capacity and durability of sandy soil45,46. When xanthan gum and guar gum were mixed into sandy soil, the soil particles were cemented together by a reaction with minerals in the sand, effectively improving the bonding strength between soil particles. On a macro level, this results in an increase in soil cohesion, which is consistent with previous studies47. The cementation formed by the reaction of Gellan glue and gelatin with minerals in the soil can effectively improve the bonding strength between sand particles and increase the bearing capacity of sand.
Crust thickness analysis
As shown in Fig. 11, the undisturbed sand exhibits minimal crust thickness, while the samples treated with four types of biopolymer can reach up to 30 mm in crust thickness. The samples treated only with stimulation solution and cementation solution show a crust thickness of 21 mm, which increases over time and stabilizes after 60 days. Among the four types of biopolymer, gelatin produces the thickest skin, while xanthan gum yields the smallest thickness, consistent with the specimen bearing capacity described earlier.
The process of stimulation and cementation solution penetrating the sample proceeds from the exterior towards the interior. As the outer layer develops into a cementing matrix, biopolymers undergo hydration to solidify the surface soil, filling pores between particles and enhancing particle adhesion. Guar gum’s binding properties increase sand crust thickness, while gellan gum directly interacts with soil particles by forming a network matrix. Both gellan gum and xanthan gum have short molecular structures and charges that enable direct interaction with fine sand particles to form biopolymer and increase crust thickness. Furthermore, xanthan gum and gellan gum create pore spaces resembling a honeycomb structure in improved silt, leading to increased sandy soil crust thickness. Gelatin’s strong cementation effect on soil particles not only enhances structural connections but also strengthens particle attraction within the soil. Its inherent gelling and film-forming properties cover loose particles in the soil, resulting in increased sample crust thickness.
Analysis of weight loss due to wind erosion
As illustrated in Fig. 12, the undisturbed sand experienced the greatest weight loss among the various samples, On the 30th day, the mass loss of the samples combined with cementation solution and four distinct types of biopolymer tended to stabilize. The mass loss of the samples augmented with biopolymer was lower than that of the samples merely containing cementation solution. The mass loss of the samples was in a descending order as follows: xanthan gum, gellan gum, guar gum, and gelatin. Which is consistent with previous research findings7,20. As depicted in Fig. 12, the mass loss rate of samples supplemented with biopolymer is significantly lower than that of the other two samples. This can be attributed to fine soil particles forming matrix cementation with biopolymer when biopolymer was added, thereby resisting tension stress and maintaining soil structure stability.
Additionally, a high content of biopolymer can adsorb cementation solution to form a highly viscous suspension and fill pores between sand particles48. Consequently, closer bonding between sand particles occurs as well as an increase in contact area and establishment of bridges between discrete sand particles due to accumulation and coverage by biopolymer on their surfaces49. The interaction between biopolymer and sand particles directly results in a stable structure that strengthens sand, inhibits crack formation, reduces wind erosion susceptibility, and enhances resistance to wind erosion.
Analysis of freeze–thaw cycle test results
As depicted in Fig. 13, following a single of freezing–thawing cycles, the undisturbed sand undergoes a weight loss of up to 6.2% and a bearing capacity reduction of up to 8%. The samples treated with cementation solution and stimulation solution exhibit a weight loss of up to 1.97% and a bearing capacity decrease of up to 2.1%. In contrast, the addition of xanthan gum to MICP samples resulted in a reduction of weight loss and bearing capacity by 0.78% and 1.09%, respectively. In this study, the addition of gelatin to MICP was found to have a positive effect. Following a single of freeze–thaw cycle, there was a decrease in weight loss and bearing capacity of 0.25% and 0.5%, respectively. With an increase in the number of freeze–thaw experiment cycles, there is a diminish in both mass loss and bearing capacity. This suggests that the samples enhanced with biopolymer display superior durability. Additionally, gelatin demonstrates the least amount of weight loss and bearing capacity reduction, followed by guar gum, gellan gum, and xanthan gum in that order. These findings are consistent with previous research45,46.
The primary causes of mass decrease during freeze–thaw cycles include water infiltration, ice expansion, biopolymer cracks, and an increase in sand porosity. As the temperature decreases during the freezing process of sand, ice crystals within the sand gradually increase. The adsorption force between unfrozen water and ice crystals becomes stronger than the cementation effect between water and sand particles, weakening cohesive forces between sand particles and leading to an overall reduction in strength-consistent with previous research results50. Water forms ice and expands in volume, creating expansion stress. When the expansion stress is significant, cracks will appear in the sand sample. The number of freeze–thaw cycles leads to increased and continuous accumulation of crack damage inside the sample, following a pattern from less to more and from narrow to wide. During the melting process, water in freeze-heave soil crystallizes into ice, causing the soil to become loose and resulting in mass loss due to surface shedding, peeling, and angulation. This leads to a deterioration in performance51. The lower mass loss rate of samples with biopolymer can be attributed to factors such as void ratio, interparticle bonding strength, and ion concentration in the liquid phase. Intergranular bond strength plays a crucial role in frost resistance. With the addition of biopolymer, there is an improvement in intergranular bond strength which offsets stress caused by frost heaving, reduces strength loss, and minimizes quality loss.
Analysis of acid resistance test results
Observation from Fig. 14 reveals that, following an acid resistance test, both weight loss and bearing capacity reduction for samples exceed those observed after undergoing freeze–thaw cycles. This phenomenon arises from a chemical reaction between calcium carbonate present in these specimens with acid leading to CO2 generation, thereby causing weight reduction. Specifically, after conducting one time of acid resistance test undisturbed sand experiences up to a substantial 9% weight loss accompanied by a significant decrease in its bearing capacity by approximately 15%. In contrast, following an acid resistance test, the samples of 8 times of stimulation solution and cementation solution exhibited a weight loss of 2% and a bearing capacity loss of 12%. However, the samples treated with biopolymer gelatin showed improved results, with only a 0.6% weight loss and a 5% bearing capacity loss. Similarly, the samples treated with xanthan gum experienced a weight loss of 1.5% and a bearing capacity loss of 8%. The smallest loss of weight and loss of bearing capacity was observed in gelatin-treated samples, followed by guar gum, gellan gum, and finally xanthan gum. This reflects the better bonding effect of gelatin followed by guar gum, gellan gum and xanthan gum. With the increasing number of acid resistance tests, the reduction in weight and bearing capacity is gradually diminishing.
These results illustrate superior durability compared to untreated samples. Furthermore, incorporation of biopolymer results in lower levels of both bearing capacity decline and weight loss when compared to other types of treated samples due to its ability to significantly enhance cementitious bonding among sand particles despite some impact on their strength attributed to hydrolysis reactions occurring within acidic environments affecting glycosidic bonds as well as pyruvate groups within biopolymer molecules52.
Analysis of microscopic mechanisms
SEM reveals the production of a significant amount of calcium carbonate cemented crystals and jelly-like gels during the process of MICP bonding. These crystals adhere to and fill sand particles and bioadhesive aggregates, effectively cementing them together to form cohesive sand. Additionally, the biopolymer fills the void between sand particles, providing a skeleton for the cement body and ultimately improving its strength (Fig. 15). Test results demonstrate that biopolymer enhances soil bearing capacity, crust thickness, wind erosion resistance, acid resistance, and freeze–thaw resistance. Furthermore, it tightens the microscopic arrangement of sand, reduces pore size and number, limits displacement of sand particles, affects internal friction angle of sand, and improves overall bearing capacity.
Figure 15b–e shows the SEM image of the sample with biopolymer added. A comparison with the images in Fig. 15a reveals that the addition of biopolymer results in a glue layer on the surface of soil particles, forming a network structure and fibrous substance. This increases the number of contact points between sand particles, enhancing overall cohesion, stability, and carrying capacity of the sand. The physical strength of the sand is also affected as hydrogen bonds between galactomannan molecular chains and hydroxyl groups on side chain monosaccharide residues combine with chemical bonds between sand particles to form a physical structure, thereby improving overall strength. As a high molecular polymer, biopolymer enhances sand’s ability to resist deformation by altering porosity, cohesion, and bonding force to improve bearing strength. Additionally, introducing an appropriate amount of biopolymer can effectively increase retention of cementing fluid, improve utilization rate of \({\text{Ca}}^{2+}\), and increase calcium carbonate content within the sand sample. This improves molding effect and significantly increases curing strength consistent with previous studies53. On the other hand, in the process of MICP reaction, microorganisms produce urease to expedite the decomposition of urea in the stimulation solution into \({\text{CO}}_{3}^{2-}\), and \(\text{C}{a}^{2+}\) in the stimulation solution adheres to the surface of microbial cells. The chemical reaction between \({\text{CO}}_{3}^{2-}\) and \(\text{C}{a}^{2+}\) results in the formation of \({\text{CaCO}}_{3}\) crystals with a gelatinous effect, which cements sand particles together as a whole. The gaps between sand particles and \({\text{CaCO}}_{3}\) crystals, as well as between \({\text{CaCO}}_{3}\) crystals themselves, were filled with biopolymer, thereby enhancing the effectiveness of MICP in curing sand particles54.
The calcium carbonate crystals generated through microbial activity partially fill the pores, but due to the substantial interstitial spaces between particle skeletons, they were unable to completely occupy the pores along with fine sand particles. This results in visibly larger interstitial spaces between the particles. When magnified 3000 times (Fig. 15a), it was evident that the smooth surface of sand particles prevents complete wrapping by calcium carbonate crystals. In contrast, these crystals form and grow on bacterial cells, using them as nucleation sites55, indicating less bacterial adhesion on the surface of sand particles. Consequently, the filling and bonding effects of calcium carbonate crystals on pores and between particles were suboptimal. As the microbial reaction progresses, calcium carbonate crystals continue to stack and envelop surfaces. With biopolymer aiding in filling actions between biopolymer and sand particles, particle shapes gradually blur while contact modes shift from point-to-point to face-to-side and face-to-surface contacts (Fig. 15b–e). This leads to closer connections between particles and significantly improves overall uniformity and compactness of samples.
Figure 16 illustrates the relative content of calcite mineral mass in samples with different bioadhesives added. The samples with gelatin showed a relative content of calcite mineral mass at 6.2%, while the samples with guar gum had a relative content of calcite mineral mass at 5.0%. In comparison, the relative content of calcite mineral mass in samples with gellan gum and xanthan gum was 3.5% and 2.5%, respectively, whereas the samples without biopolymer exhibited a relative content of calcite mineral mass at only 2.0%. Calcite is a common calcium carbonate mineral found in nature, displaying various crystal shapes and forming crystal clusters. In this study, the relative content of calcite mineral mass was utilized to assess the formation of calcium carbonate crystals in the MICP process, and the impact on crystal content after adding biopolymer was observed as depicted in Fig. 16. The addition of biopolymer has been shown to enhance the effectiveness of MICP in forming calcium carbonate crystals, bridging gaps between sand grains, improving sand bearing capacity, and enhancing wind erosion resistance. Additionally, it was observed that biopolymer themselves also exhibited bonding effects. Under the conditions studied here, gelatin demonstrated the most significant improvement effect on MICP performance followed by Guar gum, Gellan gum and Xanthan gum-consistent with previous SEM observations.
The enhancement mechanism of biopolymer on microbiologically solidified sand mainly includes three aspects. Firstly, the formation of a biopolymer-clay matrix through hydrogen and ionic bonding with clay particles, which also acts as a filler for the pores between soil skeletons formed by sand and silt56. This results in biopolymer playing a significant role in filling the spaces between sand particles, thereby greatly reducing the size of large pores and enhancing the compactness of the solidified body. Secondly, the good adsorption effect of biopolymer effectively increases the content of bacteria in the sample, leading to an increase in both yield and distribution uniformity of calcium carbonate within the solidified body. Lastly, active substances within biopolymer participate in reactions to form composite gels with calcium carbonate crystals, further enhancing cementation properties and compactness within the solidified body.
In order to analyze the calcium ion content in five samples, EDS scanning was conducted. As shown in Fig. 17, the normalized quantity of calcium ions of 8 + 8, Xanthan gum, Gellan gum, guar gum and gelatin are as follows: corresponding values were 10.56%, 11.28%, 12.1%, 12.58% and 12.86%. It is evident that the calcium content varied among the different samples. Upon comparison, it was observed that samples without added biopolymer had lower calcium content, while gelatin combined with MICP showed higher levels of calcium content. These data reflect changes in calcium carbonate content across different samples laterally, and the change in normalized mass content of calcium ions aligns with variations in XRD calcium carbonate content as well as SEM image results and sample outcomes. This indicates that adding biopolymer can effectively promote full and uniform reaction between stimulation solution and nutrient solution with sand to generate more calcium carbonate crystals. The gel effect of biopolymer itself can also enhance connections between sand particles, thereby improving sand bearing capacity, wind erosion resistance, and durability.
Discussions
In the treated samples, the content of calcite treated by biostimulatory MICP witnessed a significant increase57. Through SEM, it is evident that the particle surfaces are coated with biopolymer, leading to a significant reduction in internal pores within the sample. This enhancement in inter-particle connectivity among sand particles mitigates potential mesostructural damage mechanisms, such as weakened particle connections and complex pore morphologies58,59. EDS analysis confirmed the presence of calcium ions, while XRD detected calcite, calcium carbonate crystals, particularly those in the form of calcite, play a crucial role in enhancing the cementation properties of sandy soil, thereby improving its structural stability and mechanical performance60. The precipitation of calcite-form calcium carbonate crystals in the interstitial spaces between particles facilitates effective cementation of sandy soil by forming robust bridges that connect sand particles. Consequently, these calcium carbonate precipitates significantly contribute to the improvement of the mechanical properties of sand61. Biopolymer molecules exhibit a negative charge at their termini, possess high viscosity and adhesion, hydrophilicity, and long-chain characteristics. These properties enable them to provide nucleation sites for bacterial enrichment, attract calcium ions, reduce seepage rates, retain cementitious solutions, and form “biopolymer-calcium carbonate” aggregates within sand particles. Consequently, this enhances the bridging effect between sand particles and improves both the pore structure and mechanical performance39.
Furthermore, a considerable body of research has investigated EICP as a technique for solidifying desert sand and forming a hardened shell layer on its surface6,62,63,64. Although these studies have shown improvements in sand stabilization, significant variations exist in their methodologies. Notably, differences are observed in the types of microorganisms employed, the timing and volume of applications, and the sources of sand used, which encompass both laboratory and field settings. As a result, wind speed parameters also vary across different studies. Several studies65 have integrated microbial mineralization technology with BMDS containing Polyacrylic Acid (PAA) modifiers to mitigate secondary dust pollution. In contrast, the effectiveness of BMDS in consolidating pulverized coal is significantly influenced by the concentration of PAA. Concerning durability enhancement, while this study utilizes biopolymer, study66 demonstrates that incorporating polyvinyl acetate (PVAc) into the cementing solution can also enhance resistance to rainfall erosion in MICP treated materials. Similarly, study67 shows that adding PVAc or polyethylene glycol (PEG) to the replacement solution improves both shear strength and rain erosion resistance in EICP treated dust. Observations indicate that both MICP and EICP possess sand solidification capabilities, and the inclusion of PAA or biopolymer in the cementation solution enhances the durability of the samples. As indicated by the research findings68, the incorporation of PAA can effectively suppress dust generation, while significantly enhancing the surface strength, wind erosion resistance, and rainfall erosion resistance of the samples. In addition, the application of optimization techniques along with additives, such as xanthan gum and fibers, can further enhance the mechanical strength, treatment uniformity, and toughness of biocementified soils69.
Conclusion
The MICP biostimulatory method supplemented with biopolymer can not only effectively enhance the uniformity of calcium carbonate crystals generated by MICP but also the gelling property of the biopolymer, enabling the beneficial substances in the stimulatory and cementing fluid to remain in the sand for a longer duration. Thus, the stimulation solution can fully react with the bacteria in the sand to generate more calcium carbonate crystals. This article presents the results of laboratory experiments that examined the integration of MICP with biopolymer-stabilized sand. Four distinct biopolymers were assessed, and it was found that MICP in combination with gelatin yielded significantly superior curing effects compared to the other three biopolymers (guar gum, Gellan gum, and xanthan gum), which showed progressively enhanced performance. All four biopolymers effectively reduced wind erosion losses and improved the bearing capacity of the sand. During the acid resistance test and freeze–thaw cycle test, it was observed that samples treated with biopolymer could effectively resist corrosion under acidic conditions and maintain better bearing capacity with less mass loss. Microscopic analysis revealed that the biopolymer dissolved in the sand to form a gelled substance, binding the sand particles and calcium carbonate crystals together. This process effectively improved the bearing capacity of the sand, consolidated the sand particles, formed a thicker crust, and mitigated wind erosion.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Zhao, Q. H., Xin, X. L. & Wang, H. Research on the High-Quality Development Path of Foreign Trade in the Kashi Region of Xinjiang Against the Background of “One Belt and One Road”. External economic and trade activities of the province. 86–91 (2023).
Shi, G. Y. & Zhao, S. X. Some scientific issues in the research of sandstorms. Atmospheric Sci. 27(4), 592–598 (2003).
Jia, L. H., Li, H. Y., Li, R. Q., Tang, H. & Huo, W. Numerical simulation and diagnostic analysis of severe sandstorm weather in “3.12” in southern Xinjiang. Deserts China 32(4), 1136–1138 (2012).
Joseph, P. V., Raipal, D. K. & Deka, S. N. “ANDHI”: The convective dust storms of northwest India. Mausam 31, 431–442 (1980).
Liu, H. T., Qu, X. J., Yang, X. L. & Li, X. C. Climate characteristics, causes and countermeasures of sandstorm in the Hotan Area of Southern Xinjiang. Resour. Environ. Arid Areas 25(6), 169–175 (2011).
He, J. et al. Recent development on optimization of bio-cementation for soil stabilization and wind erosion control. Biogeotechnics 1(2), 100022. https://doi.org/10.1016/j.bgtech.2023.100022 (2023).
Wei, C. & Qu, J. L. Biostimulation was employed to augment the resistance of soil against wind erosion. Water Transp. China 23(2), 61–63 (2023).
Naeimi, M. & Chu, J. Comparison of conventional and bio-treated methods as dust suppressants. Environ. Sci. Pollut. Res. 24(29), 23341–23350 (2017).
Tian, K., Wu, Y., Zhang, H., Li, D. & Nie, K. Increasing wind erosion resistance of aeolian sandy soil by microbially induced calcium carbonate precipitation. Land Degrad. Dev. 29(12), 4271–4281 (2018).
Zhan, Q., Qian, C. & Yi, H. Microbial-induced mineralization and cementation of fugitive dust and engineering application. Constr. Build. Mater. 121, 437–444 (2016).
Barabesi, C. et al. Bacillus subtilis gene cluster involved in calcium carbonate biomineralization. J. Bacteriol. 189(1), 228–235 (2007).
Tang, C. S., Pan, X. H. & Lü, C. Biogeoengineering technology and the applications. Geol. J. China Univ. 27(6), 625 (2021).
Chen, Y. G., Jiang, S. M., Fu, J., Zhou, H. & Wen, Z. H. Cultivation optimization and mineralization mechanism of Bacillus pasteurella solidified contaminated soil. J. Tongji Univ. (Nat. Sci.) 53(4), 635–643. https://doi.org/10.11908/j.issn.0253-374x.23290 (2025).
Dong, B. W. et al. Natural seawater based on microbial-induced calcium carbonate precipitation Evaluation of the effect of reinforcing calcareous sand. Rock Soil Mech. 42(4), 1104–1114. https://doi.org/10.16285/j.rsm.2020.1068 (2021).
Van, P. L. Biogrout ground improvement by microbially induced carbonate precipitation (Netherlands Delft University of Technology, 2009).
Gao, Y. F., Yang, E. J. & Jia, H. Experimental investigation on wind-proof sand fixation based on microbiome-induced calcium carbonate deposition. Sci. Henan 37(1), 144–150 (2019).
Bang, S. C., Min, S. & Bao, S. Application of microbiologically induced soil stabilization technique for dust suppression. Int. J. Geo-Eng. 3(2), 27–37 (2011).
Meyer, F. D., Bang, S., Min, L. D., Stetler, S. & Bang, S. Microbiologically-induced soil stabilization: Application of sporosarcina pasteurii for fugitive dust control. Geo-Frontiers Congress. 4002–4011 (2011).
Raveh-Amit, H., Aviv, G. B., Kesem, A. & Michael, T. Mitigation of aeolian erosion of loess soil by Bio-Stimulated microbial induced calcite precipitation. CATENA 237, 107808 (2024).
Meng, H., Gao, Y. F., He, J., Qi, Y. S. & Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 383, 114723 (2021).
Shafii, I., Shidlovskaya, A. & Briaud, J. L. Investigation into the effect of enzymes on the erodibility of a low-plasticity silt and a silty sand by EFA testing. J. Geotech. Geoenviron. Eng. 145, 04019001 (2019).
Bang, S. S., Bang, S., Frutiger, S., Nehl, L. M. & Comes, B. L. Application of novel biological technique in dust suppression. 0831 (Transportation research board 88th annual meeting. Washington DC, United States, 2009).
Gomez, M. G., Martinez, B. C., Dejong, J. T., Hunt, C. E. & Dworatzek, S. W. Biomediated soil improvement field study to stabilize mine sands. (Proceedings of GeoMontreal. Montreal, Canada, 2013).
Maleki, M., Ebrahimi, S., Asadzadeh, F. & Tabrizi, M. E. Performance of microbialinduced carbonate precipitation on wind erosion control of sandy soil. Int. J. Environ. Sci. Technol. 13(3), 937–944. https://doi.org/10.1007/s13762-015-0921-z (2016).
Meyer, F. D., Bang, S., Min, S., Stetler, L. D. & Bang, S. S. Microbiologically-induced soil stabilization: application of Sporosarcina pasteurii for fugitive dust control. 4002–4011 (GSP 211. ASCE. Reston. VA. USA, 2011). https://doi.org/10.1061/41165(397)409
Stabnikov, V., Chu, J., Myo, A. N. & Ivanov, V. Immobilization of sand dust and associated pollutants using bioaggregation. Water Air Soil Poll. 224(9), 1631. https://doi.org/10.1007/s11270-013-1631-0 (2013).
Wang, Z., Zhang, N., Ding, J., Lu, C. & Jin, Y. Experimental study on wind erosion resistance and strength of sands treated with microbial-induced calcium carbonate precipitation. Adv. Mater. Sci. Eng. 2018(1), 3463298. https://doi.org/10.1155/2018/3463298 (2018).
Zomorodian, S. M. A., Ghaffari, H. & O’Kelly, B. C. Stabilisation of crustal sand layer using biocementation technique for wind erosion control. Aeolian Res. 40, 34–41. https://doi.org/10.1016/j.aeolia.2019.06.001 (2019).
Abdullah, A., Kehinde, L., Mohamed, G. A. & Ahmed, A. Mitigating wind erosion of sand using biopolymer-assisted EICP technique. Soils Found. 60, 356–371 (2020).
Zhao, Z. et al. Biomimetic hydrogel composites for soil stabilization and contaminant mitigation. Environ. Sci. Technol. 50(22), 12401–12410 (2016).
Fatehi, H., Ong, D. E. L., Yu, J. & Chang, I. Biopolymers as green binders for soil improvement in geotechnical applications: A review. Geosciences 11(7), 291 (2021).
Chang, I. et al. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 24, 100385 (2020).
Chang, I., Prasidhi, J. I. & Cho, G. C. Soil strengthening using thermo-gelation biopolymers. Constr. Build. Mater. 77, 430–438 (2015).
Kavazanjian, J., Iglesias, E. & Karatas, I. Biopolymer soil stabilization for wind erosion control. Stand Alone. 881–884 (2009). https://doi.org/10.3233/978-1-60750-031-5-881
Alsanad, A. Novel biopolymer treatment for wind induced soil erosion (Arizona State University United States-Arizona, 2011).
Chen, R., Lee, I. & Zhang, L. Biopolymer stabilization of mine tailings for dust control. J. Geotech. Geoenviron. Eng. 141(2), 04014100 (2014).
Chen, R., Ding, X., Lai, H. & Zhang, L. Improving dust resistance of mine tailings using green biopolymer–An experimental and numerical investigation. Environ. Geotech. 8(6), 382–391 (2019).
Cui, M. J., Zheng, J. J. & Zhang, R. J. Influence of cementation level on the strength behaviour of bio-cemented sand. Acta Geotech. 12(5), 971–986 (2017).
An, R., Deng, C., Zhang, X. W., Ge, X. H., Yin, S. & Wang, Y. X. MICP Study on mechanical properties and microscopic mechanism of Xanthan gum enhancedmicrobial solidification of coral sand. Engineering science and Technology. (2024). https://link.cnki.net/urlid/51.1773.TB.20240920.0943.002
Rashmi, D., Animesh, J., Arjun, D. & Aloke, K. Microbially induced calcite precipitation using Bacillus velezensis with guar gum. PLoS ONE 13, 023674. https://doi.org/10.1371/journal.pone.023674 (2020).
Gomez, M. G., Charles, M. R., DeJong, J. T., Nelson, D. C. & Tsesarsky, M. Stimulation of native microorganisms for biocementation in samples recovered from field-scale treatment depths. J. Geotech. Geoenviron. Eng. 144(1), 04017098 (2018).
Zhang, S. J., Wang, Ou., Wang, T. L., Wang, L. & Liu, S. S. Strength improvement and microscopic mechanism of tailings sands using xanthan gum and guar gum. J. Eng. Geol. 31(2), 441–448. https://doi.org/10.13544/j.cnki.jeg.2022-0333 (2023).
Yang, T. B., Li, Q., Zhang, X. & Chen, J. B. Determination of calcium carbonate in Marine sediments by EDTA complexometric titration. The Analyzer 2, 45–48 (2016).
Wang, T. L., Wang, L., Liu, S. S., Zhang, S. J. & Wang, O. Experimental study on mechanical properties of expansive soil improved by xanthan gum and guar gum. China Railway Sci. 44(2), 1–10 (2023).
Latifi, N. & Horpibulusk, S. Improvement of problematic soils with biopolymer - An environmentally friendly soil stabilizer. J. Mater. Civ. Eng. 29(2), 04016204 (2017).
Thombare, N., Jha, U. & Mishra, S. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 88, 361–372 (2016).
Acharya, R., Pedarla, A., Bheemasetti, T. V. & Puppala, A. J. Assessment of guar gum biopolymer treatment toward mitigation of desiccation cracking on slopes built with expansive soils. Transp. Res. Record J. Transp. Res. Board 2657(1), 78–88 (2017).
Whitcomb, P. J. & Macosko, C. W. Rheology of xanthan gum. J. Rheol. 22(5), 493–505 (1978).
Chang, I., Im, J., Prasidhi, A. K. & Cho, G. C. Effects of xanthan gum biopolymer on soil strengthening. Construct. Build. Mater. 74, 65–72 (2015).
Shi, H. C., Zhang, J. & Wang, Z. J. Investigation on the freeze-thaw strength and index of sand. Rep. Heilongjiang Univ. Eng. 10(3), 27–31. https://doi.org/10.13524/j.2095-008x.2019.03.035 (2019).
Guo, J. & Peng, H. Study on the influence factors of compressive strength of slag-metakaolin-based geopolymer. J. Transp. Sci. Eng. 38(3), 33–39. https://doi.org/10.16544/j.cnki.cn43-1494/u.2022.03.007 (2022).
Sutherland, I. W. Structure-function relationships in microbial exopolysaccharides. Biotechnol. Adv. 12(2), 393–444 (1994).
Lin, H. B., Wei, R. J., Li, L. L., Wen, Z. L. & Pen, J. Experimental study on the strengthening effect of MICP enhanced by Xanthan gum. Sci. Henan 40(4), 618–627 (2022).
Zúñiga-Barra, H. et al. Improving the sustainable management of mining tailings through microbially induced calcite precipitation: Are view. Miner. Eng. 189, 107855 (2022).
Chang, I. & Cho, G. C. Shear strength behavior and parameters of microbial Gellan gum-treated soils: From sand to clay. Acta Geotech. 14(2), 361–375 (2019).
Dhami, N. K., Reddy, M. S. & Mukherjee, A. Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites. J. Microbiol. Biotechnol. 23(5), 707 (2013).
Tiwari, N., Satyam, N. & Sharma, M. Micro-mechanical performance evaluation of expansive soil biotreated with indigenous bacteria using MICP method. Sci. Rep. 11, 10324 (2021).
Duan, S. Q. et al. A new perspective on the semi-quantitative meso-structural failure mechanism of deep weak interlayer zone under different stress paths. Rock Mech. Rock Eng. 57, 3171–3195. https://doi.org/10.1007/s00603-024-03760-6 (2024).
Wang, P. et al. Anti-seepage reinforcement property and pollution control effect of bio-cemented fracture zone in electrolytic manganese residue dump. Sci. Total Environ. 958, 177928. https://doi.org/10.1016/j.scitotenv.2024.177928 (2025).
Tang, C. S. et al. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 79, 1–23. https://doi.org/10.1007/s12665-020-8840-9 (2020).
Mujah, D., Mohamed, A. S. & Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol J. 34(6), 524–537. https://doi.org/10.1080/01490451.2016.1225866 (2017).
Miao, L. C., Wu, L. Y. & Sun, X. H. Enzyme-catalysed mineralisation experiment study to solidify desert sands. Sci. Rep. 10(1), 10611. https://doi.org/10.1038/s41598-020-67566-6 (2020).
Sun, X. H., Miao, L. C., Wang, H. X., Wu, L. Y. & Zhang, J. Z. Enzymatic calcification to solidify desert sands for sandstorm control. Clim. Risk Manag. 33, 100323. https://doi.org/10.1016/j.crm.2021.100323 (2021).
Miao, L. C., Wang, H. G., Sun, X. H. & Wu, L. Y. Biomineralization for reducing and controlling sand-dust storms. Adv. Sci. 11(32), 03961. https://doi.org/10.1002/advs.202403961 (2024).
Diao, Y., Li, P. J., Hu, Q. S., Huang, J. Y. & Guo, X. Investigation on coal dust prevention by biomimetic mineralized dust suppressant with polyacrylic acid modifier. J. Environ. Chem. Eng. 11(6), 111223. https://doi.org/10.1016/j.jece.2023.111223 (2023).
Sun, X., Miao, L., Yuan, J., Wang, H. & Wu, L. Application of enzymatic calcification for dust control and rainfall erosion resistance improvement. Sci. Total Environ. 759, 143468. https://doi.org/10.1016/j.scitotenv.2020.143468 (2021).
Sun, X. H., Miao, L. C., Wang, H. X., Yuan, J. H. & Fan, G. C. Enhanced rainfall erosion durability of enzymatically induced carbonate precipitation for dust control. Sci. Total Environ. 791, 148369 (2021).
Wang, H. X. et al. Erosion resistance of treated dust soils based on the combined enzymatically induced carbonate precipitation and polyacrylic acid. Biogeotechnics 1(4), 100050. https://doi.org/10.1016/j.bgtech.2023.100050 (2023).
Miao, L. C., Wang, H. X., Sun, X. H., Wu, L. Y. & Fan, G. G. Effect analysis of biomineralization for solidifying desert sands. Biogeotechnics 2(1), 100065. https://doi.org/10.1016/j.bgtech.2023.100065 (2024).
Funding
The project was funded by items of Research on Key Technology of Sand fixation and Dust Prevention by Microbial Mineralization in Kashi Area, No: 2022E01046 (Xinjiang Autonomous Region Science and Technology Agency); Research on long-term weathering and corrosion resistance of soil reinforced by microorganism, No: DL2022013001 (Ministry of Science and Technology of P.R.China).
Author information
Authors and Affiliations
Contributions
"H. T. is responsible for drafting the trial protocol and writing the paper. J.L. Q. is in charge of revising the paper, supervising the trial, and securing funding. Y.D. H. is responsible for conducting data searches, analyzing data, and refining the paper. P.P.J. contributed to a portion of the reference survey and processing."
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tao, H., Jiang, P., Qu, J. et al. MICP incorporated biopolymer to mitigate wind erosion of sand. Sci Rep 15, 21710 (2025). https://doi.org/10.1038/s41598-025-06612-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-06612-7